Published online Sep 16, 2025. doi: 10.12998/wjcc.v13.i26.108052
Revised: April 28, 2025
Accepted: June 7, 2025
Published online: September 16, 2025
Processing time: 110 Days and 18.8 Hours
Adenoid cystic carcinoma (ACC) of the Bartholin’s gland represents an exceptio
This case series details two cases of primary ACC cases involving the Bartholin’s gland treated with radical surgical resection of the vaginal lesions. Notably, divergent therapeutic approaches resulted in contrasting prognoses: The patient receiving adjuvant radiotherapy following surgery maintained disease-free status with no locoregional recurrence or metastatic progression through 58 months of surveillance. Conversely, the non-radiated patient experienced disease recurrence within 18 months postoperatively.
Our findings suggest that postoperative radiation therapy may significantly de
Core Tip: In two cases of primary adenoid cystic carcinoma (ACC) involving the Bartholin’s gland treated with radical surgical resection of vaginal lesions, different therapeutic approaches resulted in contrasting prognoses: The patient who received adjuvant radiotherapy after surgery remained disease-free with no locoregional recurrence or metastatic progression through 58 months of surveillance, whereas the untreated patient experienced disease recurrence within 18 months postoperatively. Postoperative radiotherapy may significantly reduce local recurrence rates in Bartholin’s gland ACC, potentially influencing long-term disease control.
- Citation: Liu P, Huang HQ, Bian C, Quan Y. Adenoid cystic carcinoma of the Bartholin’s gland: Two case reports and review of literature. World J Clin Cases 2025; 13(26): 108052
- URL: https://www.wjgnet.com/2307-8960/full/v13/i26/108052.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i26.108052
Bartholin’s gland carcinoma (BGC) is an exceptionally rare malignancy, constituting approximately 0.1%–5% of vulvar cancers and 0.001% of all gynecologic neoplasms[1]. Histological subtypes encompass squamous cell carcinoma, epithe
Case 1: In April 2020, a 52-year-old Chinese multiparous woman (gravida 6, para 1) presented to our institution with a progressively enlarging mass in the right Bartholin’s gland region.
Case 2: A 46-year-old female presented to a local clinic with a 12-month history of a progressively enlarging, asymptoma
Case 1: Her medical history included a Bartholin’s cyst managed conservatively with local heat therapy a decade prior, without prior incision, drainage, or catheterization. The current lesion had grown from approximately 1 cm to 2 cm in diameter over 2-3 months, accompanied by persistent discomfort during sitting. She reported no additional symptoms, denied a history of sexually transmitted infections, and had a recent normal cervical Pap smear.
Case 2: 18 months after the initial treatment, the patient presented to our institution with a new left vulvar mass.
Case 1: She reported no history of hypertension or any other medical conditions.
Case 2: She reported no history of hypertension or any other medical conditions.
Case 1: No personal or family history was available.
Case 2: No personal or family history was available.
Case 1: Digital examination revealed unremarkable external and internal genitalia, with a firm, immobile 2.5 cm mass localized to the right Bartholin’s gland area. Vaginal mucosa and perineal skin appeared intact, and no nodularity or extension was noted on rectovaginal palpation.
Case 2: Gynecologic examination identified a 3 cm × 3 cm firm, tender lesion in the left vestibular gland region.
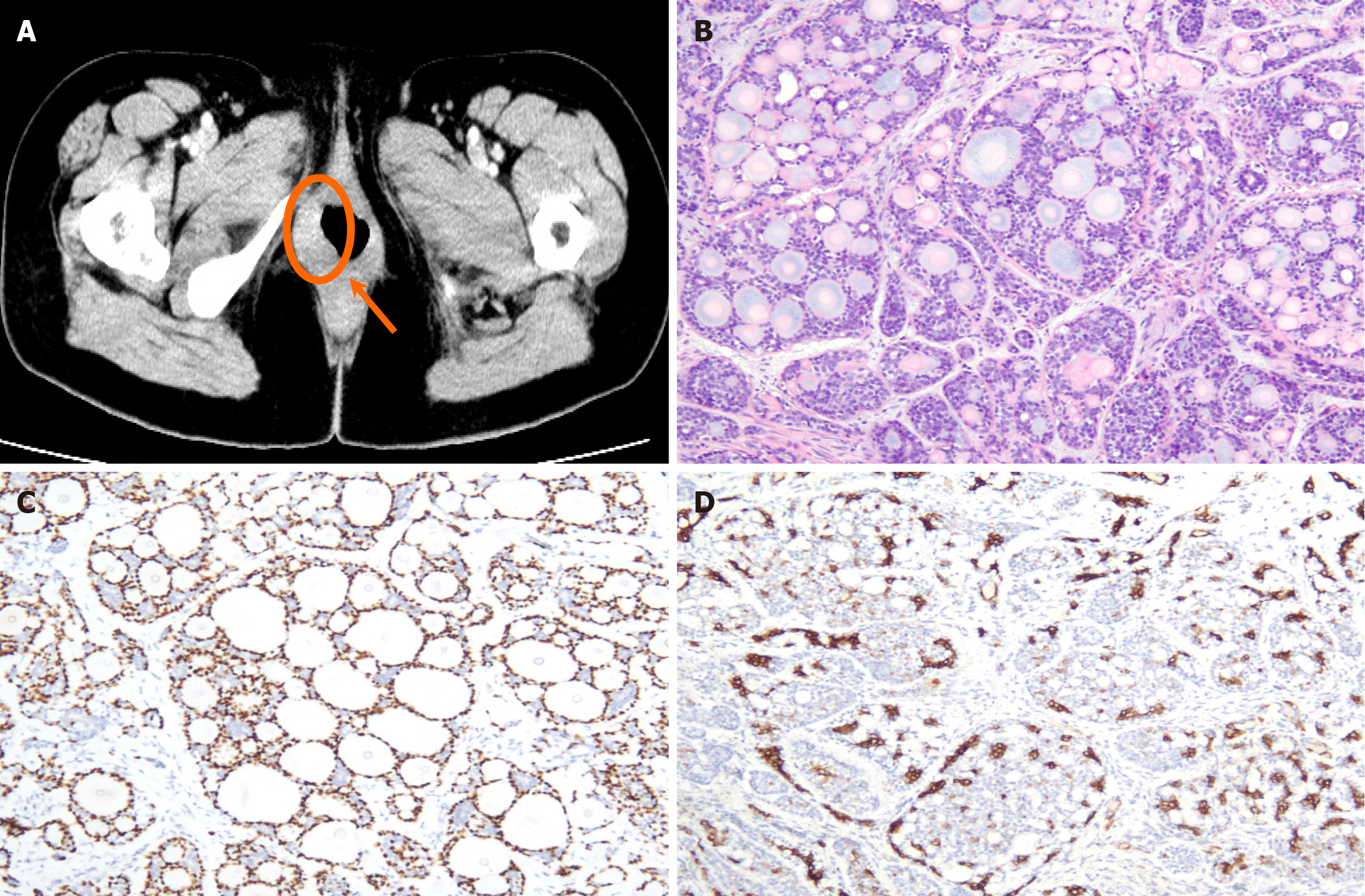
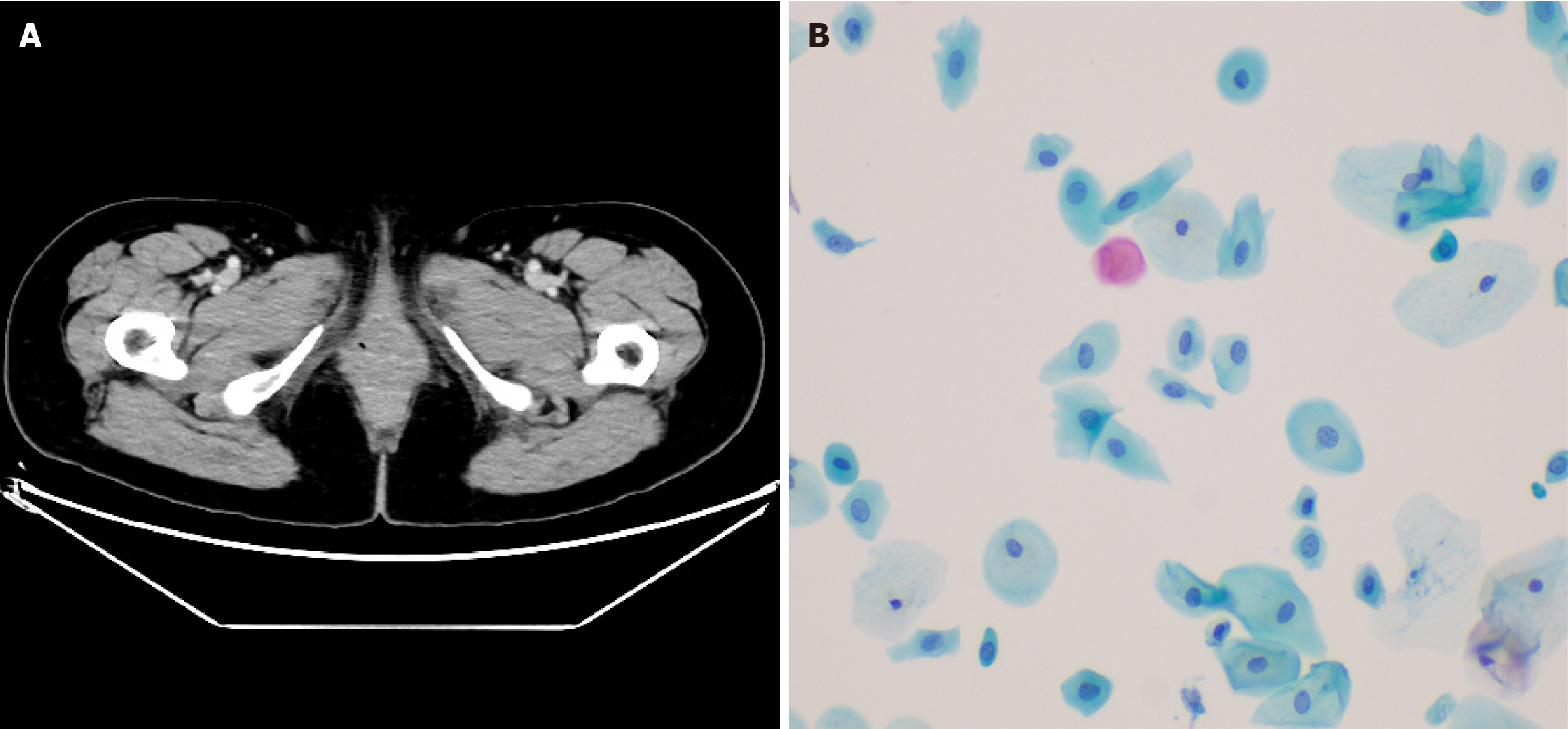
Case 1: Admission laboratory studies, including complete blood count and renal/hepatic function panels, were within normal limits. Histological analysis of the biopsy specimen revealed carcinoma with myoepithelial differentiation, characterized by neoplastic cells forming irregular papillary and tubular structures. Immunohistochemistry confirmed the myoepithelial lineage, with strong positivity for p63 and smooth muscle actin (SMA) in the peripheral cell layers of the tubular formations (Figure 1).
Case 2: Tumor marker panels (e.g., CA-125, CEA) were within normal limits. Initial biopsy confirmed ACC, characterized by infiltrative nests of basaloid cells with cribriform architecture.
Case 1: Contrast-enhanced pelvic computed tomography (CT) demonstrated a 1.8 cm × 1.5 cm heterogeneously enhan
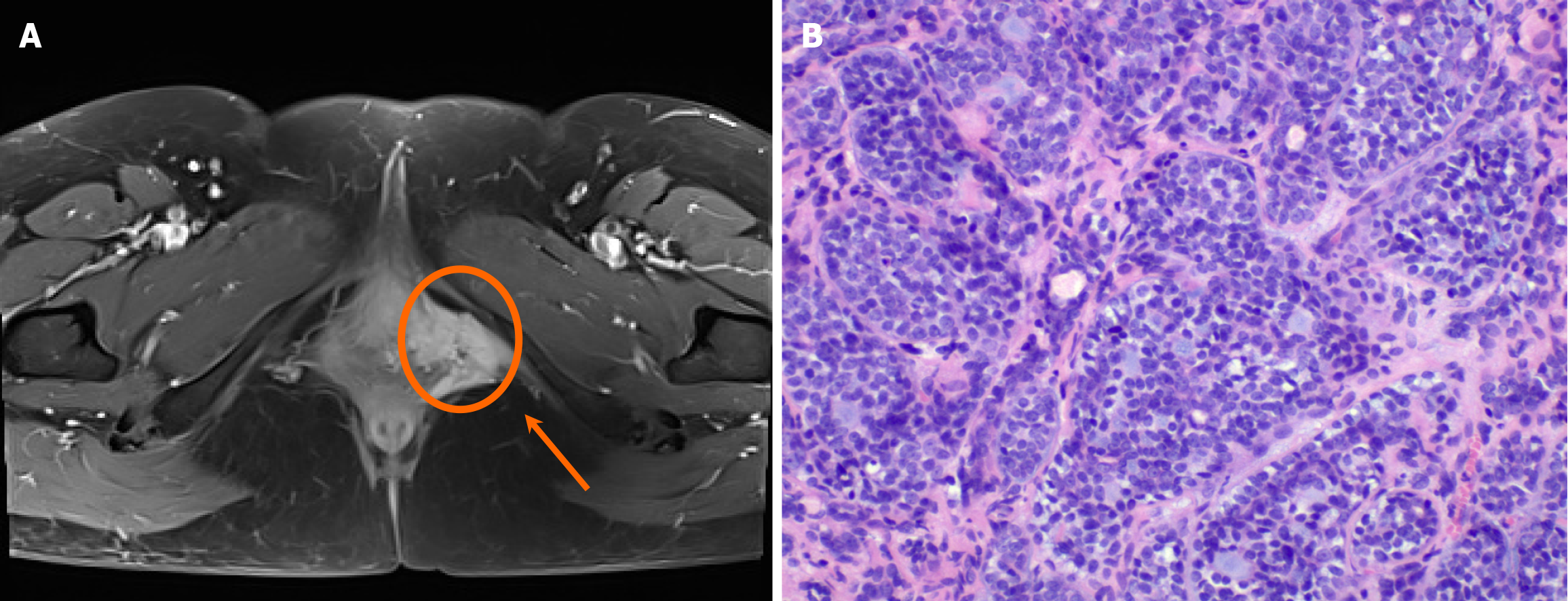
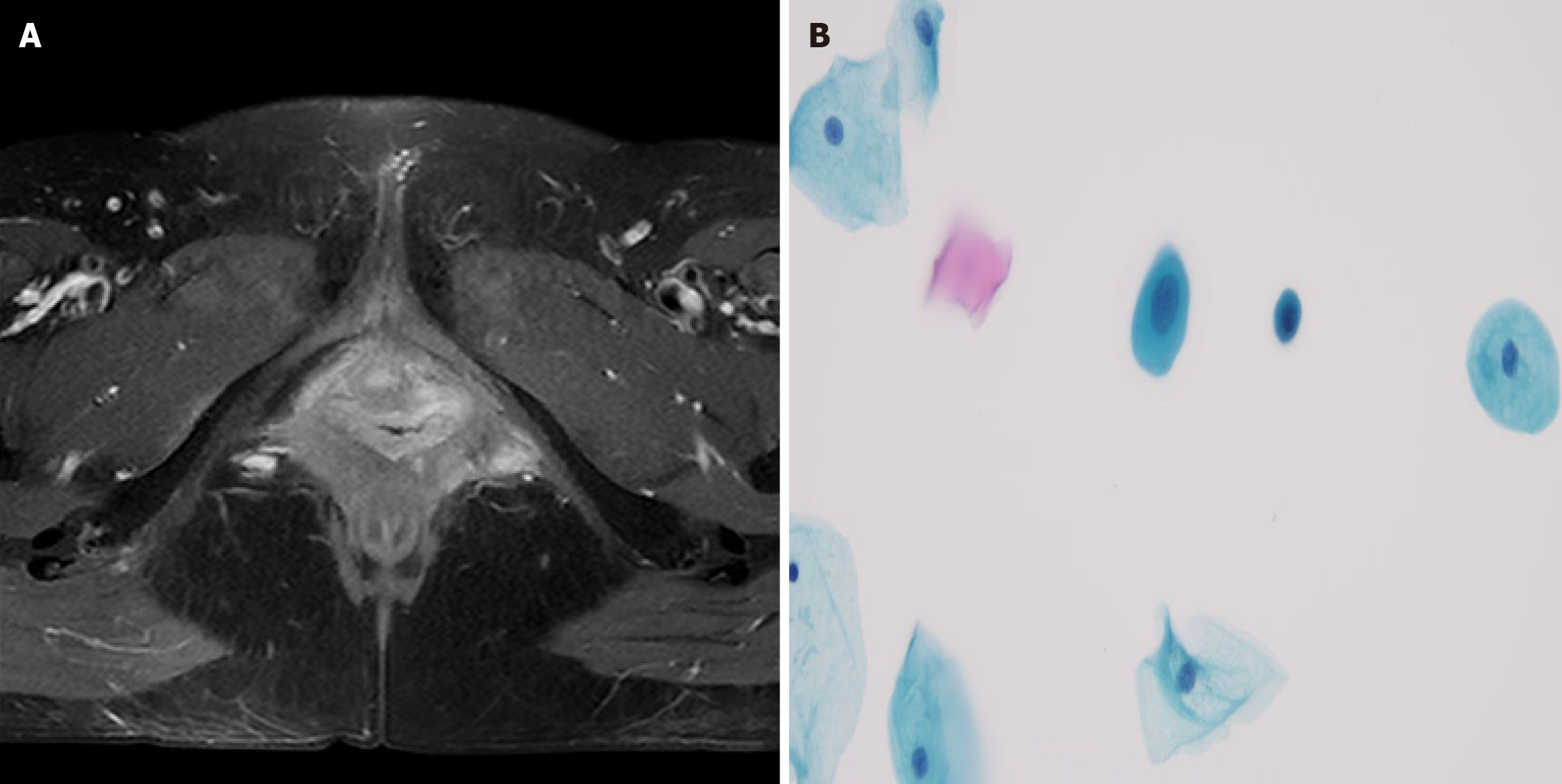
Case 2: Pelvic magnetic resonance imaging (MRI; in April 2022) demonstrated a 3 cm × 4 cm × 3 cm × 3 cm heterogeneous soft tissue mass in the left posterolateral vaginal introitus and anteromedial infiltration of the vaginal wall without midline crossing (Figure 2). A-repeat biopsy confirmed ACC recurrence.
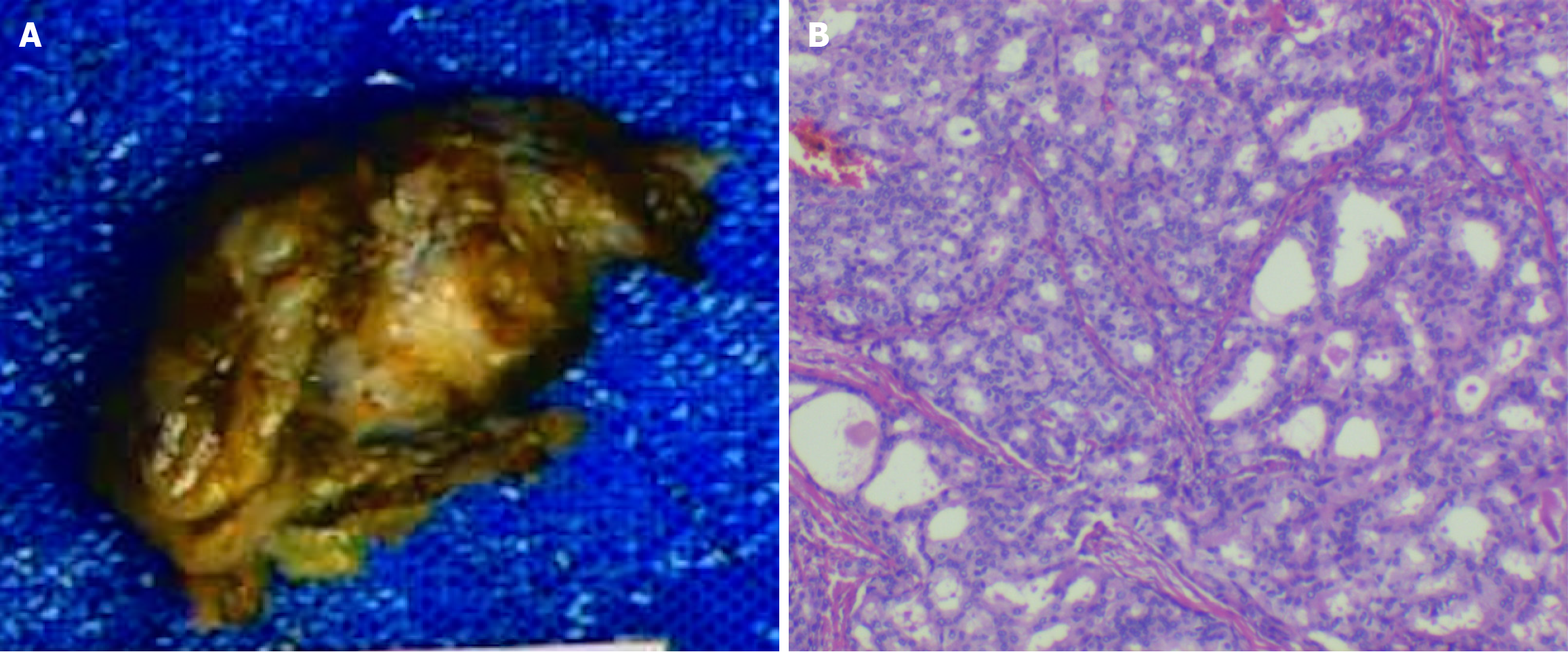
Case 1: The preoperative diagnosis was FIGO Stage II vulvar carcinoma (T2N0M0). The patient underwent radical surgical excision of the mass (Figure 3), with final pathology confirming negative resection margins.
Case 2: The diagnosis suggests a recurrence of the tumor.
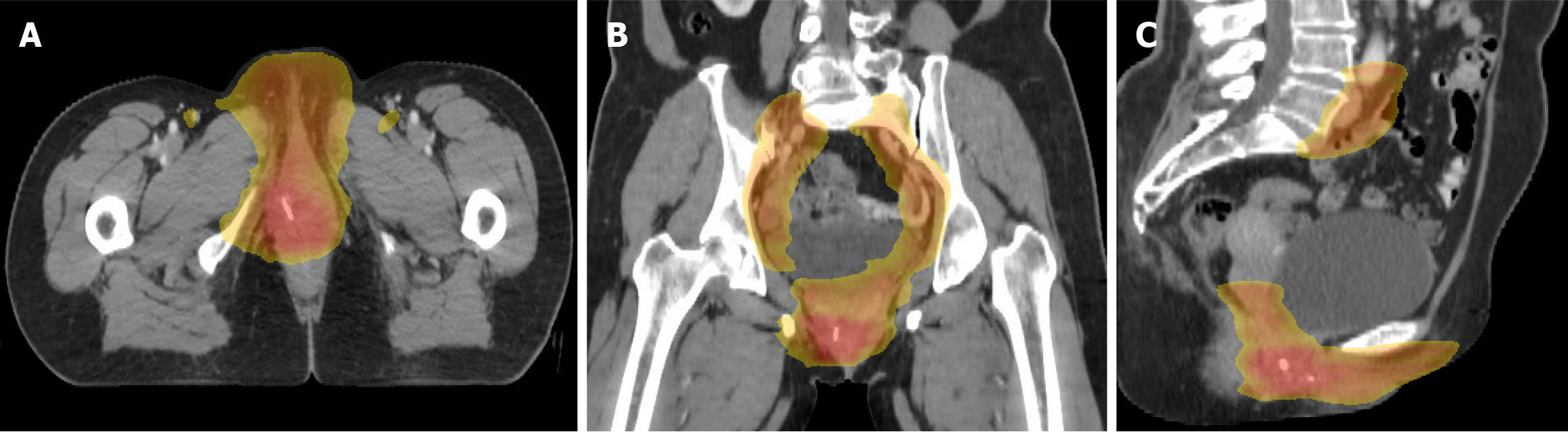
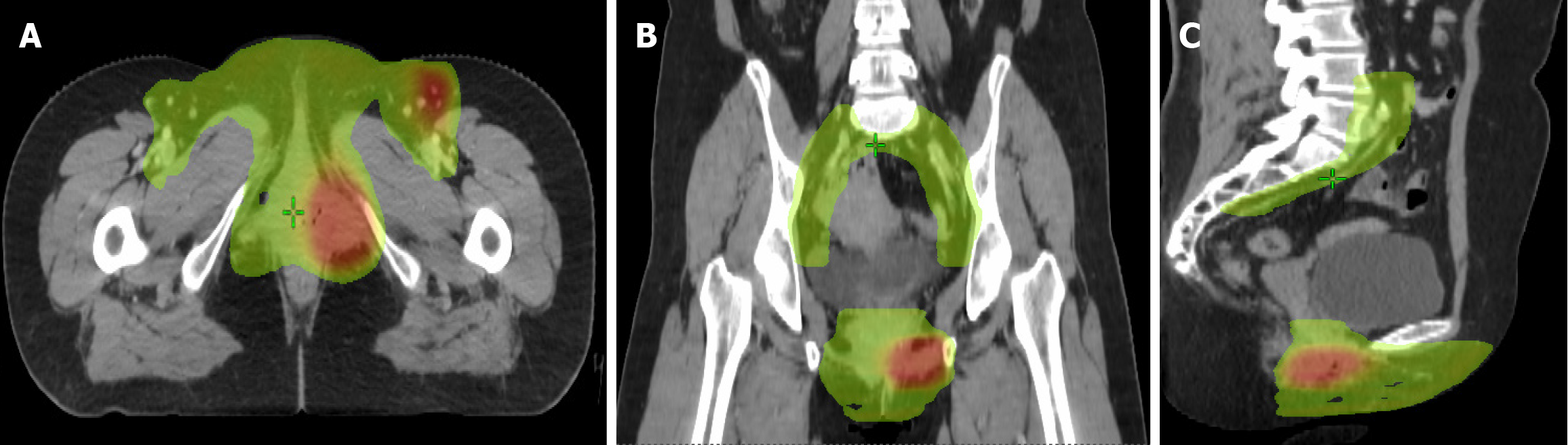
Case 1: Adjuvant intensity-modulated radiation therapy (IMRT) was administered, targeting the right inguinal region (45 Gy in 25 fractions, 1.8 Gy/fraction) and tumor bed (55 Gy in 25 fractions, 2.2 Gy/fraction) (Figure 4).
Case 2: Wide local excision of the left vulva was performed, with final pathology confirming ACC (Figure 2). Immunohistochemical profiling revealed: Ductal epithelium: CEA (+), epithelial membrane antigen (+). Myoepithelial cells: p53 (+), p63 (+), SMA (+), calponin (+), CD117 (+); S-100 (-). Proliferative index: The Ki-67 positivity rate was approximately 10%. The surgical margins were tumor-free. The patient underwent adjuvant treatment, which included external pelvic radiotherapy at a dose of 50.4 Gy divided into 28 fractions of 1.8 Gy over the left inguinal region. The tumor bed received a dose of 56 Gy divided into 28 fractions of 2.0 Gy, delivered by a technique of IMRT with concurrent weekly 40 mg/m2 cisplatin chemotherapy. Treatment tolerance was excellent, with only Grade 2 dermatitis and dysuria per Radiation Therapy Oncology Group (RTOG) criteria (Figure 5).
Case 1: Treatment tolerance was favorable, with only grade 2 dermatitis and dysuria per RTOG criteria. At 58 months of follow-up, the patient remains disease-free, with no evidence of local recurrence or metastatic progression (Figure 6).
Case 2: At the 38-month post-treatment surveillance, clinical and radiographic evaluations (MRI) showed no evidence of locoregional recurrence (Figure 7). The patient remains on a structured monitoring protocol.
ACC is a rare malignancy that occurs most frequently in the head and neck salivary glands. Clinically, it exhibits paradoxical behavior; it is slow-growing, yet locally aggressive, with a propensity for perineural invasion and late metastases. First described in 1853 by Robin and Laboulbène as “tumeur hétéradénique” and later classified as an adenocarcinoma by Foote and Frazell in 1953[5], ACC rarely manifests in the Bartholin’s glands (greater vestibular glands) of the vulva, a presentation termed BGC. BGC predominantly affects postmenopausal women with a median age of 53 years[6]. Epidemiological studies have reported a 5-year progression-free survival rate of 47% and an overall survival rate of 71%, highlighting its indolent but persistent clinical course[7]. Diagnosis is often delayed because of nonspecific symptoms (e.g., painless vulvar masses) that mimic benign Bartholin’s cysts, highlighting the need for heigh
The most common complaints associated with ACC of Bartholin’s gland are burning pain, palpable masses, and swelling, which can mimic a benign condition. Consequently, initial misdiagnosis or delayed diagnosis may occur in up to 50% of patients[8]. In the case of this malignancy manifesting in older, sexually inactive women, the diagnosis can be delayed, often resulting in the malignancy being detected at an advanced stage. By this time, urinary and rectal symp
The underlying mechanisms driving the development and progression of ACC remain incompletely understood. Although certain squamous subtype ACCs have shown a potential link to human papillomavirus (HPV) infection, nota
Molecular insights into salivary gland ACC revealed characteristic genetic alterations that affect key oncogenic pathways. Recent studies have identified recurrent mutations in chromatin regulators, including PIK3CA, ATM, and TSC1, in these tumors[14]. Togashi et al[15] detected that MYB or MY BL1 Locus rearrangements have been identified in nearly all ACCs examined, suggesting that these rearrangements may serve as effective diagnostic markers for ACCs[15]. Recently, gain-of-function mutations in NOTCH1 have been associated with poor outcomes in patients diagnosed with ACC[16]. Pure ACCs from the Bartholin gland parallel other anatomical sites, featuring characteristic MYB-NFIB and MYBL1-NFIB fusion oncoproteins that underscore shared pathogenesis mechanisms[12]. These recurrent translocations have demonstrated diagnostic utility, with MYB/MYBL1 rearrangement analysis emerging as a valuable adjunct tool for confirming AdCC-BG diagnosis[17]. Diagnostic confirmation of Bartholin gland carcinomas requires stringent clinicopa
The management of ACC, particularly at rare sites such as the Bartholin gland, remains poorly standardized because of the rarity of the disease and the absence of robust prospective trials[18]. Current strategies prioritize surgical resection as the cornerstone of treatment, with procedural selection guided by tumor characteristics and patient-specific factors. The surgical approach encompasses wide local excisions, hemivulvectomy, simple vulvectomy, and radical vulvectomy, with or without inguinal and/or femoral lymphadenectomy, which is determined on a case-by-case basis, considering factors such as patient age, reproductive status, comorbidities, and the presence of pregnancy. The European Society of Gynecological Oncology guideline recommends surgical excision margins of at least 1 cm. Surgical excision aims to achieve tumor-free margins that decrease the likelihood of local recurrence. Hemivulvectomy is recommended for lesions measuring less than 2 cm, whereas radical total vulvectomy and inguinofemoral lymph node dissection are the preferred surgical approaches for larger lesions[4]. One study revealed that 30% of patients in the radical vulvectomy group had positive resection margins compared with 48% in the simple excision group[19]. However, the role of lymph node dissection in ACC remains controversial. The prevailing opinion among experts in the field is that lymphadenectomy should not be routinely recommended unless there is clinical or radiological suspicion of lymph node involvement, as this tumor often metastasizes to distant organs before involving locoregional lymph nodes[2].
Because of the absence of prospective trials, a consensus regarding the efficacy of radiation therapy remains elusive. ACC is characterized by its propensity for frequent and often silent distant metastases, likely attributable to its proclivity for extensive perineural invasion compounded by delayed diagnosis[20]. Distant metastasis of ACC is rare, with the most prevalent sites being the lungs, followed by the liver and, in rare instances, the bone[21]. To date, guidelines for post
Several reports have emerged that support the notion that adjuvant radiotherapy confers benefits to patients diagnosed with breast cancer with positive surgical margins. A review by Hsu et al[22] reported that among 16 cases of surgical margin positivity treated with adjuvant radiotherapy, ten patients did not experience local recurrence. In contrast, six patients developed distant metastasis[6]. This finding suggests a beneficial role of adjuvant radiotherapy in reducing local recurrence. Copeland et al[7] reported nine (25%) recurrences (six local, two distant, and one local and distant), and it is noteworthy that local failure occurred in one of 14 patients (7%) who received adjuvant irradiation compared with six of 22 (27%) who did not[8,23]. Alsan et al[24] reported that radiation therapy decreased local recurrence, especially in patients with positive margins. Positive margins exhibited a 35% recurrence risk compared to a 10% recurrence risk observed in negative margins, indicating that radiotherapy can effectively manage local recurrence[21]. This finding suggests a beneficial role for adjuvant radiotherapy, at least in reducing local recurrence.
As previously indicated in our reports, the initial patient who received radiotherapy demonstrated a positive outcome at the subsequent follow-up. Conversely, patients who did not undergo radiotherapy experienced rapid recurrence. Notably, radiotherapy has been observed to reduce the likelihood of local tumor recurrence; radiotherapy was admini
Our findings suggest that postoperative radiation therapy may significantly decrease the local recurrence rates in Bartholin’s gland ACC, potentially influencing long-term disease control. This comparative outcome analysis underscores the importance of integrating adjuvant radiotherapy into treatment protocols for this rare malignancy. Given the limited number of cases presented, the possibility of cancer should be considered in any female older than 40 years of age with a lesion near the Bartholin’s glands, and the optimal management of ACC of the Bartholin’s gland should be tailored to each patient. Because recurrence may occur over a long period, long-term follow-up is required for such patients.
| 1. | DePasquale SE, McGuinness TB, Mangan CE, Husson M, Woodland MB. Adenoid cystic carcinoma of Bartholin's gland: a review of the literature and report of a patient. Gynecol Oncol. 1996;61:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Woida FM, Ribeiro-Silva A. Adenoid cystic carcinoma of the Bartholin gland: an overview. Arch Pathol Lab Med. 2007;131:796-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ramanah R, Allam-Ndoul E, Baeza C, Riethmuller D. Brain and lung metastasis of Bartholin's gland adenoid cystic carcinoma: a case report. J Med Case Rep. 2013;7:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Di Donato V, Casorelli A, Bardhi E, Vena F, Marchetti C, Muzii L, Benedetti Panici P. Bartholin gland cancer. Crit Rev Oncol Hematol. 2017;117:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Foote FW Jr, Frazell EL. Tumors of the major salivary glands. Cancer. 1953;6:1065-1133. [PubMed] [DOI] [Full Text] |
| 6. | Chraibi Z, Hebert T, Body G, Arbion F, Ouldamer L. [Bartholin's gland carcinoma]. Gynecol Obstet Fertil. 2014;42:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Copeland LJ, Sneige N, Gershenson DM, McGuffee VB, Abdul-Karim F, Rutledge FN. Bartholin gland carcinoma. Obstet Gynecol. 1986;67:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 80] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Bhalwal AB, Nick AM, Dos Reis R, Chen CL, Munsell MF, Ramalingam P, Salcedo MP, Ramirez PT, Sood AK, Schmeler KM. Carcinoma of the Bartholin Gland: A Review of 33 Cases. Int J Gynecol Cancer. 2016;26:785-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Marcinow A, Ozer E, Teknos T, Wei L, Hurtuk A, Old M, Agrawal A, Carrau R, Iwenofu OH. Clinicopathologic predictors of recurrence and overall survival in adenoid cystic carcinoma of the head and neck: a single institutional experience at a tertiary care center. Head Neck. 2014;36:1705-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Miller ED, Blakaj DM, Swanson BJ, Xiao W, Gillison ML, Wei L, Bhatt AD, Diavolitsis VM, Wobb JL, Kang SY, Carrau RL, Grecula JC. Sinonasal adenoid cystic carcinoma: Treatment outcomes and association with human papillomavirus. Head Neck. 2017;39:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Xing D, Schoolmeester JK, Ren Z, Isacson C, Ronnett BM. Lower Female Genital Tract Tumors With Adenoid Cystic Differentiation: P16 Expression and High-risk HPV Detection. Am J Surg Pathol. 2016;40:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Xing D, Bakhsh S, Melnyk N, Isacson C, Ho J, Huntsman DG, Gilks CB, Ronnett BM, Horlings HM. Frequent NFIB-associated Gene Rearrangement in Adenoid Cystic Carcinoma of the Vulva. Int J Gynecol Pathol. 2017;36:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Nakamura K, Aimono E, Tanishima S, Nomura H, Imai M, Hayashi H, Nishihara H. Genetic profiling of patients with adenoid cystic carcinoma of the Bartholin's glands reveals potential new routes for targeted therapies: a case report. Diagn Pathol. 2020;15:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Rettig EM, Talbot CC Jr, Sausen M, Jones S, Bishop JA, Wood LD, Tokheim C, Niknafs N, Karchin R, Fertig EJ, Wheelan SJ, Marchionni L, Considine M, Ling S, Fakhry C, Papadopoulos N, Kinzler KW, Vogelstein B, Ha PK, Agrawal N. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev Res (Phila). 2016;9:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Togashi Y, Dobashi A, Sakata S, Sato Y, Baba S, Seto A, Mitani H, Kawabata K, Takeuchi K. MYB and MYBL1 in adenoid cystic carcinoma: diversity in the mode of genomic rearrangement and transcripts. Mod Pathol. 2018;31:934-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Sajed DP, Faquin WC, Carey C, Severson EA, H Afrogheh A, A Johnson C, Blacklow SC, Chau NG, Lin DT, Krane JF, Jo VY, Garcia JJ, Sholl LM, Aster JC. Diffuse Staining for Activated NOTCH1 Correlates With NOTCH1 Mutation Status and Is Associated With Worse Outcome in Adenoid Cystic Carcinoma. Am J Surg Pathol. 2017;41:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | López-varela E, Oliva E, Mcintyre J, Fuller A. Primary treatment of Bartholin’s gland carcinoma with radiation and chemoradiation: a report on ten consecutive cases. Int J Gynecol Cancer. 2007;17:661-667. [DOI] [Full Text] |
| 18. | Chamlian DL, Taylor HB. Primary carcinoma of Bartholin's gland. A report of 24 patients. Obstet Gynecol. 1972;39:489-494. [PubMed] |
| 19. | Milchgrub S, Wiley EL, Vuitch F, Albores-Saavedra J. The tubular variant of adenoid cystic carcinoma of the Bartholin's gland. Am J Clin Pathol. 1994;101:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Blontzos N, Iavazzo C, Giovannopoulou E, Novkovic N, Psomiadou V, Vorgias G. Recurrence of Bartholin gland mucinous adenocarcinoma managed with posterior exenteration: a case report. J Obstet Gynaecol. 2020;40:1029-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Yang SY, Lee JW, Kim WS, Jung KL, Lee SJ, Lee JH, Bae DS, Kim BG. Adenoid cystic carcinoma of the Bartholin's gland: report of two cases and review of the literature. Gynecol Oncol. 2006;100:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Hsu ST, Wang RC, Lu CH, Ke YM, Chen YT, Chou MM, Ho ES. Report of two cases of adenoid cystic carcinoma of Bartholin's gland and review of literature. Taiwan J Obstet Gynecol. 2013;52:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Feinberg J, Da Cruz Paula A, da Silva EM, Pareja F, Patel J, Zhu Y, Selenica P, Leitao MM Jr, Abu-Rustum NR, Reis-Filho JS, Joehlin-Price A, Weigelt B. Adenoid cystic carcinoma of the Bartholin's gland is underpinned by MYB- and MYBL1- rearrangements. Gynecol Oncol. 2024;185:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Alsan CI, Vinh-Hung V, Eren F, Abacioğlu U. Adenoid cystic carcinoma of the Bartholin's gland: case report and systematic review of the literature. Eur J Gynaecol Oncol. 2011;32:567-572. [PubMed] |
| 25. | Budd GT, Groppe CW. Adenoid cystic carcinoma of the salivary gland. Sustained complete response to chemotherapy. Cancer. 1983;51:589-590. [PubMed] [DOI] [Full Text] |
| 26. | Ha H, Keam B, Ock CY, Heo DS. Efficacy of cyclophosphamide, doxorubicin, and cisplatin for adenoid cystic carcinoma, and their relationship with the pre-chemotherapy tumor growth rate. Chin Clin Oncol. 2020;9:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Thibault I, Lavallée MC, Aubin S, Jain S, Laflamme N, Vigneault É. Management of Bartholin's gland carcinoma using high-dose-rate interstitial brachytherapy boost. Brachytherapy. 2013;12:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |