Published online Aug 26, 2025. doi: 10.12998/wjcc.v13.i24.107535
Revised: April 10, 2025
Accepted: May 10, 2025
Published online: August 26, 2025
Processing time: 82 Days and 17.3 Hours
This study analyzed the dental follicle and alveolar bone of two patients with tooth eruption disorders, aiming to provide some reference for exploring the etiology and selecting treatment plans of this disease from the perspective of the influence of extracellular matrix on osteoclasts differentiation in dental follicle.
Collect dental follicle and alveolar bone tissue from one patient with single tooth eruption disorder and one patient with full permanent tooth eruption disorder, respectively. Simultaneously collect the dental follicle and alveolar bone tissue of obstructed teeth that need to be extracted due to orthodontic treatment as the control group. Hematoxylin and eosin (HE) staining was used to observe the morphology of dental follicle cells. Immunohistochemical staining was used to observe the expression of periostin, receptor activator of nuclear factor kappa B ligand (RANKL), and osteoprotegerin (OPG) protein in dental follicle and al
HE staining of two cases of dental follicle tissues showed that the volume of dental follicle cells decreased, the nuclei were condensed, and there seemed to be cellular fibrosis. The immunohistochemical staining showed that both the dental follicle and alveolar bone tissue exhibited increased expression of periostin, decreased expression of RANKL and OPG proteins, and decreased RANKL/OPG ratio. After removing resistance, the permanent tooth germ often appears to have normal eruption. Tooth eruption disorders may be accompanied by abnormal remodeling of periostin, which affects the differentiation function of osteoclasts in the dental follicle and leads to metabolic imbalance of alveolar bone, resulting in tooth eruption disorders. Whether it is a single or full permanent tooth eruption disorder, once the coronal resistance is removed, the teeth can often erupt normally.
Core Tip: This study analyzed the periostin and bone metabolic factors in the dental follicle and alveolar bone of two patients with tooth eruption disorders. And the results suggest that tooth eruption diseases may be accompanied by abnormal remodeling of periostin, which can affect the differentiation function of osteoclasts in the dental follicle, leading to an imbalance in alveolar bone metabolism and resulting in tooth eruption disorders. This provides some reference for the etiology and treatment of tooth eruption disorders from a new research perspective of the effect of extracellular matrix on the differentiation of osteoclasts in dental follicle.
- Citation: Cai J, Qin H. Mechanism analysis of periostin in osteoclasts differentiation of dental follicle: Two case reports. World J Clin Cases 2025; 13(24): 107535
- URL: https://www.wjgnet.com/2307-8960/full/v13/i24/107535.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i24.107535
Tooth eruption disorder refers to the condition where the development of the tooth root is basically completed, but the eruption period is significantly later than that of normal teeth[1]. Once the resistance of the dental crown is removed, teeth can usually erupt normally, indicating that excessive stress may be a key factor in preventing tooth eruption. The dental follicle, as an essential organ for tooth eruption, is the loose fibrous connective tissue surrounding the developing tooth germ[2]. When teeth erupt, a certain amount of stress is generated between the dental follicle, alveolar bone, and gingiva as the tooth germ develops and occlusal movement occurs. Then promote the secretion of various factors in the dental follicle, recruit macrophages into the dental follicle and fuse to form osteoclasts, leading to alveolar bone resorption and eruption channels formation[3]. This indicates that normal tooth eruption requires a certain amount of stress to act on the dental follicle and stimulate the differentiation of osteoclasts by recruiting macrophages. However, why does the dental follicle have different effects on the activation function of osteoclasts during tooth eruption disorders? The reason for this may be that excessive stress alters the molecular regulatory mechanism of the dental follicle, leading to abnormal differentiation of osteoclasts. At present, research on tooth eruption disorders both domestically and internationally mainly focuses on changes in cytokines during tooth eruption[4,5]. On the contrary, there have been no reports on how the dental follicle perceives stress to initiate osteoclast differentiation, how the morphology and function of dental follicle cells change when stress increases, and how the corresponding alveolar bone changes at this time.
Extracellular matrix is a three-dimensional network composed of large molecules and minerals synthesized and secreted by cells, distributed on the cell surface or between cells, supporting and connecting tissue structures, regulating tissue development and cellular physiological activities[6,7]. If remodeling is disrupted, it can easily lead to fibrosis in fibroblasts. Periostin, as a multifunctional extracellular matrix protein, is mainly expressed in fibrous connective tissue subjected to sustained mechanical stress[8]. It can sense stress and convert it into biological signals, regulating tissue cell function. Recent studies have shown that periostin is expressed in the dental follicle and can promote the differentiation of dental follicle cells[9]. This suggests that periostitis protein may be a key extracellular matrix that can sensitively sense stress changes during tooth eruption, and can serve as an important indicator for dynamically observing the morphology and osteoclast differentiation function of dental follicle cells.
This study collected dental follicle and alveolar bone tissue specimens from one case of single tooth and one case of full permanent tooth eruption disorder. Hematoxylin and eosin (H&E) staining methods was used to observe the morphology of dental follicle cells. And immunohistochemical staining methods was used to observe the periostin, receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) protein expression. The objective aims to offer guidance for the etiological exploration and treatment options of this type of disease from the perspective of abnormal dental follicle morphology and osteoclast differentiation caused by excessive extracellular matrix sensing stress.
Single tooth eruption disorder: A 12-year-old female patient with good overall health came to our pediatric dental department for treatment due to the failure of the left maxillary first premolar (tooth 24) to erupt.
Full mouth tooth eruption disorder: A 10-year-old male patient with overall good health came to our pediatric dental clinic for treatment because none of his permanent teeth had properly erupted or replaced.
Both patients showed no history of systemic diseases or other abnormalities during examination.
Parents of the two patients stated that they were full-term natural birth, and her/his mother had not smoked, drunk or contracted infectious diseases during pregnancy. They denied that the patients have bad eating habits and hobbies, and denied the orthodontic treatment history.
General health and oral specialist examinations were conducted on family members to understand the corresponding medical history and genetic characteristics of related diseases. The results showed that no related diseases were found.
Single tooth eruption disorder: The intraoral examination revealed permanent dentition without tooth 24. But several abnormal tooth shaped tissues can be observed in the affected area.
Full mouth tooth eruption disorder: The intraoral examination of the patient revealed deciduous dentition, deciduous tooth 54, 64 and 85 were caries, and no obvious abnormality in the remaining deciduous teeth.
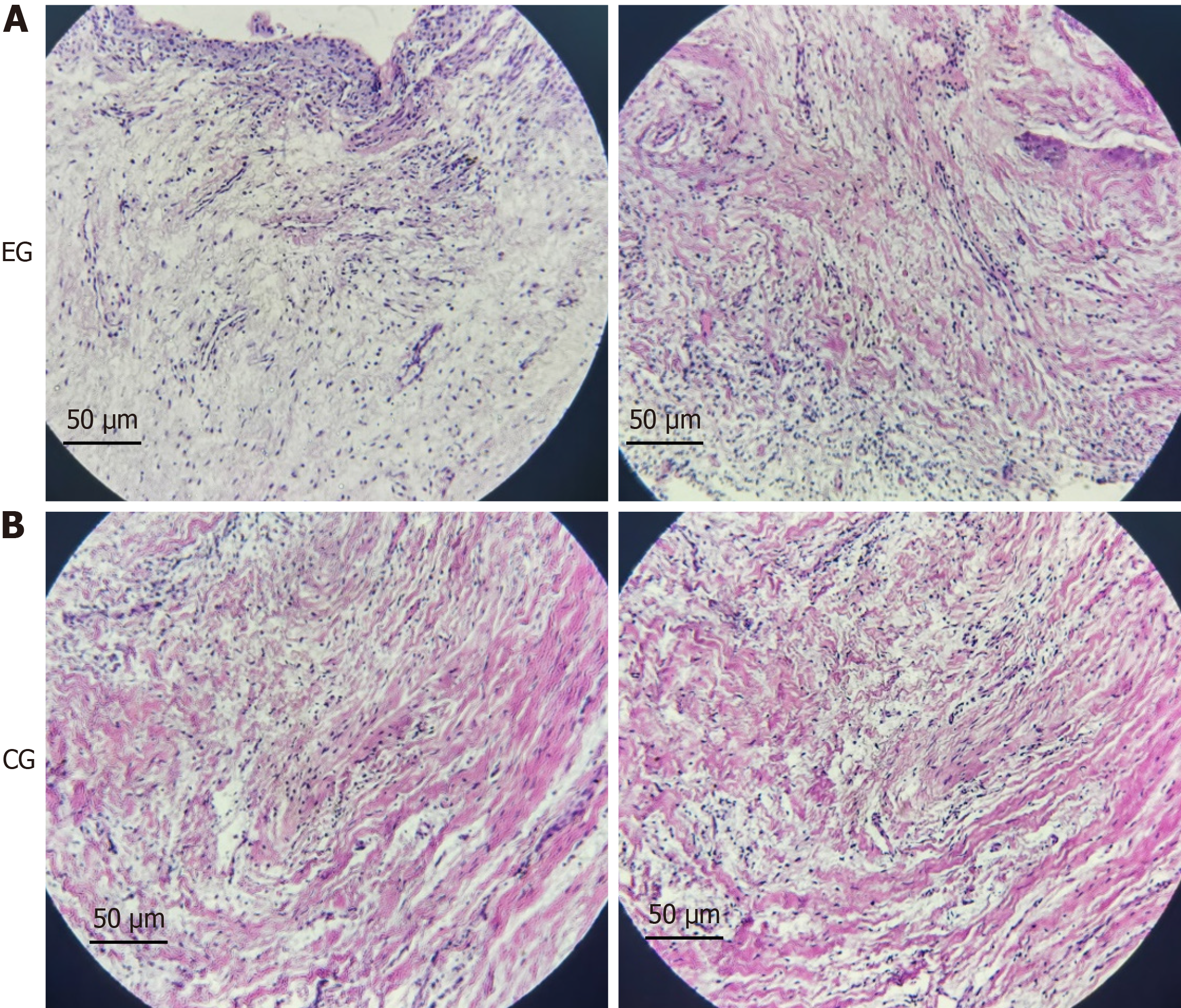
HE staining: The HE staining results of dental follicle tissues from the two patients with tooth eruption disorder showed that the volume of dental follicle cells decreased, the nuclei were condensed, and there seemed to be cellular fibrosis (Figure 1).
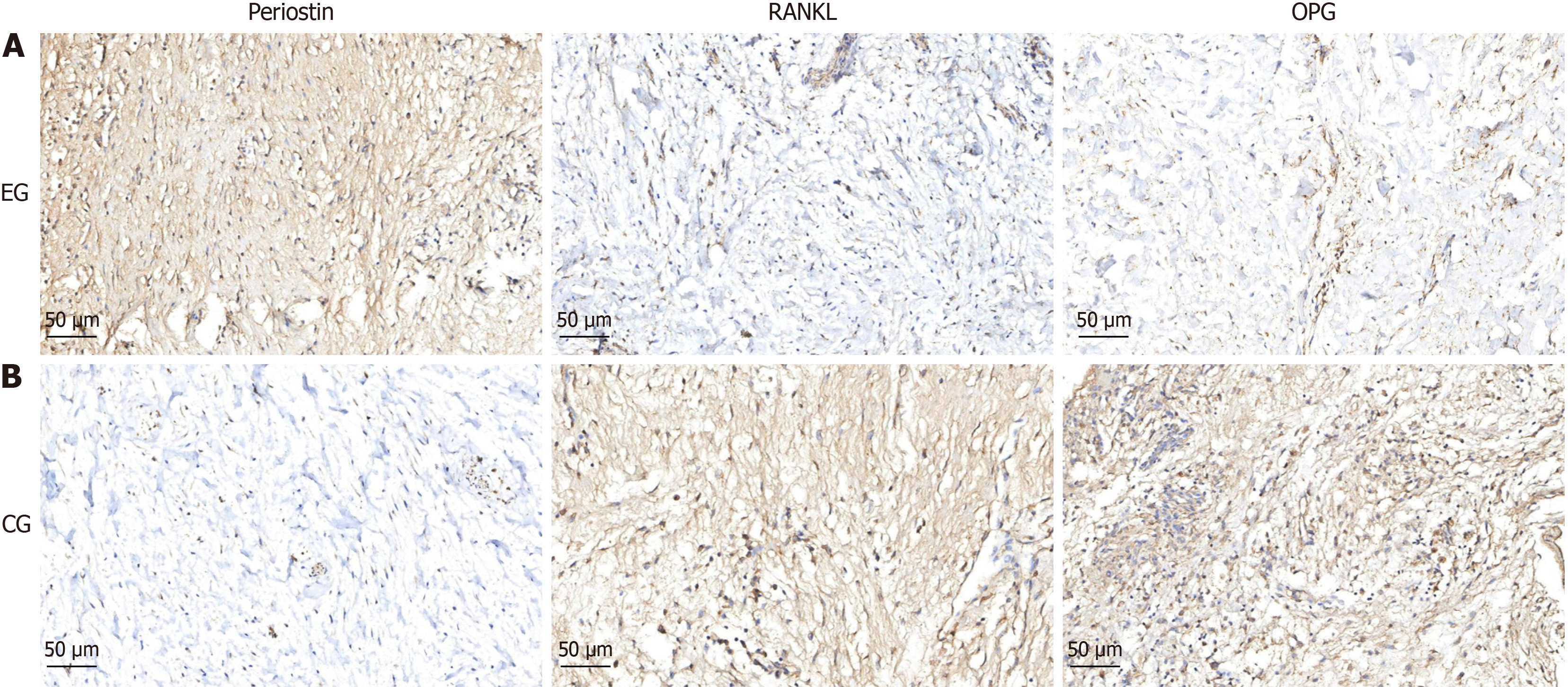
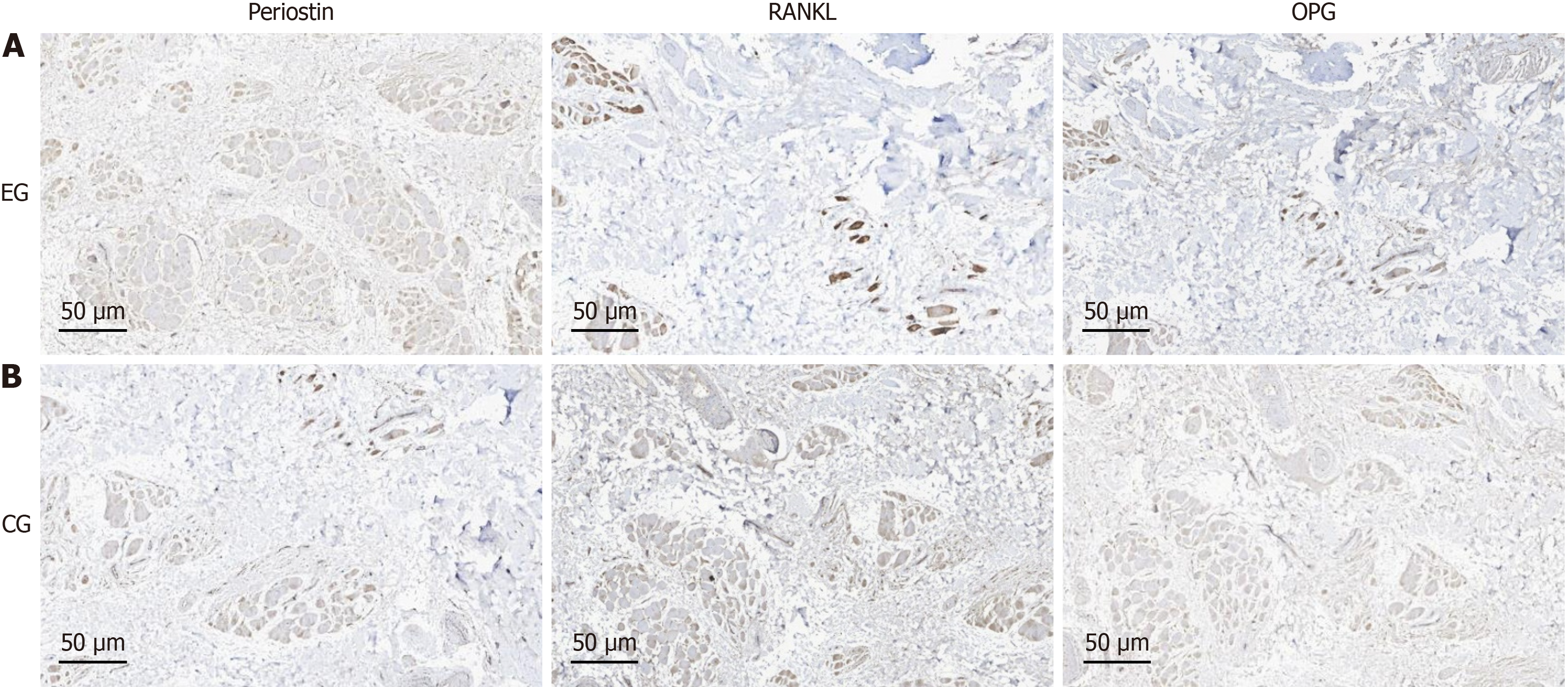
Immunohistochemical staining: Two cases of immunohistochemical staining showed that both the dental follicle and alveolar bone tissue exhibited increased expression of periostin, decreased expression of RANKL and OPG proteins, and decreased RANKL/OPG ratio (Figures 2 and 3).
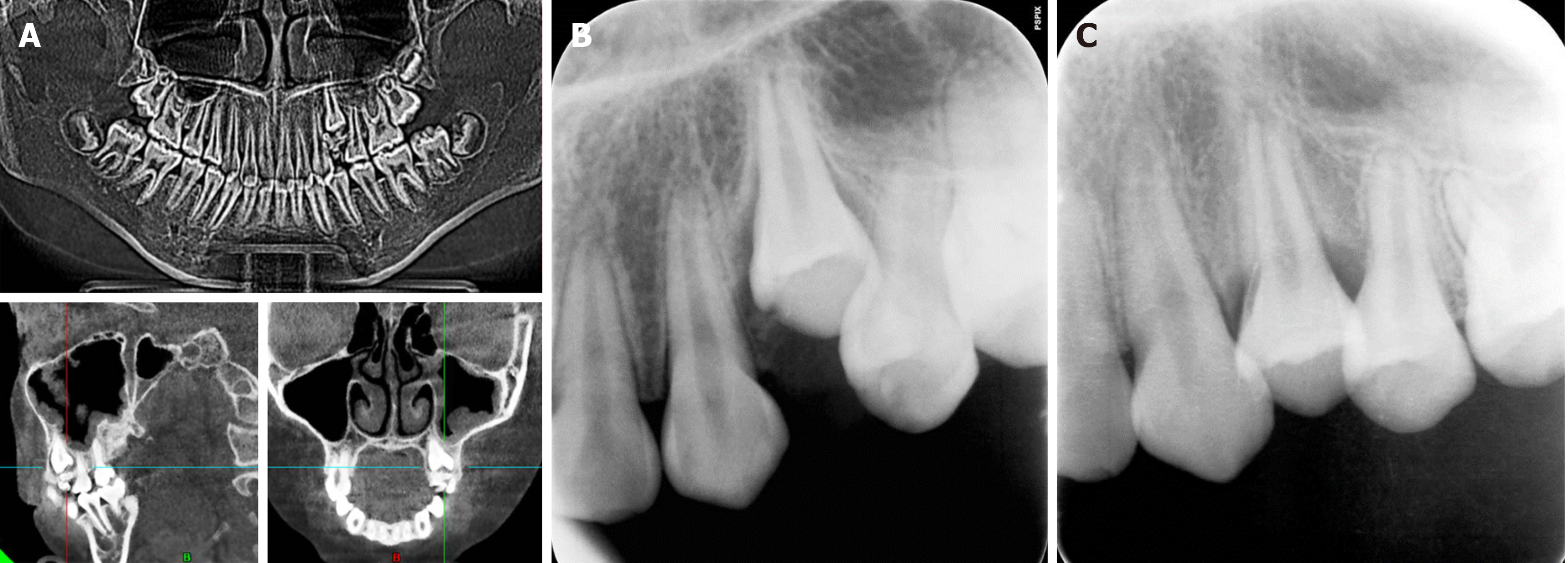
Single tooth eruption disorder: The results of cone beam computed tomography showed that image aggregates rese
The patient returned to our department for follow-up one month after surgery, and imaging showed no abnormalities above the crown region of tooth 24 (Figure 4B).
The patient had the second follow-up visit three months after the surgery. Oral examination showed that tooth 24 had almost fully erupted, and imaging data indicated no abnormalities in root development (Figure 4C).
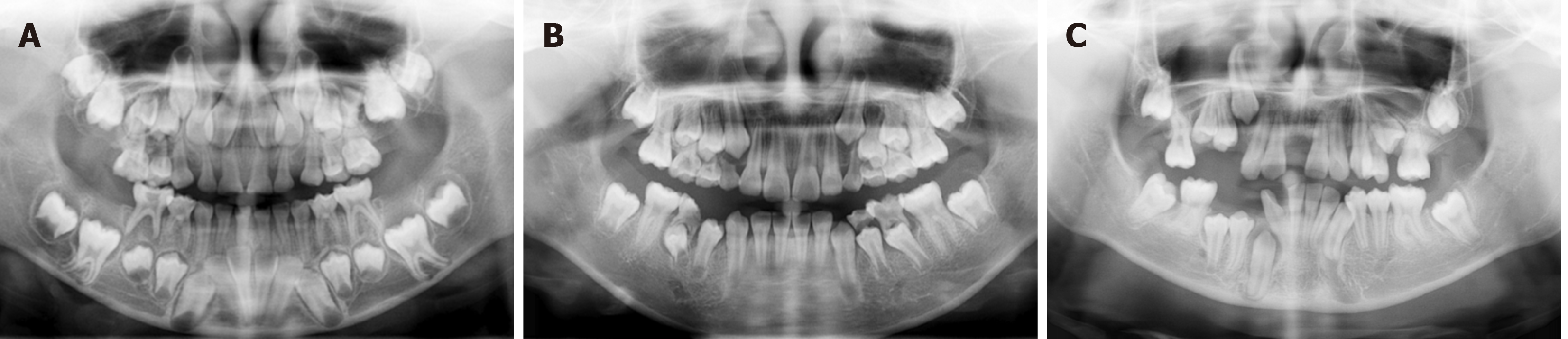
Full mouth tooth eruption disorder: His panoramic radiograph examination showed that the number of permanent tooth embryos was normal, the root of teeth 16, 26, 36, 46 were curve, root development of teeth 12-22, 32-42 was basically complete, and no obvious abnormalities were observed in alveolar bone (Figure 5A). Panoramic radiographs examination of his twin sister showed no abnormality in the replacement of primary and permanent teeth (Figure 5B). Within one year, the patient will have their retained deciduous teeth and crown alveolar bone removed in batches, fully exposing the obstructed permanent tooth germ. Two years later, the patient was returned to our clinic and the panoramic radiograph examination showed that all permanent teeth have basically erupted (Figure 5C).
The first patient was diagnosed with single tooth eruption disorder; the second patient was diagnosed with multiple tooth eruption disorders.
The first patient underwent tooth extraction under local anesthesia according to the standard of peripheral odontoma and fully exposing the obstructed permanent tooth germ.
For the second patient, the retained deciduous teeth and crown alveolar bone were extracted in batches within one year, completely exposing the obstructed permanent tooth germ.
The patient with single tooth eruption disorders was followed up one month after surgery, and imaging showed no abnormalities above the crown of the obstructed tooth. Three months after the surgery, the patient's oral examination during the follow-up showed that the obstructed teeth had erupted normally.
The patient with multiple tooth eruption disorders was followed up two years later, and oral examinations showed that all permanent teeth had mostly erupted.
The main manifestations of tooth eruption disorders are retention of single or multiple primary teeth, delayed eruption of permanent teeth, and tooth impaction[10,11]. Once the excessive stress on the crown of the obstructed tooth is removed, such as surgical removal of odontoma, extraction of retained deciduous teeth, and removal of bone above the obstructed tooth, the teeth can often erupt normally. This indicates that when the stress on the crown of the tooth germ is too high, it may affect the osteogenic differentiation ability of the tooth follicle by altering the morphology of the tooth follicle cells and molecular signal transduction mechanisms, leading to decrease in the potential for tooth eruption[12-14]. However, it is currently unclear what changes occur in dental follicle cells when subjected to excessive stress. This study observed the morphological changes of dental follicle cells through HE staining, and the results showed that when tooth eruption was hindered, the volume of dental follicle cells decreased, the nuclei were condensed, and there seemed to be cellular fibrosis. The results indicate that excessive stress on the dental crown may lead to fibrosis of dental follicle cells, thereby affecting the differentiation function of osteoclasts in the dental follicle. Nevertheless, it is currently unclear how the dental follicle sensitively perceives stress changes, why excessive stress can lead to fibrous changes in dental follicle cells, and how molecular mechanisms regulate the differentiation process of osteoclasts in dental follicles.
Extracellular matrix is a three-dimensional network composed of large molecules and minerals synthesized and secreted by cells, distributed on the cell surface or between cells, supporting and connecting tissue structures, regulating tissue development and cellular physiological activities[15,16]. If remodeling is disrupted, it can easily lead to fibrosis in fibroblasts[17]. The properties of fibroblasts in dental follicles suggest that there is a crucial regulatory role played by extracellular matrix during tooth eruption. Periostin, as a multifunctional extracellular matrix protein, is mainly expressed in fibrous connective tissue subjected to sustained mechanical stress[18]. It can sense stress and convert it into biological signals, regulating tissue cell function. The detection of periostin in the periodontal tissue of extracted mandibular teeth in mice revealed a decrease in periostin expression after loss of stress stimulation[19]. Recent studies have shown that periostin are expressed in the dental follicle and can promote the differentiation function of dental follicle cells. In this study, we found that the expression of periostin in the group with tooth eruption disorders was significantly higher than that in the control group, indicating that periostin is a key extracellular matrix for sensing stress changes during eruption and can be used as an important indicator for dynamically observing changes in tooth eruption. If the stress is too high, it may lead to dysregulation of periostin remodeling, affecting the osteoclast differentiation function of the dental follicle.
The RANKL/OPG ratio is an important indicator for measuring osteoclast differentiation[20]. An increase in the ratio indicates active osteoclast differentiation, while a decrease in the ratio indicates the opposite. The RANKL in osteoclasts is mainly expressed by stromal cells and osteoblasts, and can be activated by many factors[21]. The RANK in osteoclasts is located on the surface of the precursor cell membrane and is the only target receptor for RANKL to stimulate osteoclast activation[22]. OPG is a natural inhibitor of RANKL, which binds to RANKL and competitively blocks RANKL/RANK binding, inhibiting osteoclast differentiation and maturation[23]. RANKL positive signal is widely expressed in various tissues of tooth eruption, but the location and intensity of expression are inconsistent at different stages[24]. Animal experiments have shown that RANKL/OPG increases and osteoclasts become active during the middle stage of tooth eruption in mice, but in the early and late stages of eruption, there are relatively fewer osteoclasts in the alveolar bone, RANKL/OPG decreased, consistent with the level of bone resorption activity[25]. In this study, the ratio of RANKL/OPG in the dental follicle and alveolar bone tissues of the eruption disorder group was found to decrease synchronously. From this point, we preliminarily speculate that excessive pressure on the dental crown may lead to dysregulation of extracellular membrane protein remodeling, driving fibrosis of dental follicle cells. Thus, by changing the RANKL/OPG ratio, it affects the differentiation of osteoclasts during tooth eruption, leading to obstacles in tooth eruption.
The pathogenesis and treatment of tooth eruption disorders have always been a hot topic in the field of dentistry, but currently the limited treatment include drug-induced tooth eruption, alveolar bone repair surgery and orthodontic traction. Drug induced tooth eruption is achieved by using exogenous hormone drugs to promote tooth germ de
With the research of gene therapy and tissue engineering technology in the process of tooth eruption, it can be predicted that future treatment plans should include precise and effective site strikes to promote tooth re-eruption. This study observed the morphology of dental follicle cells and analyzed the expression of periostin, RANKL, and OPG protein in dental follicle and alveolar bone. In order to explore the molecular signal transduction mechanism of tooth eruption disorder from the perspective of extracellular matrix perception affecting cell morphology and function, and to open up novel research ideas for the protection and treatment of abnormal tooth eruption. In future research, we will expand clinical samples and establish in vitro animal models to further observe the effects of periostin on osteoclast differentiation and alveolar bone remodeling in dental follicle, providing more experimental evidence for the treatment of tooth eruption disorders.
The authors are grateful to the patients and their family members for their kind cooperation and participation.
| 1. | Chen J, Ying Y, Li H, Sha Z, Lin J, Wu Y, Wu Y, Zhang Y, Chen X, Zhang W. Abnormal dental follicle cells: A crucial determinant in tooth eruption disorders (Review). Mol Med Rep. 2024;30:168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Muthu MS, Vandana S, Akila G, Anusha M, Kandaswamy D, Aswath Narayanan MB. Global variations in eruption chronology of primary teeth: A systematic review and meta-analysis. Arch Oral Biol. 2024;158:105857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Del Rio Cantero N, Mourelle Martínez MR, Sagastizabal Cardelús B, De Nova García JM. Influence of zoledronic acid and pamidronate on tooth eruption in children with osteogenesis imperfecta. Bone. 2024;182:117069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 4. | Wagner D, Rey T, Maniere MC, Dubourg S, Bloch-Zupan A, Strub M. Primary failure of eruption: From molecular diagnosis to therapeutic management. J Oral Biol Craniofac Res. 2023;13:169-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Yamaguchi T, Hosomichi K, Shirota T, Miyamoto Y, Ono W, Ono N. Primary failure of tooth eruption: Etiology and management. Jpn Dent Sci Rev. 2022;58:258-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Malta MD, Cerqueira MT, Marques AP. Extracellular matrix in skin diseases: The road to new therapies. J Adv Res. 2023;51:149-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Mascharak S, Guo JL, Griffin M, Berry CE, Wan DC, Longaker MT. Modelling and targeting mechanical forces in organ fibrosis. Nat Rev Bioeng. 2024;2:305-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Qin H, Cai J. Effect of Periostin Silencing on the Autophagy of Osteoblasts. Cell Reprogram. 2019;21:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Wei X, Liu Q, Liu L, Tian W, Wu Y, Guo S. Periostin plays a key role in maintaining the osteogenic abilities of dental follicle stem cells in the inflammatory microenvironment. Arch Oral Biol. 2023;153:105737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Li XX, Wang MT, Wu ZF, Sun Q, Ono N, Nagata M, Zang XL, Ono W. Etiological Mechanisms and Genetic/Biological Modulation Related to PTH1R in Primary Failure of Tooth Eruption. Calcif Tissue Int. 2024;115:101-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Sobkowska Ł, Sobkowska J, Dudek D, Grabarek BO, Czajka-Jakubowska A, Przystańska A. Symptoms of the Eruption of Permanent Teeth. Int J Environ Res Public Health. 2022;19:3301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Stergiopulos O, Lagou A, Antonarakis GS, Pandis N, Kiliaridis S. The effect of occlusal loading on secondary tooth eruption: An experimental study using a rat model. J Morphol. 2024;285:e21755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Bastos VC, Gomez RS, Gomes CC. Revisiting the human dental follicle: From tooth development to its association with unerupted or impacted teeth and pathological changes. Dev Dyn. 2022;251:408-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 14. | Harano T, Asahara M. Evolution of tooth morphological complexity and its association with the position of tooth eruption in the jaw in non-mammalian synapsids. PeerJ. 2024;12:e17784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Na J, Yang Z, Shi Q, Li C, Liu Y, Song Y, Li X, Zheng L, Fan Y. Extracellular matrix stiffness as an energy metabolism regulator drives osteogenic differentiation in mesenchymal stem cells. Bioact Mater. 2024;35:549-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 16. | Yu Y, Leng Y, Song X, Mu J, Ma L, Yin L, Zheng Y, Lu Y, Li Y, Qiu X, Zhu H, Li J, Wang D. Extracellular Matrix Stiffness Regulates Microvascular Stability by Controlling Endothelial Paracrine Signaling to Determine Pericyte Fate. Arterioscler Thromb Vasc Biol. 2023;43:1887-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Younesi FS, Miller AE, Barker TH, Rossi FMV, Hinz B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat Rev Mol Cell Biol. 2024;25:617-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 163] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 18. | Feng S, Feng Q, Dong L, Lv Q, Mei S, Zhang Y. Periostin/Bone Morphogenetic Protein 1 axis axis regulates proliferation and osteogenic differentiation of sutured mesenchymal stem cells and affects coronal suture closure in the TWIST1(+/-) mouse model of craniosynostosis. J Orthop Surg Res. 2024;19:146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Qiao B, Liu X, Wang B, Wei S. The role of periostin in cardiac fibrosis. Heart Fail Rev. 2024;29:191-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Brodetska L, Natrus L, Lisakovska O, Kaniura O, Iakovenko L, Skrypnyk I, Flis P. The regulatory role of the RANKL/RANK/OPG signaling pathway in the mechanisms of tooth eruption in patients with impacted teeth. BMC Oral Health. 2020;20:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Arid J, Xavier TA, da Silva RAB, De Rossi A, da Silva LAB, de Queiroz AM, Galo R, Antunes LAA, Silva MJB, Antunes LS, Abbasoglu Z, Nelson Filho P, Küchler EC, Fukada SY. RANKL is associated with persistent primary teeth and delayed permanent tooth emergence. Int J Paediatr Dent. 2019;29:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Gao B, Deng R, Chai Y, Chen H, Hu B, Wang X, Zhu S, Cao Y, Ni S, Wan M, Yang L, Luo Z, Cao X. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J Clin Invest. 2019;129:2578-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Yamaguchi M, Takami M, Azetsu Y, Karakawa A, Chatani M, Funatsu T, Sakai N. Effects of anti-RANKL antibodies administered to pregnant mice on bone and tooth development in neonates. J Oral Biosci. 2023;65:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Shan Y, Jin Y, Zhang X, Tang Y, Lai W, Liao J, Wu M, Long H. Development of a novel hyaluronic acid/alginate/RANKL degradable microneedle patch for accelerating bone remodeling and orthodontic tooth movement through promoting osteoclastogenesis. Int J Pharm. 2025;669:124915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Liu J, Wang J, Huang R, Jia X, Huang X. The Shh-p38-NFATc1 signaling pathway is essential for osteoclastogenesis during tooth eruption. Tissue Cell. 2025;92:102643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |