Published online Sep 16, 2025. doi: 10.12998/wjcc.v13.i26.104421
Revised: April 2, 2025
Accepted: May 28, 2025
Published online: September 16, 2025
Processing time: 215 Days and 20.8 Hours
Hepatitis D virus-hepatitis B virus (HDV-HBV) co-infection accelerates liver di
A 40-year-old female developed malaise and jaundice with an alanine amino
Patients with HDV-HBV co-infection combined with AIH can achieve clinical remission following combination therapy, and the study of immunomodulatory mechanisms should be emphasized.
Core Tip: This study reports, for the first time, two patients with hepatitis D virus (HDV)-hepatitis B virus co-infection combined with autoimmune hepatitis (AIH) who were not treated with interferon and achieved serological conversion and histological remission with antiviral drugs (entecavir/tenofovir alafenamide) in combination with immunosuppression (prednisone + azathioprine). Patients with severe HDV-related liver disease should be routinely screened for autoantibodies to avoid exacerbation by interferon therapy. The combination therapy was effective in controlling HDV-hepatitis B virus co-infection associated AIH, suggesting that aberrant immunoregulation may be an important mechanism of HDV-induced AIH, providing new evidence for elucidating the pathogenesis of this complex disease, and emphasizing the necessity of immune mechanism research.
- Citation: Dou J, Zhao XY, Wang ZG, Ning ZH, Wang XZ, Guo F. Hepatitis B virus and hepatitis D virus co-infection complicated by autoimmune hepatitis: Two case reports. World J Clin Cases 2025; 13(26): 104421
- URL: https://www.wjgnet.com/2307-8960/full/v13/i26/104421.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i26.104421
Hepatitis D virus (HDV) is a satellite virus that requires the envelope proteins of hepatitis B virus (HBV) for particle assembly and propagation. Co-infection with HBV and HDV can lead to severe viral hepatitis, accelerating the pro
Case 1: A 40-year-old female presented to Department of Hepatology with fatigue and jaundice for one month.
Case 2: A 58-year-old male presented with vague pain in the right side for one month.
Case 1: In September 2011, the patient presented with right-sided distension, pain and malaise. Positive hepatitis B markers were found and a hepatic puncture was performed. The patient refused treatment. In 2012, due to abnormalities in liver function, she underwent a second liver biopsy in an external hospital, which did not rule out primary biliary cholangitis. She was started on ursodeoxycholic acid capsules at 750 mg/day with intermittent follow-up. In 2014, she was started on antiviral therapy with tibivudine. By 2015, due to the diagnosis of AIH, she was advised to switch to antiviral therapy with entecavir along with prednisone. Due to the unknown nature of a pelvic mass, the patient and her family cautiously postponed prednisone treatment. The mass was later clarified to be an ovarian luteal cyst, and no pelvic mass was seen on routine gynecological ultrasound during late follow-up. In 2017, antiviral therapy was switched to tenofovir disoproxil fumarate during pregnancy, and immunosuppressive therapy with azathioprine was added in 2018, and then prednisone 20 mg/day was added in 2019. Following the development of splenomegaly and leukopenia (1.62 × 109/L), her treatment was adjusted to tenofovir disoproxil fumarate plus prednisone 10 mg/day, and azathioprine was discontinued. In 2021, the patient presented to an outpatient hospital and upper abdominal magnetic resonance imaging revealed probable cirrhosis. The treatment regimen was adjusted to tenofovir disoproxil fumarate, methylprednisolone 16 mg/day, merti-mescaline 0.5 g/day, compound glycyrrhizin to protect the liver and reduce enzymes, and tapered down to methylprednisolone 12 mg/day. In July 2023, the patient discontinued the use of ursodeoxycholic acid and methylprednisolone, and continued antiviral therapy with tenofovir disoproxil fumarate only. In August 2023, the patient presented to the clinic with complaints of malaise and jaundice for one month.
Case 2: The patient reported that hepatitis B was identified on physical examination 20 years previously, but no antiviral treatment was given, and since then there has been no regular review. He was admitted to the hospital with the main complaint of pain in the right hypochondrium for one month.
Case 1: The patient had tuberculous pleurisy in 2003 which was cured.
Case 2: The patient has had fatty liver for one year.
Case 1: The patient was employed as a clerk, with no tobacco or alcohol addiction, and denied any family history of disease or hereditary disease.
Case 2: A smoking history of 20 years, with an average of 15 cigarettes/day, and occasional alcohol consumption in the past, with abstinence for 3 years.
Case 1: Physical examination revealed mild yellowing of the skin, mucous membranes and sclera over the entire body, liver palms (+), and mild edema of both lower limbs.
Case 2: No special features.
Case 1: In 2011, liver function tests showed the following: Alanine aminotransferase 161 U/L, aspartate aminotransferase 118 U/L, alkaline phosphatase 38.6 U/L, and gamma-glutamyl transferase 43.6 U/L. HBV DNA titers were below the limit of detection, quantitative hepatitis B surface antigen was 1387 IU/mL and anti-hepatitis B e antibody was 0.09 IU/mL. Anti-nuclear antibody titer was 1:100 and anti-mitochondrial M2 antibody was positive.
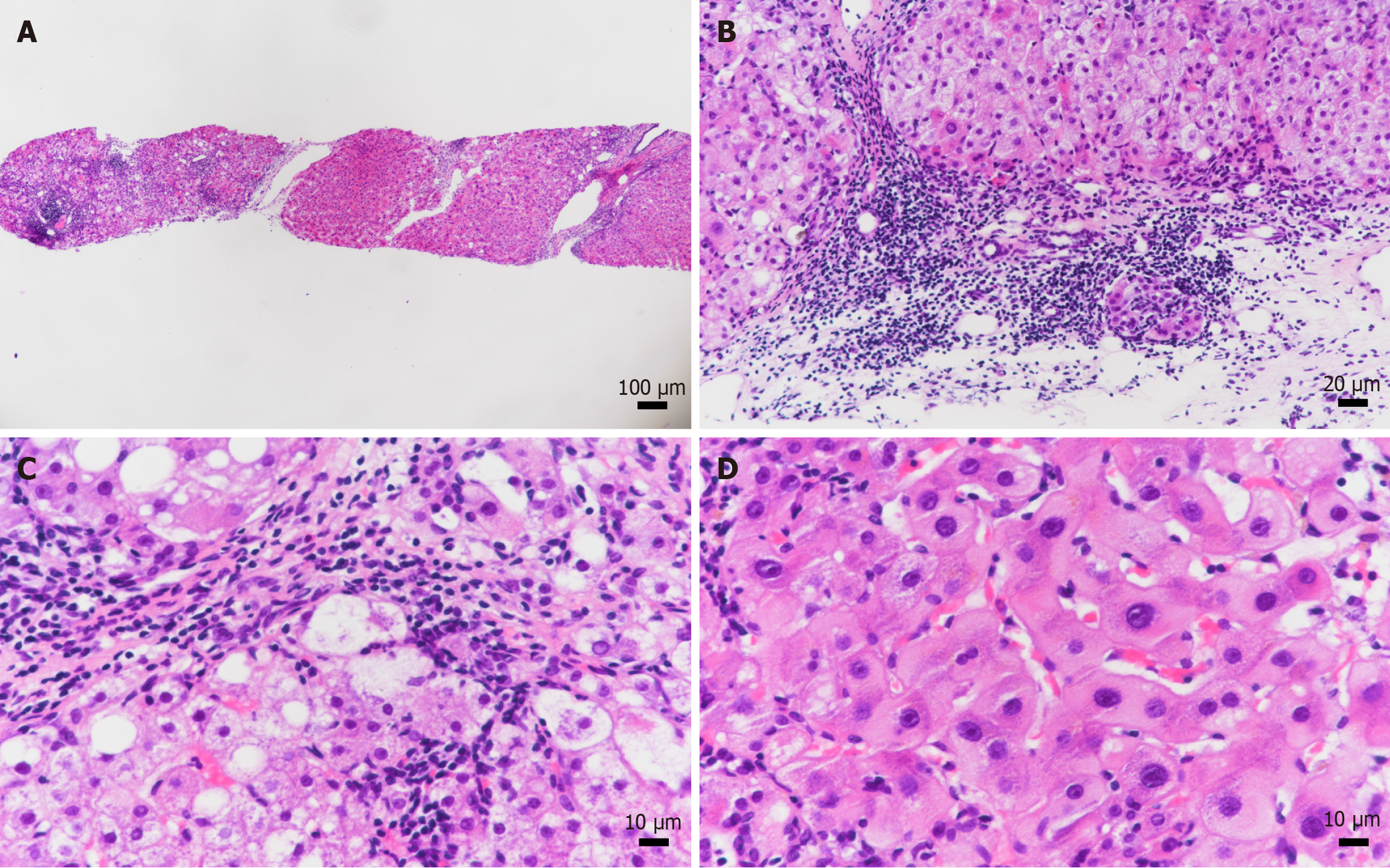
In August 2023, high-sensitivity HBV DNA was 1.12 × 102 IU/mL, alanine aminotransferase 985.00 U/L, aspartate aminotransferase 2115.00 U/L, alkaline phosphatase 198.83 U/L, gamma-glutamyl transferase 112.60 U/L, total bilirubin 97.20 μmol/L, plasminogen time 26.80 seconds, international normalized ratio 2.29, albumin 27.38 g/L, IgG 47.1 g/L, HDV antibody IgG and IgM positive, HDV RNA 1.6 × 107 copies/mL (Tables 1 and 2), and liver biopsy showed G3S4 (Figure 1).
| Test item | August 2023 | November 2023 |
| Current antiviral medication | Tenofovir propofol fumarate | Tenofovir propofol fumarate |
| Cirrhosis stage | Decompensated | Decompensated |
| ALT (U/L) | 985.00 | 17.20 |
| AST (U/L) | 2115.0 | 23.50 |
| GGT (U/L) | 161.61 | 28.93 |
| ALP(U/L) | 198.83 | 57.50 |
| Alb (g/L) | 27.38 | 41.10 |
| TBIL (μmol/L) | 97.20 | 45.90 |
| DBIL (μmol/L) | 60.22 | 23.60 |
| IBIL(μmol/L) | 36.98 | 22.30 |
| LDH (U/L) | 653.11 | 267.88 |
| CHE (U/L) | 2240 | 1310.00 |
| TBA (μmol/L) | 126.40 | 176.20 |
| TP (g/L) | 80.56 | 61.80 |
| Glb (g/L) | 53.18 | 20.70 |
| PT (seconds) | 26.80 | 22.20 |
| PTA (%) | 27.70 | 40.50 |
| INR | 2.29 | 1.73 |
| WBC (109/L) | 1.55 | 1.57 |
| HGB (g/L) | 105.00 | 73.00 |
| PLT (109/L) | 103.00 | 60.00 |
| IgG (g/L) | 47.10 | 13.62 |
| AFP (ng/mL) | 16.80 | 3.84 |
| HBsAg (IU/mL) | 12286.00 | 14214.00 |
| HBeAg (IU/mL) | 0.12 | 0.13 |
| HBV DNA (IU/mL) | 1.12 × 102 | < 20 |
| Anti-HDV IgM | Positive | Positive |
| Anti-HDV IgG | Positive | Positive |
| Liver stiffness (kPa) | 16.5 | 17.6 |
| Fat attenuation (db/m) | 268 | 246 |
| Non-alcoholic fatty liver disease | Present | Present |
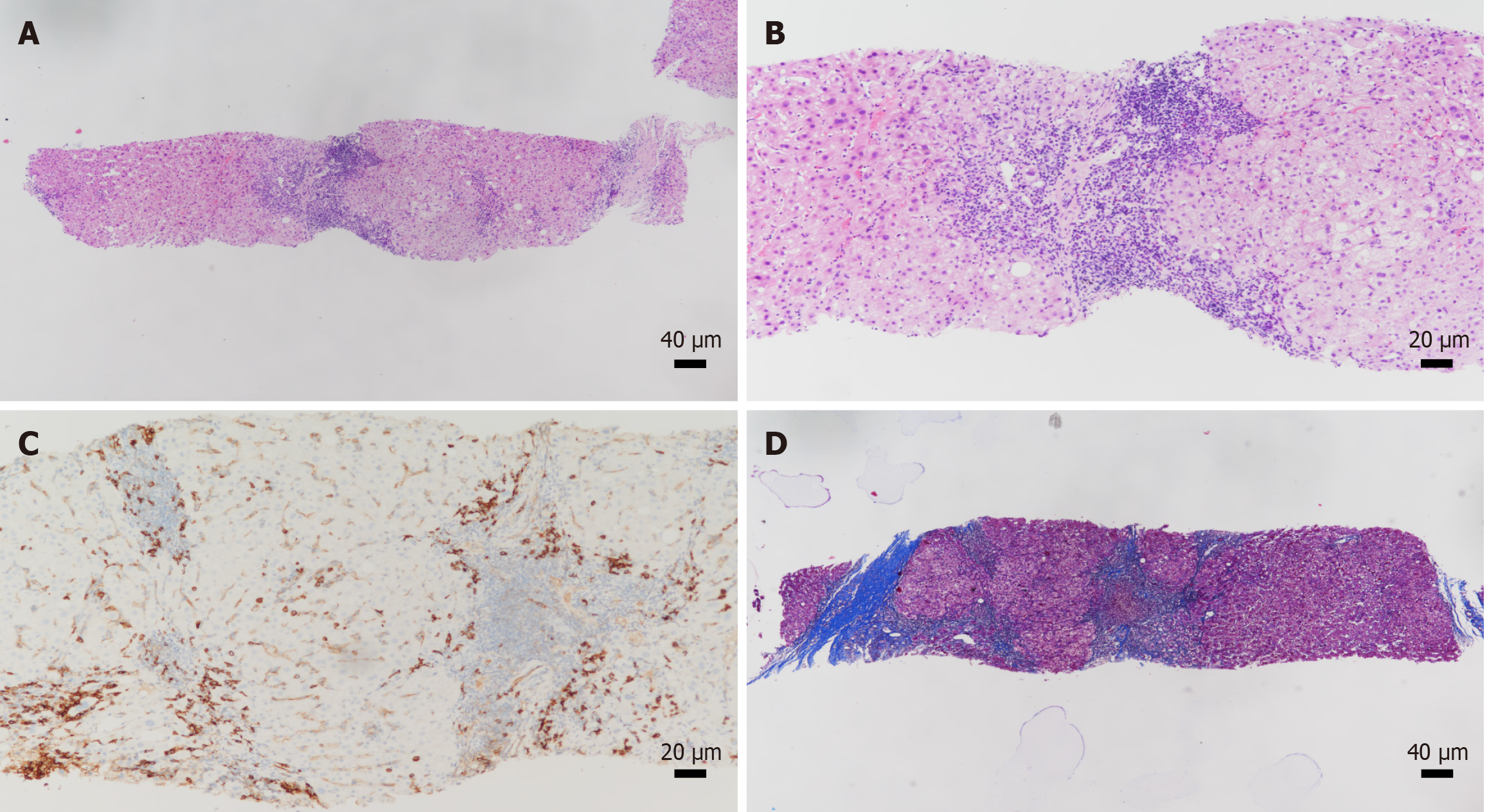
| Test/feature | Case 1 | Case 2 | |
| Anti-nuclear antibody | 1:320 | 1:320 | |
| IgG (g/L) elevation | 47.10 | 22.60 | |
| Liver kidney microsomal type 1 antibody | - | + | |
| Anti-soluble liver antigen antibody | - | - | |
| Pathology | Portal inflammation | Moderate | Moderate |
| Interface hepatitis | Mild to moderate | Moderate | |
| Plasma cell infiltration | Present | Present | |
| Rosette formation | Absent | Absent | |
| Piecemeal necrosis | Absent | Absent | |
| Inflammation grade (G) | G3 | G2 | |
| Fibrosis stage (S) | S4 | S3 | |
Case 2: Alanine aminotransferase 77.20 U/L, aspartate aminotransferase 60.80 U/L, alkaline phosphatase 142.50 U/L, gamma-glutamyl transferase 93.58 U/L, HDV antibody IgG and IgM positive, HDV RNA negative, high-sensitivity HBV DNA < 20 IU/mL, quantitative hepatitis B surface antigen 7755.00 IU/mL, quantitative hepatitis B e antigen 0.09 IU/mL, anti-liver and kidney microsomal type 1 antibody positive, antinuclear antibodies IgG 1:320, IgG 22.60 g/L (Tables 2 and 3), and liver biopsy showed G2S3 (Figure 2).
| Test item | June 2023 | January 2024 |
| Current antiviral medication | Entecavir | Entecavir |
| Cirrhosis stage | Compensated | Compensated |
| ALT (U/L) | 77.20 | 47.60 |
| AST (U/L) | 60.80 | 32.90 |
| GGT (U/L) | 93.58 | 86.89 |
| ALP (U/L) | 142.50 | 100.40 |
| Alb (g/L) | 40.90 | 36.40 |
| TBIL (μmol/L) | 13.20 | 7.20 |
| DBIL (μmol/L) | 2.90 | 1.80 |
| IBIL (μmol/L) | 10.30 | 5.40 |
| LDH (U/L) | 218.10 | 214.00 |
| CHE (U/L) | 7380 | 6470.00 |
| TBA (μmol/L) | 5.60 | 21.10 |
| TP (g/L) | 78.80 | 67.90 |
| Glb (g/L) | 37.90 | 31.50 |
| PT (seconds) | 13.20 | 13.50 |
| PTA (%) | 83.30 | 95.0 |
| WBC (109/L) | 4.70 | 6.53 |
| HGB (g/L) | 144.00 | 134.00 |
| PLT (109/L) | 143.00 | 129.00 |
| IgG (g/L) | 22.60 | 14.22 |
| AFP (ng/mL) | 10.07 | 7.40 |
| HBsAg (IU/mL) | 7755 | 9605.00 |
| HBeAg (IU/mL) | 0.09 | 0.12 |
| HBV DNA (IU/mL) | < 20 | < 20 |
| Anti-HDV IgM | Positive | Positive |
| Anti-HDV IgG | Positive | Positive |
| Liver stiffness (kPa) | 21.6 | 22.7 |
| Fat attenuation (db/m) | 294 | 283 |
| Non-alcoholic fatty liver disease | Present | Present |
Case 1: Nuclear magnetic resonance imaging showed cirrhosis with diffuse intrahepatic regenerative nodules, splenomegaly, portal hypertension, splenic varicose vein, and open umbilical vein.
Case 2: Enhanced computed tomography of the upper abdomen showed cirrhosis, splenomegaly, portal hypertension and periumbilical vein opening.
The patient was diagnosed as HBV, HDV, and AIH.
The patient was diagnosed as HBV, HDV, and AIH.
Treatment consisted of hepatoprotective and anti-yellowing, anti-infective, artificial liver support therapy, and antiviral therapy with prednisone 40 mg/day and tenofovir disoproxil fumarate 25 mg/day.
The patient was treated with antiviral therapy with entecavir 0.5 mg/day and immunosuppressive therapy with pred
Currently, prednisone has been reduced to 20 mg/day, and the Child-Turcotte-Pugh score has improved to grade B. The patient’s condition was stable.
The patient’s liver function and immunoglobulin level were normalized and his condition was stable.
Chronic HDV infection accelerates the disease progression in patients with chronic hepatitis B. A meta-analysis showed that for every 3% increase in HDV prevalence, the detection rate of HDV RNA increased by 10%, with HDV accounting for 18% (95% confidence interval: 10%-26%) of cirrhosis cases and 20% (95% confidence interval: 8%-33%) of HCC cases[8]. Drug use and male homosexual behavior are high-risk factors for HDV infection[9]. Patients co-infected with HDV typically exhibit higher levels of transaminases compared to those with HBV mono-infection. Compared to patients with HBV and hepatitis C virus (HCV) co-infection, those with HBV and HDV co-infection are likely to experience more severe liver disease, accelerated fibrosis progression, and up to a threefold increased risk of developing cirrhosis and HCC[10]. The two cases reported here progressed to the cirrhosis stage, corroborating these research findings to some extent.
There are also studies on the co-occurrence of AIH in viral hepatitis. One study analyzed the clinical characteristics of patients with HBV-HCV co-infection and AIH, suggesting that in cases of HBV or HCV with unexplained increases in transaminase and IgG levels (especially in patients previously treated with IFN-α), where liver histology is consistent with AIH, and non-organ-specific autoantibodies, particularly anti-nuclear antibodies and anti-smooth muscle antibodies, or other more AIH-specific antibodies are detected, along with low or absent viremia, concurrent AIH should be highly suspected[11]. Similarly, another study found that patients with chronic hepatitis D are more likely to exhibit high non-organ-specific autoantibodies titers compared to those with chronic hepatitis B, suggesting that autoantibodies are often detected as a co-infection characteristic in chronic hepatitis D patients[4].
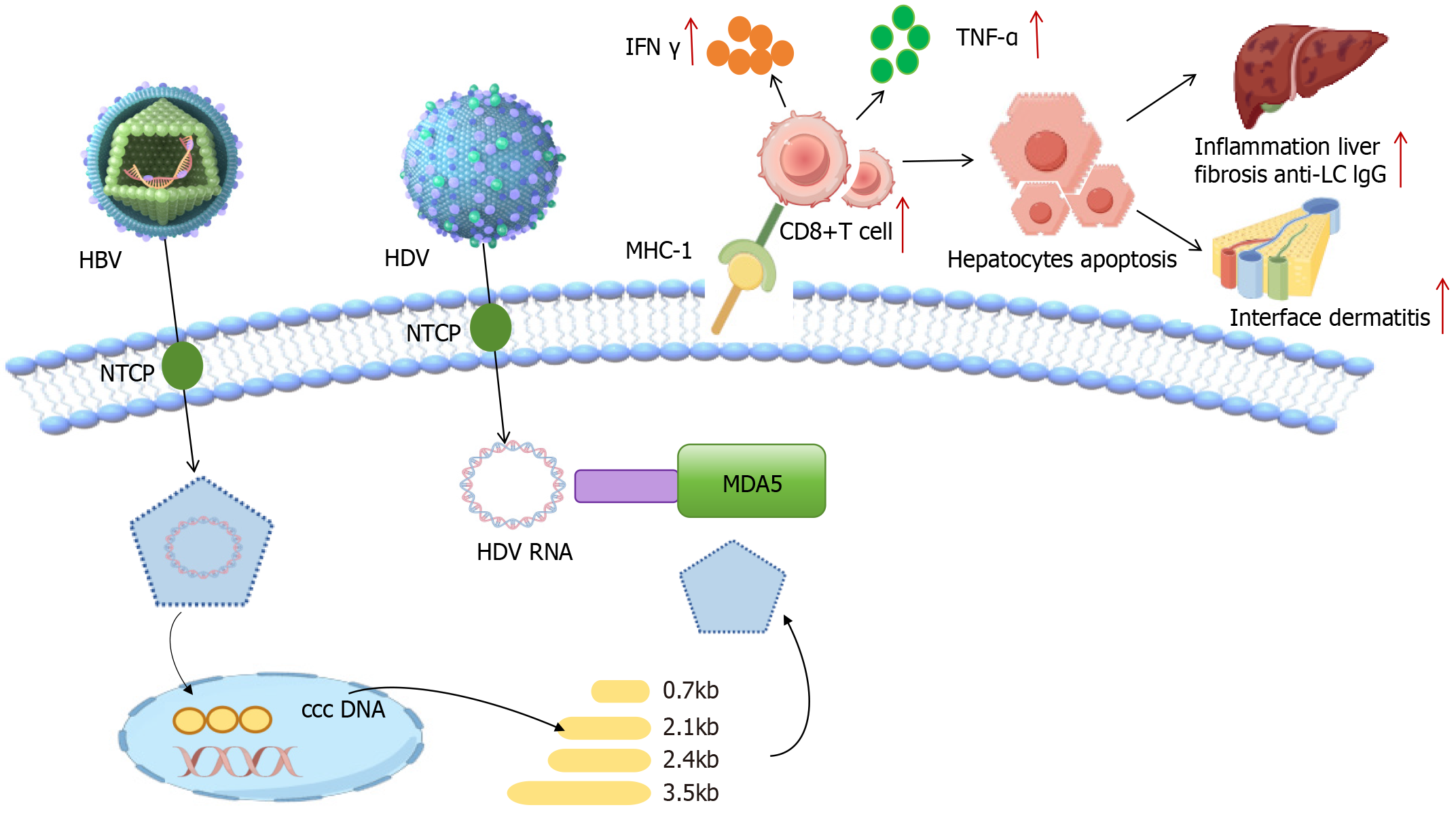
The pathogenesis of HDV infection has not been fully elucidated, but its close association with abnormalities in im
Notably, there appears to be a paradoxical immune exploitation strategy for HDV. The IFN-β/λ-mediated enhance
Viral hepatitis can induce autoimmunity through molecular mimicry[21] and the immune system uses the major histocompatibility complex to detect foreign pathogens. This locus encodes the human leukocyte antigen (HLA) genes and various immune response genes that defend against pathogens. There are two main types of HLA antigens, HLA class I and class II. The role of HLA class I is to recognize infected cells, while HLA class II molecules deliver peptides to receptors on CD4+ helper T cells. HDV is fully adapted for recognition by CD8+ T cells, while CD8+ T cells are restricted by conventional HLA class I alleles. Furthermore, in persistent HBV-HDV co-infection, the rapid progression of cirrhosis is attributed to immune-mediated mechanisms, initiated by innate pathways of the IFN system or triggered by dysfunctional T cells[22,23]. The molecular mimicry theory provides an important explanation for the autoimmune mechanism. HLA class I molecules are responsible for presenting endogenous antigens to CD8+ T cells and their expression is significantly upregulated in HDV-infected hepatocytes[24]. This enhanced antigen-presenting capacity, superimposed on the adaptation of HDV to the conventional HLA class I antigen-presenting pathway, may induce a cytotoxic immune response. The release of IFN-γ and tumor necrosis factor-α by activated CD8+ T cells may not only exacerbate auto
Clinical case analyses revealed the following key issues. Both patients included in this report progressed to the stage of cirrhosis despite effective control of viral replication with immunosuppressive therapy. The persistent liver function abnormalities in the HDV co-infected case (case 1) suggest that virus-induced immune activation may outweigh direct antiviral benefits, whereas progression of the patient with no HDV RNA (case 2) suggests the presence of non-viral immunopathological damage. This phenomenon supports that HDV infection accelerates hepatic fibrosis through two pathways. On the one hand, virus-specific immune responses partially inhibit HBV replication, and on the other hand, the persistently activated immune system maintains the chronic inflammatory state of the liver.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis delta virus. J Hepatol. 2023;79:433-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 2. | Zhang Z, Filzmayer C, Ni Y, Sültmann H, Mutz P, Hiet MS, Vondran FWR, Bartenschlager R, Urban S. Hepatitis D virus replication is sensed by MDA5 and induces IFN-β/λ responses in hepatocytes. J Hepatol. 2018;69:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Vlachogiannakos J, Papatheodoridis GV. New epidemiology of hepatitis delta. Liver Int. 2020;40 Suppl 1:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Hermanussen L, Lampalzer S, Bockmann JH, Ziegler AE, Piecha F, Dandri M, Pischke S, Haag F, Lohse AW, Lütgehetmann M, Weiler-Normann C, Zur Wiesch JS. Non-organ-specific autoantibodies with unspecific patterns are a frequent para-infectious feature of chronic hepatitis D. Front Med (Lausanne). 2023;10:1169096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 5. | Chida T, Ishida Y, Morioka S, Sugahara G, Han C, Lam B, Yamasaki C, Sugahara R, Li M, Tanaka Y, Liang TJ, Tateno C, Saito T. Persistent hepatic IFN system activation in HBV-HDV infection determines viral replication dynamics and therapeutic response. JCI Insight. 2023;8:e162404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Fasano R, Malerba E, Prete M, Solimando AG, Buonavoglia A, Silvestris N, Leone P, Racanelli V. Impact of Antigen Presentation Mechanisms on Immune Response in Autoimmune Hepatitis. Front Immunol. 2021;12:814155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Enc F, Ulasoglu C. A case of autoimmune hepatitis following pegylated interferon treatment of chronic hepatitis delta. North Clin Istanb. 2020;7:407-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Stockdale AJ, Kreuels B, Henrion MYR, Giorgi E, Kyomuhangi I, de Martel C, Hutin Y, Geretti AM. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 428] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 9. | Tan YC, Lee GH, Huang DQ, Lim SG. Future anti-HDV treatment strategies, including those aimed at HBV functional cure. Liver Int. 2023;43:1157-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Koh C, Heller T, Glenn JS. Pathogenesis of and New Therapies for Hepatitis D. Gastroenterology. 2019;156:461-476.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Rigopoulou EI, Zachou K, Gatselis N, Koukoulis GK, Dalekos GN. Autoimmune hepatitis in patients with chronic HBV and HCV infections: patterns of clinical characteristics, disease progression and outcome. Ann Hepatol. 2013;13:127-135. [PubMed] |
| 12. | Jung S, Altstetter SM, Protzer U. Innate immune recognition and modulation in hepatitis D virus infection. World J Gastroenterol. 2020;26:2781-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 13. | Zhang Z, Urban S. New insights into HDV persistence: The role of interferon response and implications for upcoming novel therapies. J Hepatol. 2021;74:686-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Urban S, Neumann-Haefelin C, Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70:1782-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 15. | Zhang Z, Urban S. Interplay between Hepatitis D Virus and the Interferon Response. Viruses. 2020;12:1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Chen H, Han Z, Fan Y, Chen L, Peng F, Cheng X, Wang Y, Su J, Li D. CD4+ T-cell subsets in autoimmune hepatitis: A review. Hepatol Commun. 2023;7:e0269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Rathi C, Pipaliya N, Choksi D, Parikh P, Ingle M, Sawant P. Autoimmune Hepatitis Triggered by Treatment With Pegylated Interferon α-2a and Ribavirin for Chronic Hepatitis C. ACG Case Rep J. 2015;2:247-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Kawashima H, Kato N, Ioi H, Nishimata S, Watanabe C, Kashiwagi Y, Takekuma K, Hoshika A, Szenborn L, Bergman K. mRNA expression of T-helper 1, T-helper 2 cytokines in autoimmune hepatitis in childhood. Pediatr Int. 2008;50:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | García-Buey L, García-Monzón C, Rodriguez S, Borque MJ, García-Sánchez A, Iglesias R, DeCastro M, Mateos FG, Vicario JL, Balas A. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology. 1995;108:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Sirbe C, Simu G, Szabo I, Grama A, Pop TL. Pathogenesis of Autoimmune Hepatitis-Cellular and Molecular Mechanisms. Int J Mol Sci. 2021;22:13578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Loglio A, Ferenci P, Uceda Renteria SC, Tham CYL, Scholtes C, Holzmann H, van Bömmel F, Borghi M, Perbellini R, Rimondi A, Farina E, Trombetta E, Manunta M, Porretti L, Prati D, Ceriotti F, Zoulim F, Bertoletti A, Lampertico P. Safety and effectiveness of up to 3 years' bulevirtide monotherapy in patients with HDV-related cirrhosis. J Hepatol. 2022;76:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Oberhardt V, Hofmann M, Thimme R, Neumann-Haefelin C. Adaptive Immune Responses, Immune Escape and Immune-Mediated Pathogenesis during HDV Infection. Viruses. 2022;14:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Karimzadeh H, Kiraithe MM, Kosinska AD, Glaser M, Fiedler M, Oberhardt V, Salimi Alizei E, Hofmann M, Mok JY, Nguyen M, van Esch WJE, Budeus B, Grabowski J, Homs M, Olivero A, Keyvani H, Rodríguez-Frías F, Tabernero D, Buti M, Heinold A, Alavian SM, Bauer T, Schulze Zur Wiesch J, Raziorrouh B, Hoffmann D, Smedile A, Rizzetto M, Wedemeyer H, Timm J, Antes I, Neumann-Haefelin C, Protzer U, Roggendorf M. Amino Acid Substitutions within HLA-B*27-Restricted T Cell Epitopes Prevent Recognition by Hepatitis Delta Virus-Specific CD8(+) T Cells. J Virol. 2018;92:e01891-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Tham CYL, Kah J, Tan AT, Volz T, Chia A, Giersch K, Ladiges Y, Loglio A, Borghi M, Sureau C, Lampertico P, Lütgehetmann M, Dandri M, Bertoletti A. Hepatitis Delta Virus Acts as an Immunogenic Adjuvant in Hepatitis B Virus-Infected Hepatocytes. Cell Rep Med. 2020;1:100060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol Immunol. 2022;19:158-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |