Published online Aug 26, 2025. doi: 10.12998/wjcc.v13.i24.107555
Revised: April 17, 2025
Accepted: May 13, 2025
Published online: August 26, 2025
Processing time: 82 Days and 18.7 Hours
68Ga (gallium)-PSMA PET-CT (prostate-specific membrane antigen-directed Positron emission tomography-computed tomography) has established its role in prostate cancer management as targeted molecular imaging. However, limited studies are available on the diagnostic accuracy of 99mTc (Technetium)-PSMA-SPECT/CT. Due to its cost effectiveness and better feasibility, it needs to be ex
To analyse the diagnostic accuracy of 99mTc-PSMA-SPECT/CT for detection of primary prostate carcinoma.
As a prospective study in a tertiary hospital, 99mTc-PSMA-SPECT/CT was per
Nineteen of twenty-nine patients were positive on 99mTc-PSMA-SPECT/CT, of which 16 (84.2%) had prostate cancer on histopathology, while the remaining ten were negative on imaging, of which three had prostate cancer, leading to an overall sensitivity, specificity, and accuracy of 84.2%, 70%, and 79.3%, respectively, on visual analysis. Prostate:background and prostate:liver ratios were 37.18 ± 48.85 and 5.35 ± 7.35 in the malignant group, while 6.65 ± 5.17 and 1.14 ± 0.56 in the benign group, respectively. The area under the curve values for prostate:background and prostate:liver ratios were 0.833 (95% confidence interval [CI]: 0.677-0.990, P = 0.005) and 0.767 (95%CI: 0.596-0.937, P = 0.024), respectively, on receiver operator curve analysis. A cut-off value > 10.45 for prostate:background ratio (sensitivity 85% and specificity 88.9%), and > 1.15 for prostate:liver ratio (sensitivity 75% and specificity of 77.8% respectively) was found to be pertinent to differentiate between the malignant vs benign groups.
99mTc-PSMA-SPECT/CT shows a promising role in the diagnosis of primary prostate cancer.
Core Tip: 99mTc-PSMA-SPECT/CT has good diagnostic accuracy in the detection of primary prostate cancer and the results are comparable with those of its 68Ga-PSMA positron emission tomography/computed tomography counterpart. The former is more cost-effective, feasible, and accessible, and thus can be incorporated in routine clinical practice in remote places or facilities with only gamma camera.
- Citation: Agrawal K, Patro PSS, Singhal T, Satpati D, Nayak P, Mandal S, Kumar N, Padhy BM, Sable M, Meher BR. Diagnostic performance of 99mTc-PSMA SPECT/CT in primary prostate carcinoma. World J Clin Cases 2025; 13(24): 107555
- URL: https://www.wjgnet.com/2307-8960/full/v13/i24/107555.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i24.107555
Prostate cancer (PCa) represents a significant health concern worldwide. It ranks second among cancers affecting men, and fourth in overall global cancer diagnoses, and is the fifth leading cause of cancer-related death in men globally[1,2]. PCa has an annual rise of incidence by 2%-3% with a swift increase in developing countries like India and a rise of age-standardized incidence rate by 29.8% during the last 2 decades[3]. Early detection and management reduce overall morbidity and mortality in PCa.
Conventional imaging modalities like computed tomography (CT)/magnetic resonance imaging (MRI) have limited diagnostic value in detection, staging, and restaging of prostate cancer. As rising cancer prevalence dictated, there was a constant need to improve the diagnostic capabilities for PCa, leading to the development of prostate-specific membrane antigen (PSMA)-targeted imaging. PSMA is a type II transmembrane glycoprotein with a large extracellular structural domain (accounts for 95% protein, ideal target for imaging and treatment), a transmembrane domain, and an intracellular structural domain, known to be overexpressed (approximately 100-1000 times in PCa than benign tissue). PSMA was initially believed to be specific for PCa but was later found to be expressed in various other tissues such as the kidneys, proximal small intestine, and salivary glands[4]. However, PSMA gets significantly upregulated in PCa tissues than normal prostate tissue and this rises further when PCa becomes hormone-resistant/metastatic. This overexpression holds great promise for both imaging and treating patients with PCa[5]. In recent years, it has raised interest in improving PCa imaging. While the 68Ga(gallium)-PSMA has established its role as a PET (Positron emission tomography) tracer in PCa recurrence assessment and staging, its most widespread use is in the detection of relapsed cases in biochemical recurrence. However, 99mTc(technetium)-PSMA is not widely studied for its role as a diagnostic tracer in diagnosing PCa[6].
99mTc-PSMA presents a compelling alternative to 68Ga-PSMA as a single photon emission computed tomography (SPECT) tracer. Early preclinical studies have demonstrated its efficacy in visualizing PCa lesions with positive PSMA expression. Studies have shown that utilizing 99mTc-PSMA-SPECT/CT with elevated PSA levels of > 4 ng/mL, demonstrates exceptional detection rates in cases of biochemical recurrence, proving its utility not only for initial staging but also for the re-evaluation of recurrent and advanced PCa cases[7]. Moreover, its rapid clearance from circulation through the kidneys, favourable radiation uptake profile, and less secretion in the bowel make it an appealing option for clinical use. Furthermore, in contrast to its PET tracer counterpart, 68Ga-PSMA, the cost-effectiveness, half-life, less instrumentation, and widespread availability of 99mTc-PSMA make it a potential imaging tracer on a broader scale. With PCa incidence rates steadily climbing, the need for precise and accessible diagnostic tools has never been more pressing. A recent study demonstrated that incorporating 99mTc-PSMA-SPECT/CT led to a shift in therapeutic strategies for 79% of the patients[8], highlighting its influential role in guiding the treatment approach.
This study aimed to explore the diagnostic utility of 99mTc-PSMA-SPECT/CT, focusing on its role in identifying the primary lesion of PCa, augmenting PCa management strategies, and contributing to the ongoing efforts in improving PCa care and patient outcomes.
This was a prospective study, performed after approval from the local Institutional Ethics Committee. All the outpatients with a suspicion of PCa within the study period were included. All the patients underwent 99mTc PSMA imaging with regional SPECT-CT of the abdomen and pelvis. The scan findings were compared to histopathology reports as the gold standard for final diagnosis.
The patients whose final diagnosis could not be confirmed due to the absence of histopathology report were excluded from the study.
No specific patient preparation was needed. Patients were injected with 740 MBq (20 mCi) 99mTc-PSMA intravenously (inhouse pharmacy prepared). Images were acquired on a large field of view dual head gamma camera with a low-energy, high-resolution parallel hole collimator (GE Discovery NM/CT 670) with energy peak at 140 KeV (± 10%). Planar images were acquired in anterior and posterior views at 3 hours at a scan speed of 18 cm/minute, matrix size 1024 × 256, followed by SPECT/CT of the abdomen and pelvis. SPECT was acquired in two bed positions, each with matrix size 128 × 128, 60 projections per 360° orbit, 12 seconds per step. OSEM (Ordered Subset Expectation Maximization) was used for image reconstruction (with two iterations and ten subsets). Non-contrast low-dose helical CT was acquired of the same region for attenuation correction and anatomical localization. Fused SPECT/CT image processing was done in the volumetrix MI software on Xeleris workstation (GE Healthcare) for further analysis.
The images were evaluated separately by two experienced Nuclear Medicine physicians who were blinded to the patient details, and concurrent interpretation by two readers separately was considered final. In case of any discrepancy, help from a third Nuclear Medicine physician was sought. Both planar and SPECT-CT images were reviewed and detection rate was evaluated. Furthermore, for semi-quantitative evaluation, a fixed 200 cm3 region of interest (ROI) was drawn over the prostate gland lesion, with liver and gluteal muscle used as background on the fused SPECT/CT images avoiding the areas of necrosis if present and surrounding unwanted structures or urine activity. Total counts in each ROI were determined. Subsequently, prostate:background and prostate:liver count ratios were calculated.
Data analyses were performed using SPSS v 26.0. Based on the histopathology reports, patients were divided into malignant and benign groups. All the semi-quantitative parameters in the two groups are presented as the mean ± SD. Normalcy of distribution was assessed using the Shapiro-Wilk test, and the Mann-Whitney U test was employed to assess the difference of mean between the two groups in non-parametric data. Receiver operating characteristic (ROC) curve was also drawn to determine a cut-off of these ratios to differentiate between the two groups. P value < 0.05 was considered statistically significant.
The current study included a total of 29 patients with suspected PCa with a median age of 66 (range: 50-82) years. Histopathological examination revealed a final diagnosis of benign in 9 and malignant pathology in 20 patients. Mean serum prostate-specific antigen (S.PSA) level in the benign group was 18.18 ± 18.36 ng/mL while it was 123.3 ± 193.39 nm/mL in the malignant group (Table 1).
| Sl.No | Age (years) | S.PSA (ng/mL) | PSMA uptake | HPE for malignancy (GS) | ||
| Primary | Lymph nodes | Distant | ||||
| 1 | 76 | 80.46 | No uptake | - | - | Positive (GS-4+5) |
| 2 | 73 | 7.19 | No uptake | - | - | Negative |
| 3 | 66 | 4.2 | No uptake | - | - | Negative |
| 4 | 75 | 48.8 | Mild diffuse | - | - | Negative |
| 5 | 50 | 7.7 | Mild diffuse | - | - | Negative |
| 6 | 72 | 8.1 | No uptake | - | - | Negative |
| 7 | 70 | 100 | Increased | - | Bones | Positive (GS-5+5) |
| 8 | 80 | 10.4 | No uptake | - | - | Negative |
| 9 | 62 | 10.2 | No uptake | - | - | Positive (GS-3+3) |
| 10 | 82 | > 100 | Increased | Left pelvic | - | Positive (GS-5+5) |
| 11 | 55 | > 100 | Increased | Pelvic | Lungs, bones | Positive (GS-5+5) |
| 12 | 54 | > 100 | Increased | Abdomino-pelvic | Bones | Positive (GS-5+3) |
| 13 | 73 | > 100 | Increased | Abdomino-pelvic, left sclv. | Bones | Positive (GS-4+5) |
| 14 | 59 | > 100 | Increased | Abdomino-pelvic nodes | - | Positive (GS-5+5) |
| 15 | 65 | 932 | Increased | Abdomino-pelvic, mediastinal | Lung, bones | Positive (GS-5+5) |
| 16 | 64 | > 100 | Increased | - | Bones | Positive (GS-3+3) |
| 17 | 66 | 55.5 | Increased | Pelvic | - | Positive (GS-3+3) |
| 18 | 66 | > 150 | Increased | Pelvic-mediastinal | Bones | Positive (GS-5+4) |
| 19 | 69 | 24.8 | Increased | Abdomino-pelvic, left sclv. | - | Positive (GS-4+5) |
| 20 | 59 | 86.5 | Increased | Abdo-pelvic nodes | - | Positive (GS-3+4) |
| 21 | 64 | > 100 | Increased | Mediastinal-abdomino-pelvic, left sclv. | Bones | Positive (GS-5+5) |
| 22 | 66 | 51 | No uptake | - | - | Negative |
| 23 | 59 | 44.9 | Increased | - | - | Positive (GS-3+4) |
| 24 | 70 | > 100 | Increased | Abdomino-pelvic | Bones | Positive (GS-5+5) |
| 25 | 77 | 25.7 | No uptake | - | - | Negative |
| 26 | 80 | 8.5 | Mild diffuse | - | - | Negative |
| 27 | 54 | 17.5 | No uptake | - | - | Negative |
| 28 | 62 | 100 | Increased | Retroperitoneal, abdomino-pelvic, inguinal, and sclv. | Bones | Positive (GS-5+3) |
| 29 | 58 | 55.8 | No uptake | - | Bones | Positive (GS-3+4) |
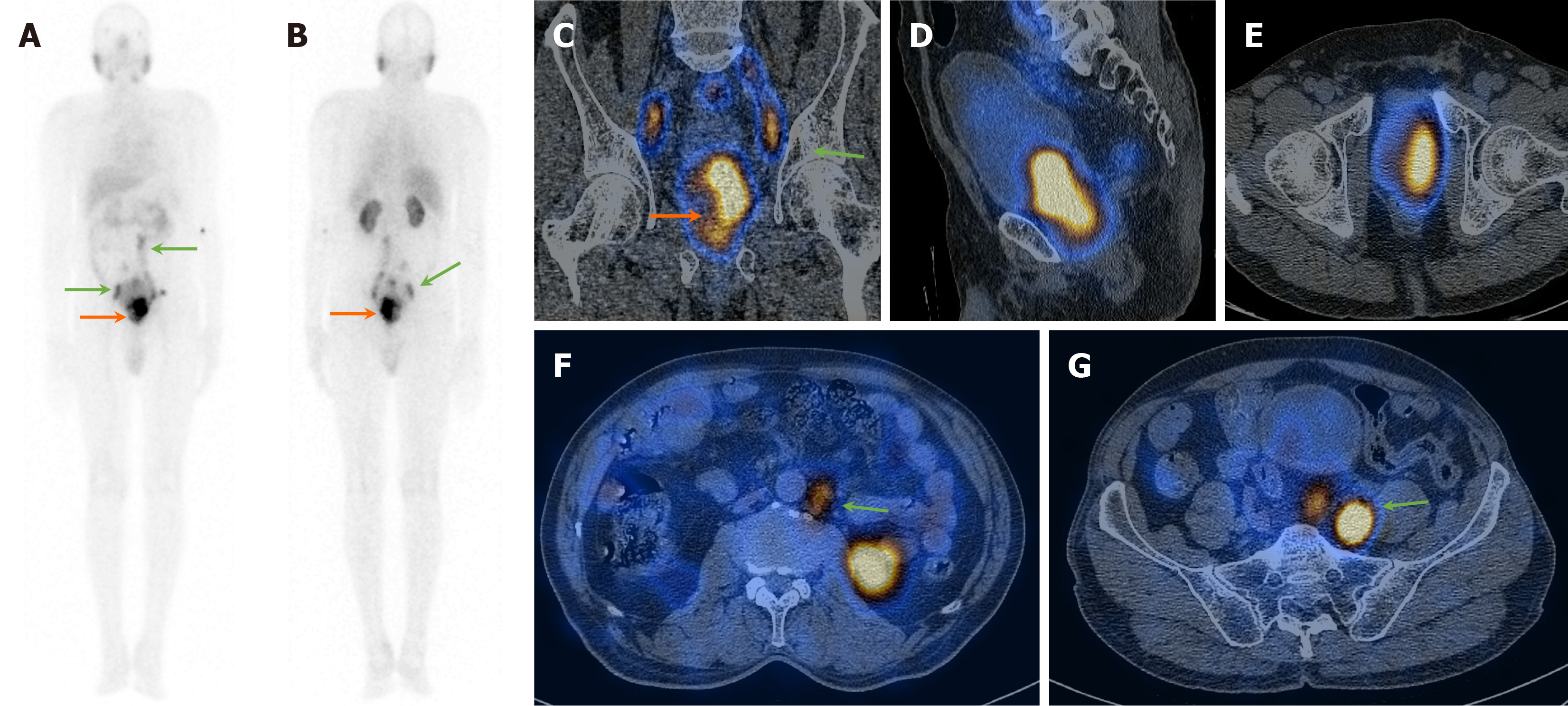
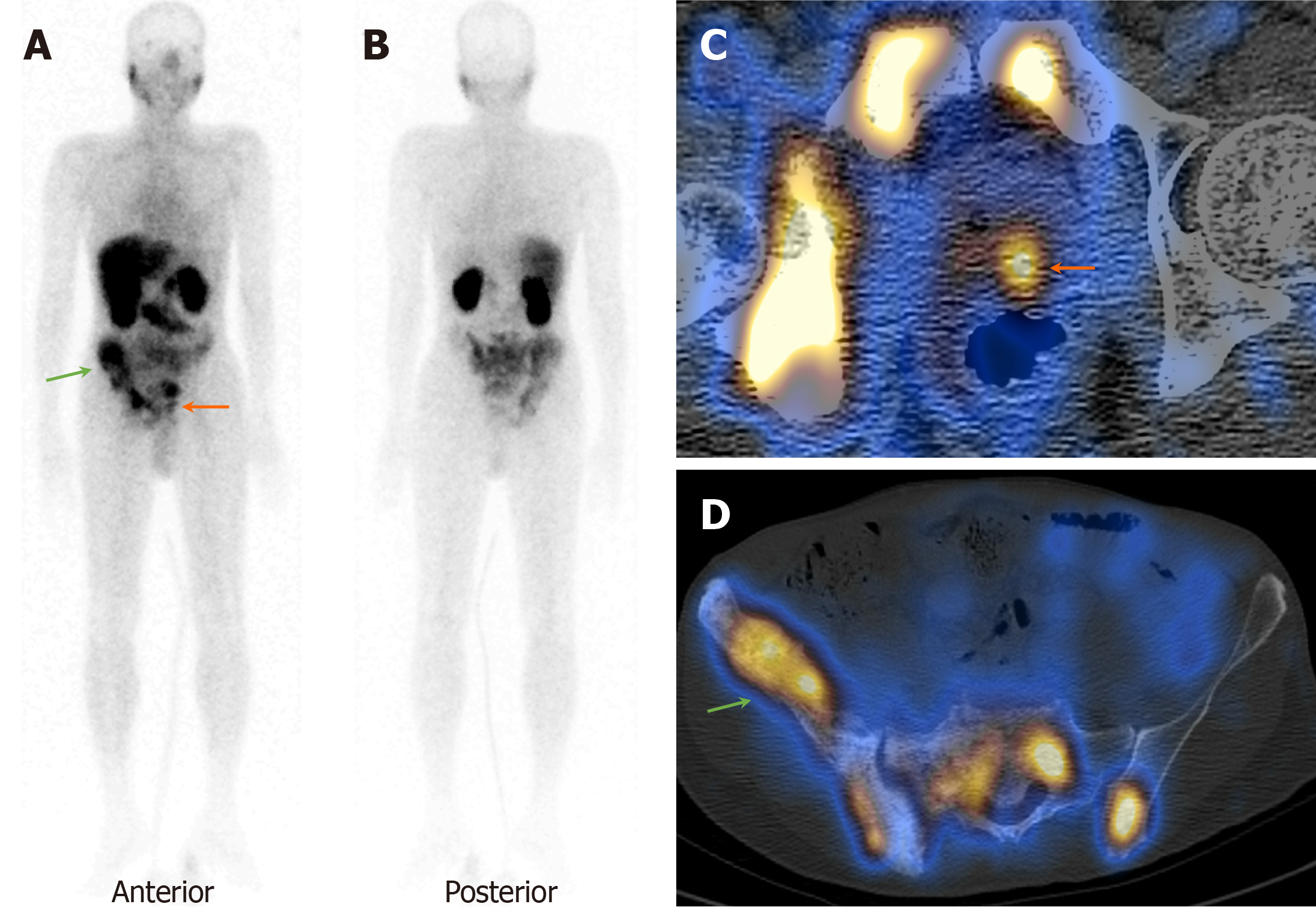
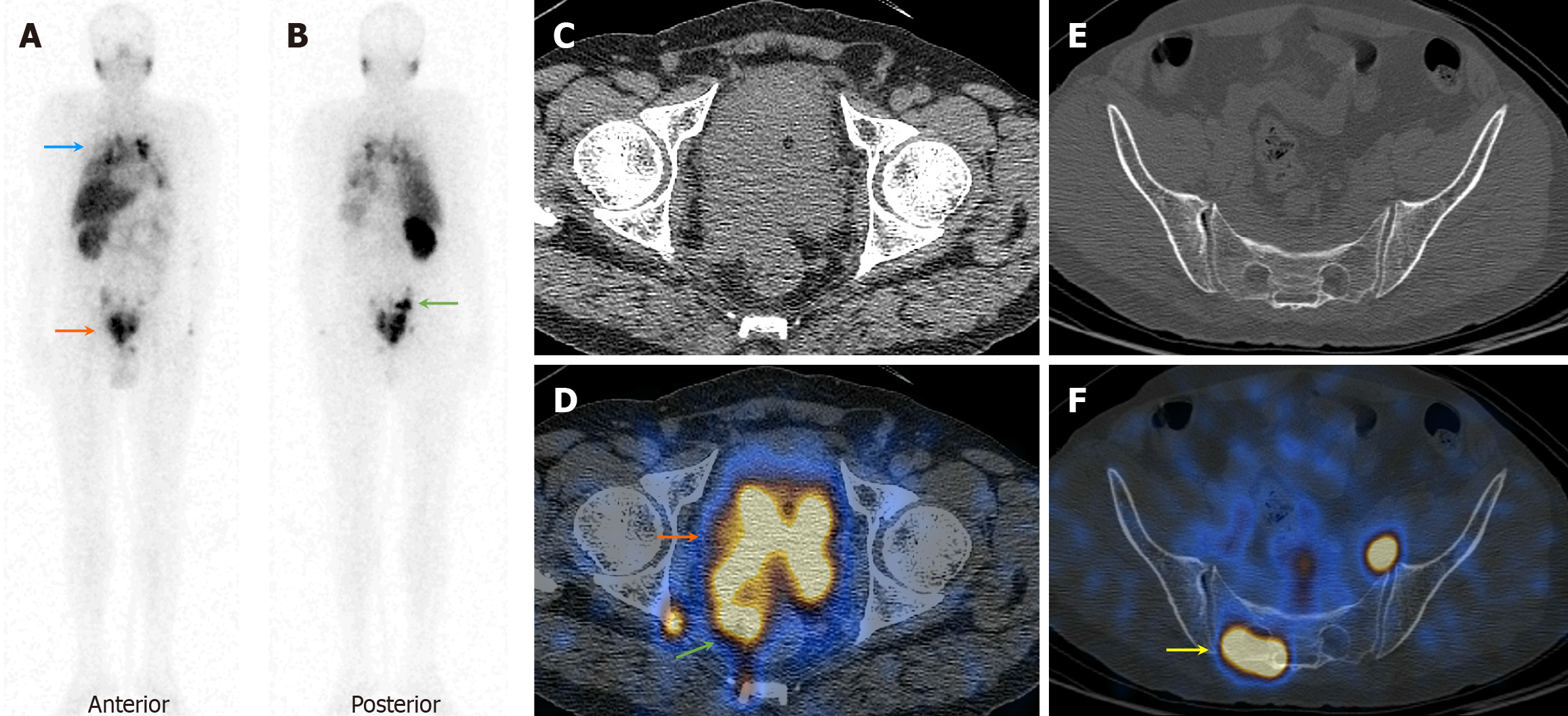
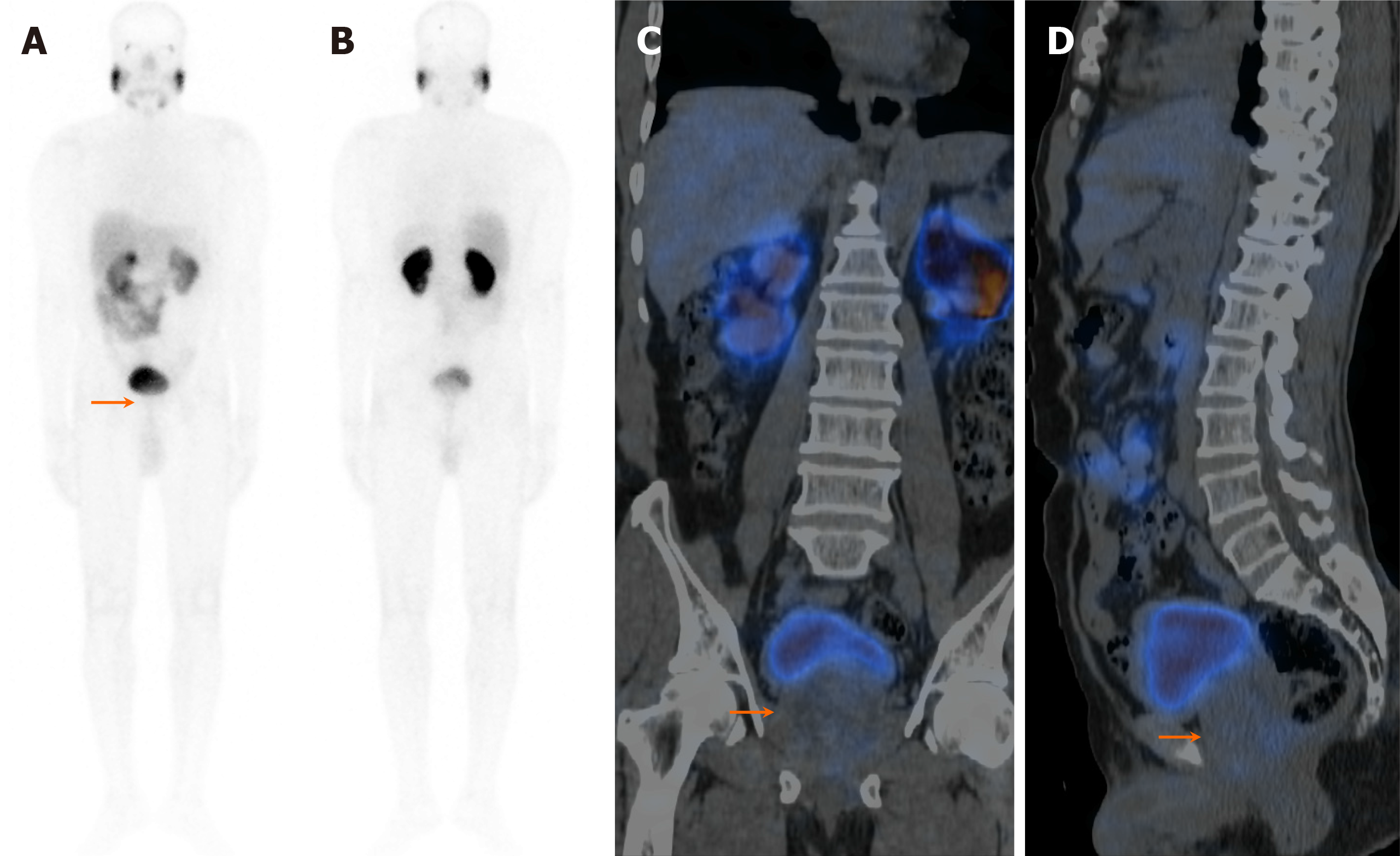
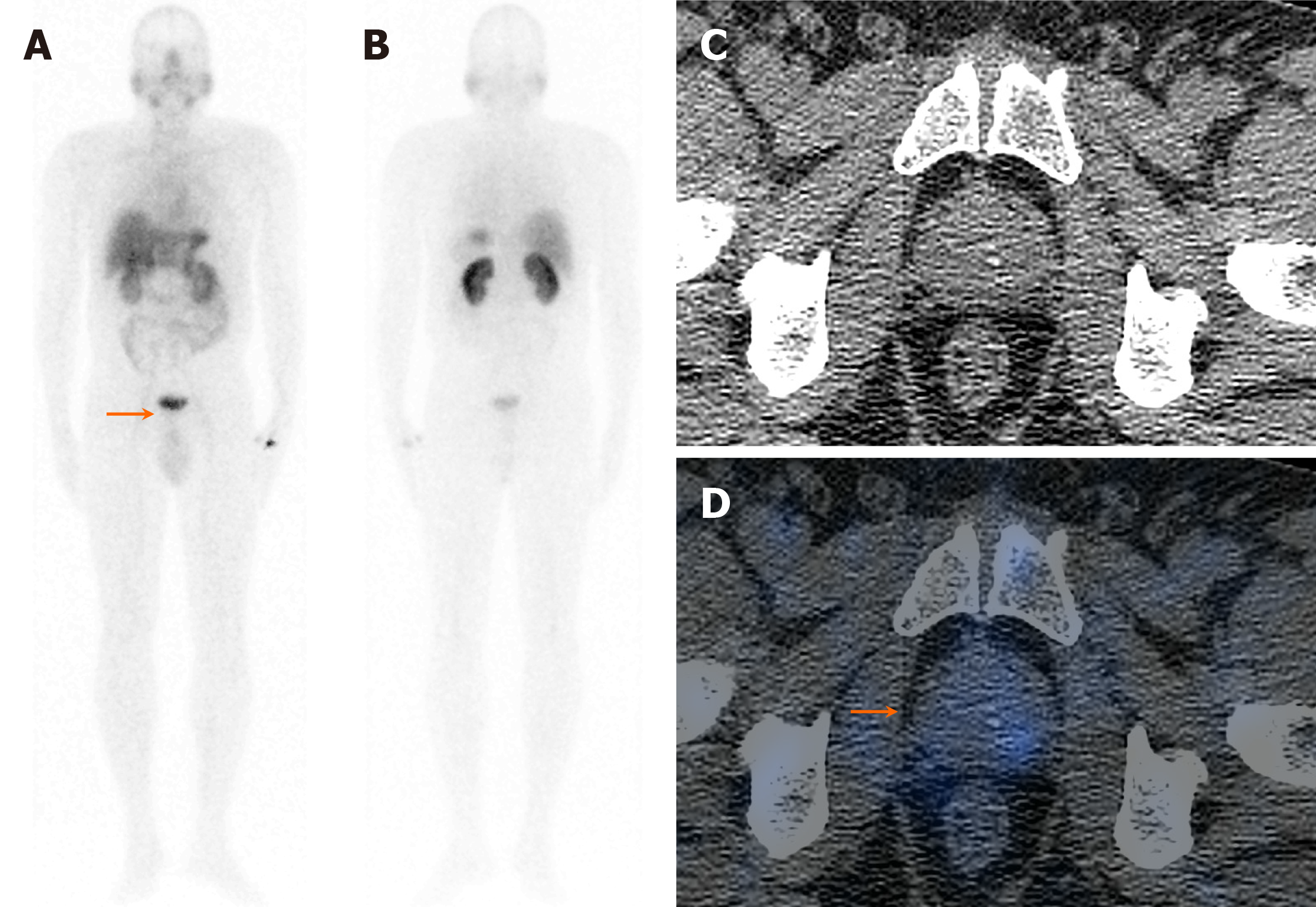
On visual analysis of 99mTc-PSMA planar images, 19 patients showed localisation of the tracer in the prostate gland. Histopathological evaluation revealed disease in 16 of these 19 (84.2%) patients. Few of these cases showed nodal disease and distant metastasis in the lungs and bones on 99mTc-PSMA-SPECT-CT (Table 1, Figures 1, 2 and 3). The three false-positive patients had diffuse homogeneous uptake in the prostate gland on SPECT/CT imaging which was in contrast to the heterogeneous or focal uptake seen in the histologically positive cases (Figure 4). Among these two patients were diagnosed to have benign hyperplasia (with S. PSA 7.7 and 4.2 ng/mL) and the third one had prostatitis (with S. PSA 48.8 ng/dL) (Table 1).
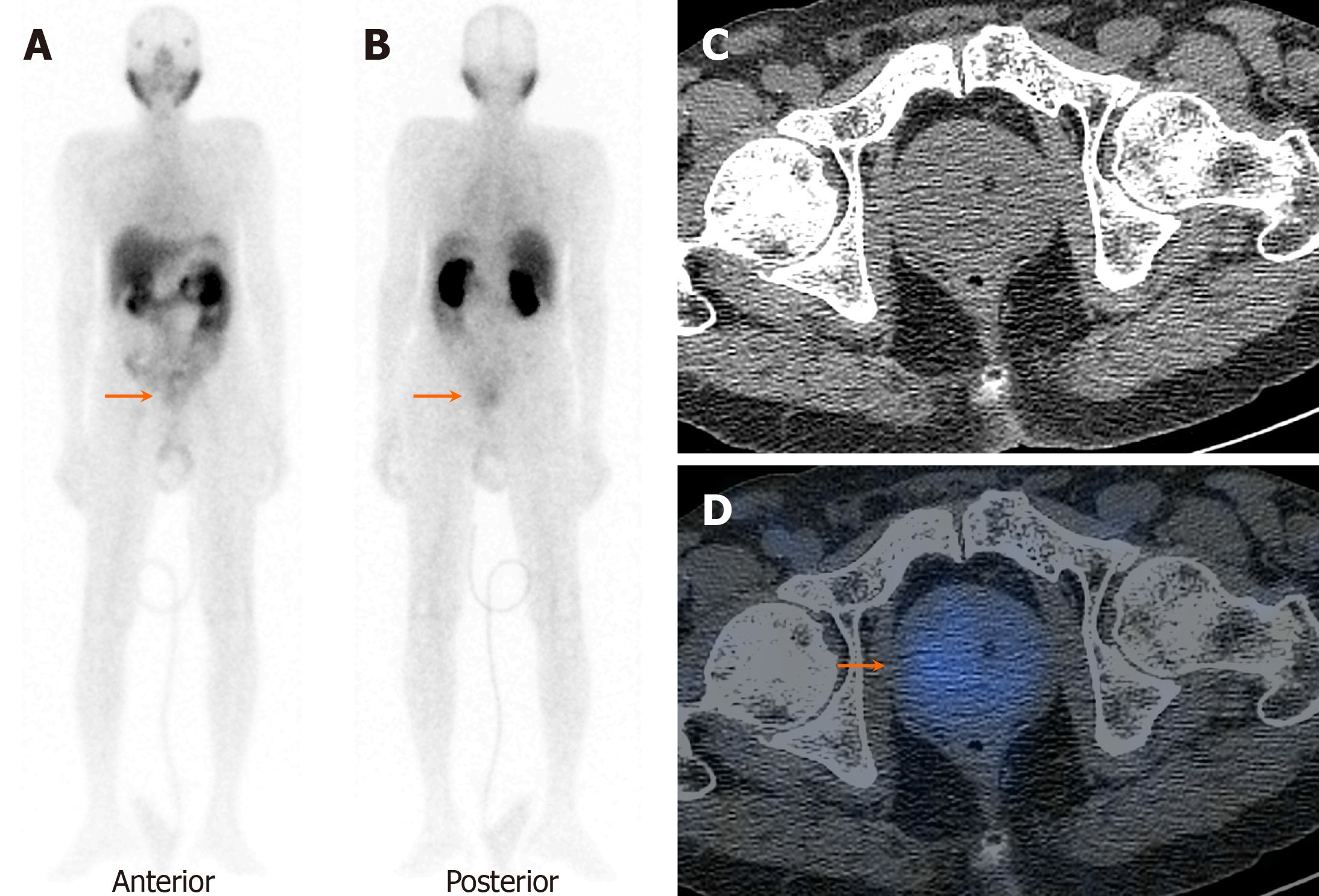
The remaining ten patients did not show any significant uptake in prostate bed. Histopathological evaluation revealed disease in 3/10 patients (Figure 5), thus demonstrating an overall sensitivity, specificity, and accuracy of 84.2%, 70%, and 79.3%, respectively, on visual analysis (Figure 6). Among the false-negative cases on imaging, two had Gleason score (GS 3+3 or 3+4) indicating low/medium-risk tumours (Table 1).
On semi-quantitative analysis, prostate:background and prostate:liver count ratios were 37.18 ± 48.85 and 5.35 ± 7.35 in the malignant group, while 6.65 ± 5.17 and 1.14 ± 0.56 in the benign group, respectively. The semi-quantitative parameters were significantly different in the two groups (Table 2).
| Parameter | mean ± SD in benign group (n = 9) | mean ± SD in malignant group (n = 20) | P value |
| Prostate:background count ratio | 6.65 ± 5.17 | 37.18 ± 48.85 | 0.004 |
| Prostate:liver count ratio | 1.14 ± 0.56 | 5.35 ± 7.35 | 0.023 |
On ROC curve analysis, the area under curve (AUC) values for prostate:background and prostate:liver count ratios were 0.833 (95% confidence interval [CI]: 0.677-0.990, P = 0.005) and 0.767 (95%CI: 0.596-0.937, P = 0.024), respectively. Furthermore, for differentiation of histologically malignant from benign groups, a cut-off value > 10.45 for pro
| Parameter | AUC | 95%CI | P value | Cut-off (ROC) | Sensitivity | Specificity |
| Prostate:background ratio | 0.833 | 0.677-0.990 | 0.005 | 10.45 | 85% | 88.9% |
| Prostate:liver ratio | 0.767 | 0.596-0.937 | 0.024 | 1.15 | 75% | 77.8% |
Due to small sample size, extensive analysis based on risk stratification could not be made, but few patients with both high S.PSA (80.4 ng/dL) and low S.PSA (10.2 ng/mL) were falsely negative on imaging (Table 1), which suggests that there might not be impact of S.PSA value on imaging findings.
PSMA-targeted imaging and therapeutic interventions have revolutionized the management of PCa. PSMA-targeted PET-imaging has become the standard imaging in PCa, offering an effective and early diagnosis of primary malignancy at subcentimetric size, accurate nodal staging and metastatic work-up, and recurrence detection, as well as treatment response assessment. PSMA PET imaging has also been incorporated in the management guidelines including the National Comprehensive Cancer Network, European Society for Medical Oncology, and European Association of Urology guidelines[9-11].
However, there is a constant unmet need for PSMA-based imaging studies particularly in developing countries with significant disease burden and limited centres equipped with PET imaging. Thus, PSMA-targeted SPECT imaging can effectively cater for PCa patients owing to its relatively wide availability and lower cost.
Available literature suggests that 99mTc-PSMA imaging carries efficacy approaching PSMA PET-CT targeted imaging for metastatic work-up. Albalooshi et al[12] compared the diagnostic efficacy of 99mTc-PSMA against 68Ga-PSMA PET/CT in 28 PCa patients. The authors found no significant difference in nodal and distant disease detection between the two imaging modalities (P > 0.05). However, 99mTc-PSMA performance was superseded by 68Ga-PSMA PET/CT in localization of primary malignancy.
In a similar study by Fallahi et al[13] including 22 PCa patients, the authors found a prostate bed lesion detection rate of 60% when compared to 68Ga PET-CT imaging. However, the authors found a comparable detection rate for nodal as well as distance metastasis. Ćwikła et al[14] found 99mTc-PSMA-T4 WB-SPECT/CT to be a cost-effective diagnostic tool in patients with PCa with a sensitivity/specificity of 92%/100%, 83%/100%, 100%/95%, and 100/100% for primary cancer, pelvic lymph nodes disease, other lymph nodes and soft tissue involvement, and bone metastasis, respectively.
A recent meta-analysis on diagnostic accuracy of 99mTc-PSMA-SPECT/CT, showed a pooled sensitivity, specificity, and AUC of 0.89 (95%CI: 0.84–0.93), 0.92 (95%CI: 0.67–0.99), and 0.93 (95%CI: 0.90–0.95), respectively[15].
The current study found the sensitivity & specificity of 99mTc-PSMA-SPECT/CT for primary disease to be 85% and 88.9%, respectively, with an overall accuracy of 88.8% when the prostate:background count ratio cut off was > 10.45. This was in line with the sensitivity and accuracy demonstrated in the study by Albalooshi et al[12], where the authors found a sensitivity and accuracy of 80% and 82%, respectively. However, the authors compared the results with 68Ga-PSMA PET-CT as the gold standard, and hence yielded no false-positive cases, giving a specificity of 100%. The current study addresses this limitation and has compared the efficacy of 99mTc-PSMA against histopathology as the gold standard.
Among the three false-negative cases, two had a Gleason’s score ≤ 7, indicating low to intermediate risk, which could suggest some impact of low tumour burden on the 99mTc-PSMA uptake by the primary lesion. Furthermore, this study showed no significant correlation of S.PSA levels with the imaging results. However, sample size is small to accurately comment on these. Hence, further extensive studies are required to evaluate the association between 99mTc-PSMA expression with S.PSA value and tumour aggressiveness/Gleason’s score.
In a preliminary study evaluating the role of semi-quantitative assessment of PSMA-SPECT/CT scans by Farkas et al[16], the authors compared the PSMA uptake as well as semi-quantitative parameters in PCa patients and healthy volunteers and found a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for primary PCa to be 86%, 100%, 100%, 83%, and 92%, respectively. However, the study design was retrospective and the control group included healthy volunteers, rather than suspected cancer patients which were supposed to be encountered regularly and will help in the tailored management in a real-world scenario. The current study addresses the limitation of this study and evaluated the role of 99mTc-PSMA in cases with suspected PCa prospectively, taking histopathology as the gold standard.
However, the current study has a limitation of being a preliminary study with small sample size, in a limited population which could have caused some bias in the statistics. In addition, whether external factors like economic, social, or technological changes could have influenced the study variables was not extensively studied. Hence, larger diverse, multi-centric prospective and longitudinal studies with more robust data analysis are warranted based on this preliminary study for external validation with broader population, before 99mTc-PSMA can be incorporated into routine standard practice.
In conclusion, PSMA-SPECT/CT represents a valuable addition to the diagnostic tools for PCa, providing a reliable and cost-effective alternative to 68Ga-PSMA PET-CT imaging. Its integration into routine practice allows for effective primary staging, guided biopsy, and differentiation between benign and malignant intraprostatic tracer uptake, particularly with the use of semi-quantitative parameters. Furthermore, 99mTc-PSMA-SPECT/CT holds the potential for selecting suitable candidates for 177Lu-PSMA therapy and radio-guided surgery. These attributes position 99mTc-PSMA-SPECT/CT as a versatile and accessible imaging modality, enabling broader adoption in PCa care and improved patient outcomes.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8187] [Article Influence: 8187.0] [Reference Citation Analysis (2)] |
| 2. | Li B, Duan L, Shi J, Han Y, Wei W, Cheng X, Cao Y, Kader A, Ding D, Wu X, Gao Y. Diagnostic performance of 99mTc-PSMA SPECT/CT for biochemically recurrent prostate cancer after radical prostatectomy. Front Oncol. 2022;12:1072437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Dizon DS, Kamal AH. Cancer statistics 2024: All hands on deck. CA Cancer J Clin. 2024;74:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 4. | Singhal T, Singh P, Parida GK, Agrawal K. Role of PSMA-targeted PET-CT in renal cell carcinoma: a systematic review and meta-analysis. Ann Nucl Med. 2024;38:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Kasperzyk JL, Finn SP, Flavin R, Fiorentino M, Lis R, Hendrickson WK, Clinton SK, Sesso HD, Giovannucci EL, Stampfer MJ, Loda M, Mucci LA. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:2354-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Duncan I, Ingold N, Martinez-Marroquin E, Paterson C. An Australian experience using Tc-PSMA SPECT/CT in the primary diagnosis of prostate cancer and for staging at biochemical recurrence after local therapy. Prostate. 2023;83:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Werner P, Neumann C, Eiber M, Wester HJ, Schottelius M. [(99cm)Tc]Tc-PSMA-I&S-SPECT/CT: experience in prostate cancer imaging in an outpatient center. EJNMMI Res. 2020;10:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Su HC, Zhu Y, Ling GW, Hu SL, Xu XP, Dai B, Ye DW. Evaluation of 99mTc-labeled PSMA-SPECT/CT imaging in prostate cancer patients who have undergone biochemical relapse. Asian J Androl. 2017;19:267-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, Eberli D, De Meerleer G, De Santis M, Farolfi A, Gandaglia G, Gillessen S, Grivas N, Henry AM, Lardas M, van Leenders GJLH, Liew M, Linares Espinos E, Oldenburg J, van Oort IM, Oprea-Lager DE, Ploussard G, Roberts MJ, Rouvière O, Schoots IG, Schouten N, Smith EJ, Stranne J, Wiegel T, Willemse PM, Tilki D. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2024;86:148-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 360] [Article Influence: 360.0] [Reference Citation Analysis (0)] |
| 10. | Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, Tombal B, Gillessen S; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 622] [Article Influence: 124.4] [Reference Citation Analysis (1)] |
| 11. | Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, Bryce A, Chapin B, Cheng HH, D'Amico AV, Desai N, Dorff T, Eastham JA, Farrington TA, Gao X, Gupta S, Guzzo T, Ippolito JE, Kuettel MR, Lang JM, Lotan T, McKay RR, Morgan T, Netto G, Pow-Sang JM, Reiter R, Roach M, Robin T, Rosenfeld S, Shabsigh A, Spratt D, Teply BA, Tward J, Valicenti R, Wong JK, Shead DA, Snedeker J, Freedman-Cass DA. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1067-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 243] [Reference Citation Analysis (16)] |
| 12. | Albalooshi B, Al Sharhan M, Bagheri F, Miyanath S, Ray B, Muhasin M, Zakavi SR. Direct comparison of (99m)Tc-PSMA SPECT/CT and (68)Ga-PSMA PET/CT in patients with prostate cancer. Asia Ocean J Nucl Med Biol. 2020;8:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Fallahi B, Khademi N, Karamzade-Ziarati N, Fard-Esfahani A, Emami-Ardekani A, Farzanefar S, Eftekhari M, Beiki D. 99mTc-PSMA SPECT/CT Versus 68Ga-PSMA PET/CT in the Evaluation of Metastatic Prostate Cancer. Clin Nucl Med. 2021;46:e68-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Ćwikła JB, Roslan M, Skoneczna I, Kempińska-Wróbel M, Maurin M, Rogowski W, Janota B, Szarowicz A, Garnuszek P. Initial Experience of Clinical Use of [(99m)Tc]Tc-PSMA-T4 in Patients with Prostate Cancer. A Pilot Study. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Wang Q, Ketteler S, Bagheri S, Ebrahimifard A, Luster M, Librizzi D, Yousefi BH. Diagnostic efficacy of [(99m)Tc]Tc-PSMA SPECT/CT for prostate cancer: a meta-analysis. BMC Cancer. 2024;24:982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Farkas I, Sipka G, Bakos A, Maráz A, Bajory Z, Mikó Z, Czékus T, Urbán S, Varga L, Pávics L, Besenyi Z. Diagnostic value of [(99m)Tc]Tc-PSMA-I&S-SPECT/CT for the primary staging and restaging of prostate cancer. Ther Adv Med Oncol. 2024;16:17588359231221342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |