Published online Aug 6, 2025. doi: 10.12998/wjcc.v13.i22.106122
Revised: April 7, 2025
Accepted: April 21, 2025
Published online: August 6, 2025
Processing time: 77 Days and 16.1 Hours
Sclerosing encapsulating peritonitis (SEP), also known as abdominal cocoon syndrome, is rare in children. The etiology of primary SEP is believed to be associated with retrograde menstruation or viral peritonitis in young adolescent girls, whereas secondary SEP refers to SEP caused by other factors such as sur
We report the case of a 10-year-old girl with secondary SEP who was admitted to our center with acute bowel obstruction.
In this report, we emphasized the imaging manifestations, diagnosis, and operative management of the case. Although postoperative SEP in children is rare, the long-term prognosis is favorable when accompanied with accurate diagnosis, appropriate perioperative management, and timely follow-up.
Core Tip: Sclerosing encapsulating peritonitis (SEP) (abdominal cocoon syndrome), a rare etiology of pediatric intestinal obstruction, is predominantly secondary to prior abdominal surgery, peritoneal dialysis, or tuberculosis. Characterized by non-specific clinical presentations, it poses diagnostic challenges in children and frequently progresses to mechanical bowel obstruction. We present a 10-year-old female with postoperative SEP, highlighting its distinctive imaging features (including "cocoon-like" peritoneal encapsulation on computed tomography), intraoperative confirmation via adhesiolysis, and successful multi-disciplinary management. This case underscores the critical role of early radiological suspicion and surgical intervention in mitigating morbidity, while providing insights for differentiating SEP from other adhesive pathologies in pediatric populations.
- Citation: Zheng HJ, Zhang JD, Wang ZC, Yao LY. Abdominal cocoon syndrome in a 10-year-old young adolescent after abdominal operation: A case report and review of literature. World J Clin Cases 2025; 13(22): 106122
- URL: https://www.wjgnet.com/2307-8960/full/v13/i22/106122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i22.106122
Sclerosing encapsulating peritonitis (SEP), also known as abdominal cocoon syndrome (ACS), is a rare cause of intestinal obstruction. It is first described by Owtschinnikow in 1907 with the term peritonitis chronica fibrosa incapsulata[1]. In 1978, Foo et al[2] first reported 10 cases of primary SEP, using the term ACS, in adolescent girls. Of the patients reported, none had a history of abdominal surgery, peritonitis, or prolonged drug intake. The etiology of primary, or idiopathic SEP, is believed to be associated with factors such as retrograde menstruation or viral peritonitis. With advancements in research, clinicians' understanding of SEP has gradually improved, and SEP has been described in children, premenstrual women, and men. Notably, SEP associated with abdominal trauma or operations[3,4], abdominal tuberculosis[5], ventriculoperitoneal shunts[6], peritoneal dialysis[7], liver transplantation[8], and certain drug effects[9,10] has been termed secondary SEP. Although numerous studies by multiple scholars have reported cases of abdominal SEP or ACS, it is extremely rare in children, especially following abdominal surgery. Through a systematic literature search on PubMed, we identified only two articles published in the past 5 years that report postoperative SEP in children under 18 years of age[10,11]. In this report, we describe a case of secondary SEP in a 10-year-old premenarchal adolescent girl and discuss the surgical options and perioperative considerations for SEP in children.
A 10-year-old girl was admitted to the Emergency Department of the Pediatric Medical Center at The First Hospital of Jilin University with abdominal pain, vomiting, and cessation of flatus and defecation for half a day.
The child developed abdominal distension after breakfast, followed by simultaneous occurrence of abdominal pain and vomiting. The vomitus initially consisted of gastric contents, which progressed to bile-stained vomiting after repeated episodes. Following laxative administration and enema at a local hospital, the symptoms showed no improvement, and neither flatus nor defecation occurred during the enema procedure.
The child was diagnosed with congenital ileal atresia 1 day after birth and underwent emergency surgery at a local hospital. After surgery, the child occasionally experienced abdominal pain but did not undergo further follow-up examinations.
The child had not experienced menarche and had no history of toxin exposure, special medications, trauma, or tuberculosis. Both parents and other immediate family members were healthy, with no family history of genetic diseases.
On physical examination, the child was short and thin, with a height of 132 cm and weight of 27 kg. Abdominal examination revealed a longitudinal surgical scar approximately 4 cm above the umbilicus, with localized depression of the abdominal wall and significant scar formation. No apparent abnormalities were observed beneath the scars. The abdomen was noticeably distended, with tenderness and rebound tenderness, particularly in the lower abdomen, but without muscle rigidity. A mass measuring approximately 5 cm × 5 cm with indistinct borders was palpated slightly to the left below the umbilicus.
The complete blood count indicated an elevated white blood cell count (12.77 × 109/L), with neutrophils increased to 9.11 × 109/L. High-sensitivity C-reactive protein levels were within normal ranges. Blood biochemical tests indicated the presence of acidosis, whereas other routine emergency tests revealed no significant abnormalities.
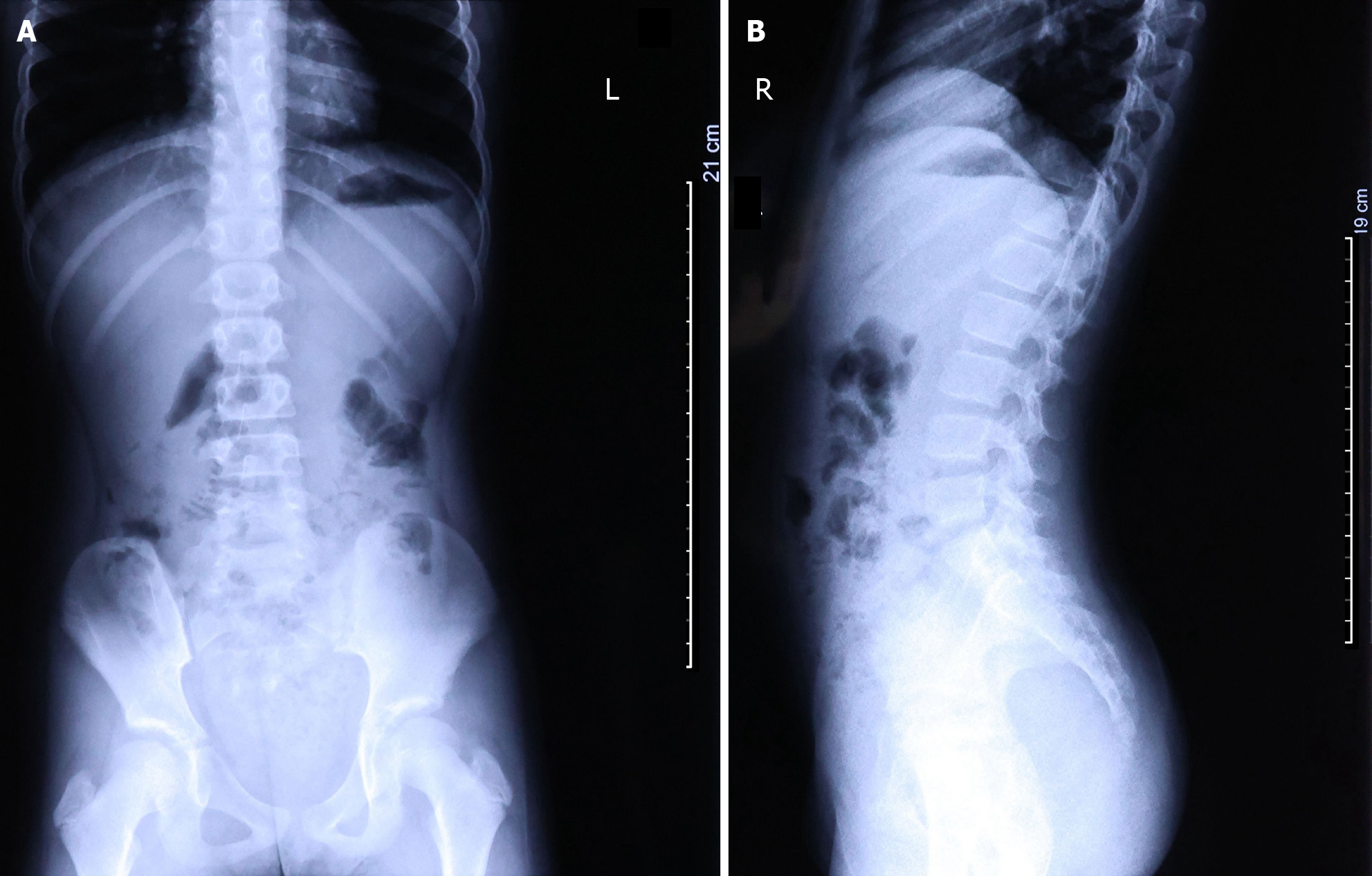
Following the physical examination, the child underwent an abdominal ultrasound, digestive system X-ray, and blood test. Abdominal ultrasound findings revealed abnormally dilated loops of the jejunum and ileum in the left and lower left abdomen, containing a large amount of chyme under high tension. The diameter of the intestinal tract was 3.1 cm. A substantial number of cord-like hypoechoic areas were visible between the intestines; a significant amount of ascites, with a depth of approximately 2.8 cm and turbid sound transmission, was present in the abdominal cavity. The mesentery was notably swollen; the ileocecal region was slightly swollen, and the wall of the terminal ileum was thickened, measuring approximately 0.6 cm. Radiographic examination revealed poor aeration of the small intestine in the lower abdomen along with the presence of air-fluid levels (Figure 1).
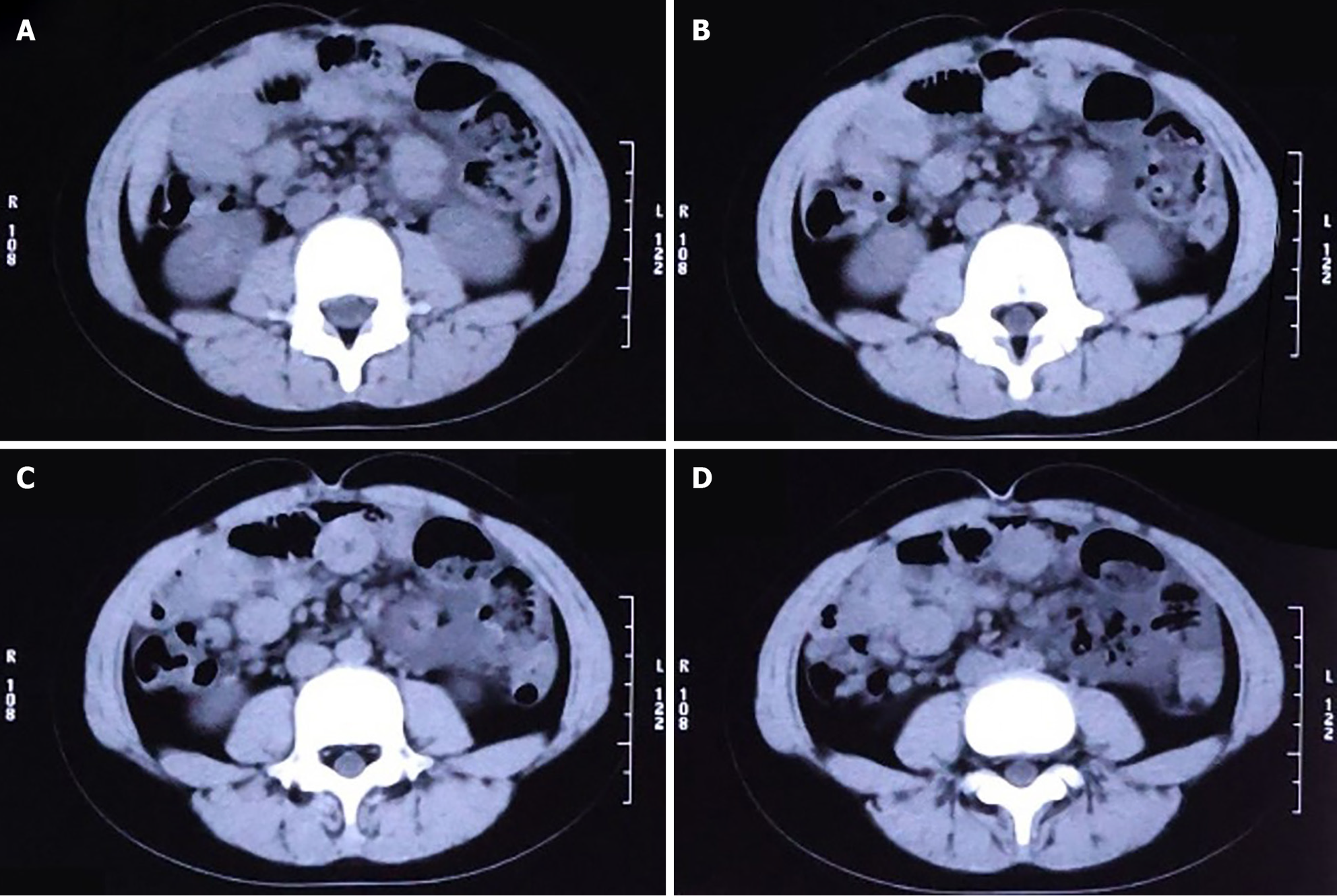
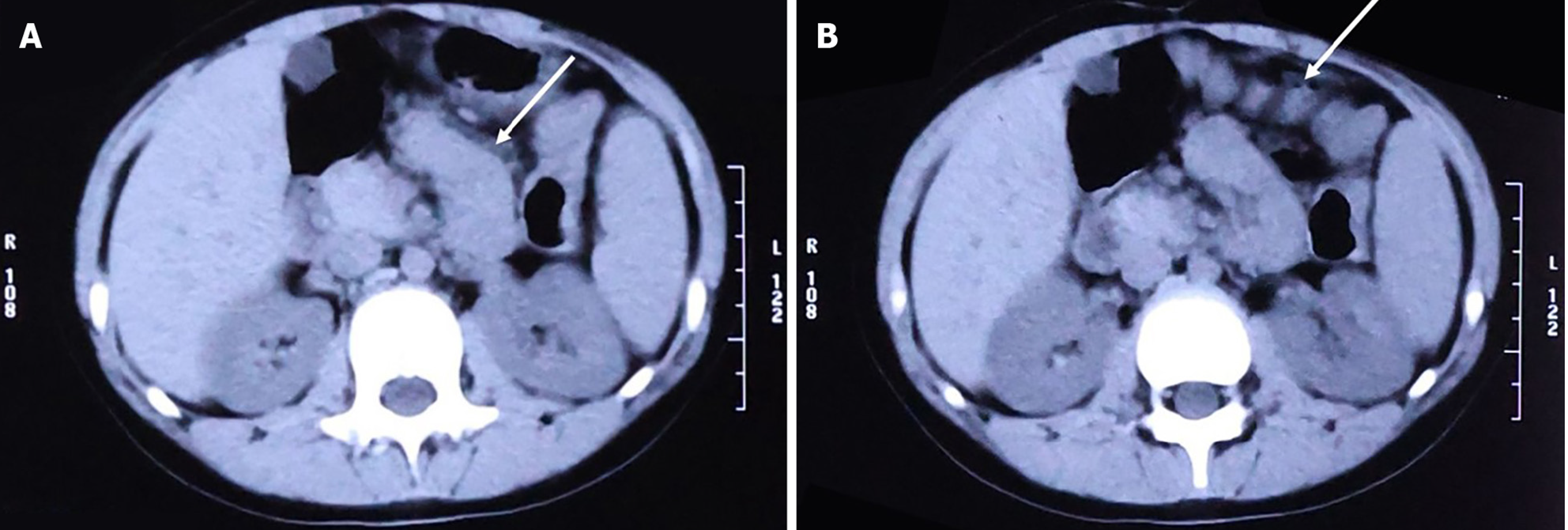
Because emergency examinations suggested signs of acute intestinal obstruction, the child was transferred to the Pediatric Surgery Department for further treatment. We proceeded with an abdominal computed tomography (CT) scan, the results of which were consistent with changes indicative of intestinal obstruction: (1) The intestinal tract within the abdominal cavity was disorganized, with a significant number of air-fluid levels visible (Figure 2A); (2) The obstruction point was located below and to the left of the umbilicus; the proximal small intestine was situated in the pelvic cavity and contained a large amount of intestinal content (Figure 2B); (3) The intestinal wall at the point of obstruction was markedly thickened, with pronounced exudation from the surrounding intestinal tract (Figure 2C); (4) The colon and rectum were collapsed (Figure 2D); and (5) Soft tissue structures were observed surrounding the small intestine, enveloping it and exhibiting a distinctive "accordion-like" appearance (Figure 3A and B).
The clinical diagnosis was acute small-bowel obstruction. Conservative treatments such as enema, anti-infection therapy, and acidosis correction were provided; however, the child’s abdominal pain could not be alleviated, and bile-like gastric fluid continued to be aspirated through gastrointestinal decompression. Consequently, emergency exploratory surgery under general anesthesia with endotracheal intubation was performed.
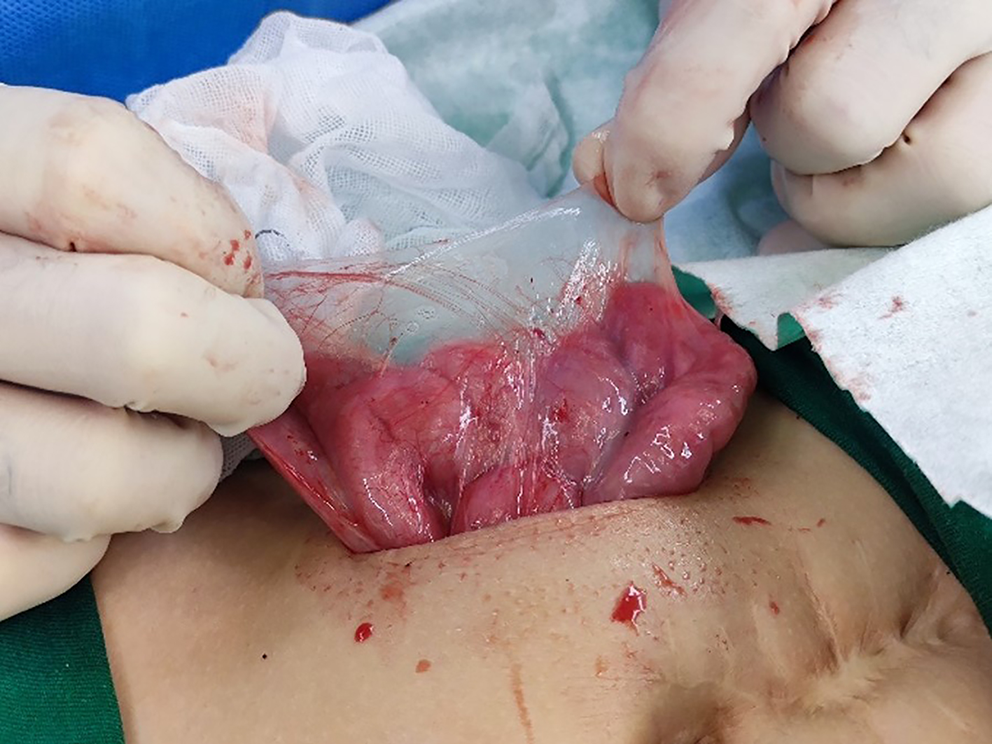
The surgery involved an incision along the lateral edge of the right rectus abdominis muscle. Upon entering the abdominal cavity, the entire surface of the small intestine, colon, and liver appeared to be covered with a dense, translucent fibrous membrane (Figure 4). The obstruction was located slightly to the left of the umbilicus, where fibrous membranes enveloped the small intestine, creating several sharp angles, one of which adhered to the abdominal wall and prevented the passage of contents from the proximal small intestine. After dissecting the fibrous membranes, the small intestine regained its patency. During surgery, we also observed the site of the previous intestinal anastomosis and found no abnormalities. Based on the preoperative presentation and surgical exploration findings, the child was diagnosed with ACS, acute small-bowel obstruction, and developmental delay.
We lysed the small intestinal adhesions and removed the obstruction-causing fibrous membranes to the maximum possible extent. The surgical procedure was completed in 3 hours and 20 minutes, with intraoperative blood loss of 50 mL. Although extensive wound bleeding occurred during the stripping process, no damage to the mesenteric vessels or serosa of the small intestine or any intestinal perforation occurred.
Following the return of bowel sounds and resumption of spontaneous flatus and defecation, the child was started on a full liquid diet orally 1 week postoperatively. However, 1 day after resuming oral intake, the abdominal pain and vomiting recurred. Repeat radiography revealed multiple air-fluid levels, although the child continued to pass gas and stool spontaneously. Abdominal ultrasonography revealed edema of the small intestinal wall, suggesting partial intestinal obstruction. We determined that the child had developed early postoperative small-bowel obstruction as a complication. After another week of fasting, the symptoms alleviated, and abdominal ultrasonography indicated a reduction in small intestinal wall edema. Consequently, we decided to initiate a residue-free diet. After 1 week of transition, the child progressed to a semi-liquid diet and was subsequently discharged in a recovered state (Table 1).
| Day | Clinical events | Diagnostic findings | Interventions |
| Day 1 | Abdominal pain, vomiting, flatus cessation, and defecation | N/A | Laxative administration and enema at a local hospital |
| Day 1 | No improvement | X-ray of the digestive system: air fluid levels; Abdominal ultrasonography: abnormally dilated loops of the jejunum and ileum; WBC 12.77 × 109/L, neutrophils 9.11 × 109/L | Transferred to the pediatric surgery department |
| Day 1 | No improvement | CT scan: Intestinal obstruction | Correction of acidosis; Gastrointestinal decompression |
| Day 1 | No improvement | Emergency exploratory surgery: abdominal cocoon syndrome, acute small bowel obstruction | Surgery procedure: Lysed the small intestinal adhesions and removed the fibrous membranes |
| Day 7 | Resumption of spontaneous flatus and defecation | Body check: Return of bowel sounds | Full liquid diet orally |
| Day 8 | Recurrence of abdominal pain and vomiting | X-ray of the digestive system: Air fluid levels; Abdominal ultrasonography: Revealed edema of the small intestinal wall; Early postoperative small bowel obstruction | Fasting |
| Day 15 | Symptoms alleviated | Abdominal ultrasonography: A reduction in small intestinal wall edema | Transition from a residue-free diet to a semi-liquid diet |
| Day 22 | Symptoms improving | Discharged home |
The child’s follow-up period was uneventful, with postoperative reviews at 3 months, 6 months, and 1 year indicating favorable recovery. Although still lagging behind her peers, the child’s height and weight were steady increasing.
While a substantial amount of literature on SEP has been published since its first report in 1907, its etiology remains unclear, and there is still no consensus on its classification method. Based on its etiology, SEP can be classified into primary (also known as idiopathic or ACS) and secondary SEP[12]. Some scholars suggest that congenital developmental anomalies, such as the absence of the greater omentum, are among the causes of primary SEP[13-15]. Nevertheless, the etiology of primary SEP, which is more common in tropical and subtropical regions, is still not fully understood[16]. Previous reports suggest that primary SEP is associated with factors such as retrograde menstruation, viral peritonitis[2], retrograde peritonitis, and cell-mediated immunological tissue damage incited by gynecological infection[17].
Secondary SEP, which is more common than primary SEP[12], is associated with multiple factors such as long-term peritoneal dialysis[18] and abdominal surgery[1], which can be responsible for inflammation and peritonitis. It may occur in all age groups[12,19,20]. In our case, the child had a history of abdominal surgery and had not experienced menarche; thus, the case was categorized as secondary SEP.
Furthermore, some scholars have divided SEP into three types[13] based on the area of the membrane: Type I—the membrane encapsulates a part of the intestine; type II—the membrane encapsulates the entire intestine; and type III—the membrane encapsulates entire intestine and other organs.
This classification is based on the extent of the encasing membrane. As surgical intervention is required to confirm the classification, it is considered to have limited clinical significance. Li et al[21] classified SEP based on whether a second enterocoelia has formed, using mainly CT imaging and clinical manifestations to determine whether conservative treatment or surgery is needed.
Although imaging manifestations lack specificity, some useful clues can still be identified, especially on CT scans. “Cauliflower-like” arrangement, “bottle-gourd” appearance, and “concertina-like” arrangement can be found on the imaging scans of SEP patients[22,23]. In recent reports, one or more of these features can be identified on CT or magnetic resonance imaging scans[13,16,24,25]. These features were also observed in our case (Figure 3A and B).
In addition to CT scans, abdominal ultrasound is also helpful for diagnosing SEP in pediatric patients due to their thinner abdominal walls[26]. Because abdominal ultrasound is radiation-free and convenient to use, it is generally employed as the initial diagnostic tool in pediatric patients[27]. An experienced ultrasonographer can also detect signs similar to those found on CT scans, as demonstrated in our case. Additionally, abdominal ultrasound possesses unique advantages. Since children with SEP often present with intestinal obstruction as the initial symptom, color Doppler ultrasound can be used to assess the mesenteric blood supply, promptly identifying intestinal ischemia or strangulation and providing valuable references for subsequent treatment.
Regarding treatment, the majority of pediatric SEP cases are managed surgically[1,26,28], although there are sporadic reports of successful conservative management[20]. This approach diverges from the management strategies employed for adult SEP or ACS, for which a considerable number of patients undergo conservative treatment. The objective of surgery is to alleviate intestinal obstruction and to excise as much of the membranous structures enveloping the bowel as possible. Although this method may injure the serosal layer of the small intestine and even lead to perforation and death[29], failure to completely remove these membranous structures can predispose patients to postoperative adhesions and potential recurrence of obstruction.
However, performing complete enterolysis of the small intestine and excision of the membranous structures in children with type III SEP is challenging. The surgery must be conducted with utmost care to expose the original anatomical structures while preventing iatrogenic injuries, inevitably leading to an extended duration of the surgical procedure[28]. In our case, the duration of the surgery was 3 hours and 20 minutes. In addition to performing enterolysis and membrane resection, we also applied chitosan within the interintestinal spaces as a barrier to prevent adhesion formation.
Unfortunately, the child in our case experienced postoperative complications: early postoperative intestinal obstruction primarily caused by extensive edema of the small intestinal wall. This edema was related to the extensive enterolysis, prolonged exposure of the intestinal tract, and damage to the serosal layer of the small intestine during the surgical procedure[30]. Despite the eventful recovery process, the child was ultimately discharged in good health, without the emergence of more severe complications[28].
Our study presents a clinically rare case of pediatric SEP, which diagnostic and therapeutic processes provide valuable insights for clinical reference. However, given the exceptional rarity of this condition, certain limitations in the diagnostic workup and management protocols warrant cautious interpretation when extrapolating these findings to broader clinical practice.
Postoperative SEP is rare in children. Based on our experience, SEP is often diagnosed during surgery. For children presenting with acute intestinal obstruction, a thorough analysis of their medical history, combined with imaging results such as abdominal ultrasound and CT scans, is essential to make the best clinical decision. In most cases, surgery is the preferred treatment for children with postoperative SEP. Although postoperative complications are rare, we should remain vigilant for early postoperative intestinal obstruction caused by intestinal wall edema. The long-term prognosis was favorable.
The authors extend their gratitude to Weiru Cheng for her invaluable assistance in data collection and literature curation, whose meticulous efforts in compiling research materials significantly enriched the foundation of this study. We also thanks Professor Chunyu Dong for providing expert clinical insights during patient diagnosis and treatment deliberations, particularly regarding therapeutic decision-making processes. Their collective contributions have been instrumental in enhancing the rigor and practical relevance of this work.
| 1. | Keese D, Schmedding A, Saalabian K, Lakshin G, Fiegel H, Rolle U. Abdominal cocoon in children: A case report and review of literature. World J Gastroenterol. 2021;27:6332-6344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 2. | Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 205] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Karona P, Blevrakis E, Kastanaki P, Tzouganakis A, Kastanakis M. Abdominal Cocoon Syndrome: An Extremely Rare Cause of Small Bowel Obstruction. Cureus. 2021;13:e14351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Liang JL, Chen ZQ, Yi Z, Kun Ming W. A case report of encapsulating peritoneal sclerosis followed by cesarean section: Clinical diagnosis and treatment experience. Medicine (Baltimore). 2022;101:e32122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Barchid A, Fadil W, Ahallat A, Aggouri Y, Aitlaalim S. Cocoon Syndrome: A Rare Cause of Bowel Obstruction Revealing Hidden Intestinal Tuberculosis. Cureus. 2025;17:e77090. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Sigaroudinia MO, Baillie C, Ahmed S, Mallucci C. Sclerosing encapsulating peritonitis--a rare complication of ventriculoperitoneal shunts. J Pediatr Surg. 2008;43:E31-E33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Chen C, Hua J, Xu Y. Abdominal cocoon in peritoneal dialysis. Int Urol Nephrol. 2023;55:2675-2676. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Lorenzo C, Chullo G, Tonina ED, Rivas E, Blasi A, Ubre M, Crespo G, Ruiz P, Colmenero J, Pera M, Fundora Y. Sclerosing Encapsulating Peritonitis: A Surgical Challenge in Liver Transplantation. Transplant Proc. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Asotibe JC, Zargar P, Achebe I, Mba B, Kotwal V. Secondary Abdominal Cocoon Syndrome Due To Chronic Beta-Blocker Use. Cureus. 2020;12:e10509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Whitlock RS, Malik T, Smith V, Mahajan P, Hayes-Jordan A, Vasudevan SA. Sclerosing Encapsulating Peritonitis in a Pediatric Patient Treated With Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J Pediatr Hematol Oncol. 2021;43:e685-e688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Tambuzzi S, Gentile G, Boracchi M, Zoja R, Gentilomo A. A forensic case of abdominal cocoon syndrome. Forensic Sci Med Pathol. 2023;19:273-279. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015;21:675-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (2)] |
| 13. | Wei B, Wei HB, Guo WP, Zheng ZH, Huang Y, Hu BG, Huang JL. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009;198:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Uzunoglu Y, Altintoprak F, Yalkin O, Gunduz Y, Cakmak G, Ozkan OV, Celebi F. Rare etiology of mechanical intestinal obstruction: Abdominal cocoon syndrome. World J Clin Cases. 2014;2:728-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol. 2007;13:3649-3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Almouwalld MN. An Idiopathic Case of Sclerosing Encapsulating Peritonitis: A Case Report. Cureus. 2024;16:e53667. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Narayanan R, Bhargava BN, Kabra SG, Sangal BC. Idiopathic sclerosing encapsulating peritonitis. Lancet. 1989;2:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Chorti A, Panidis S, Konstantinidis D, Cheva A, Papavramidis T, Michalopoulos A, Paramythiotis D. Abdominal cocoon syndrome: Rare cause of intestinal obstruction-Case report and systematic review of literature. Medicine (Baltimore). 2022;101:e29837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | AlZabali SM, AlAnazi A, Rahim KA, Faqeehi HY. Clinical improvement of encapsulating peritoneal sclerosis after challenging course and 6 months of total parenteral nutrition in child with nephronophthisis: a case report. J Med Case Rep. 2021;15:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Al Riyami MS, Altalebi A, Al Hashmi S, Elfar A, Al Maskari A, Al Gaithi B, Al Saidi S, Al Baloshi S, Al Kalbani N. Improvement of Encapsulating Peritoneal Sclerosis After Medical Treatment and Successful Deceased Donor Kidney Transplant in a Child: A Case Report. Pediatr Transplant. 2024;28:e14867. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Li S, Wang JJ, Hu WX, Zhang MC, Liu XY, Li Y, Cai GF, Liu SL, Yao XQ. Diagnosis and Treatment of 26 Cases of Abdominal Cocoon. World J Surg. 2017;41:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Gorsi U, Gupta P, Mandavdhare HS, Singh H, Dutta U, Sharma V. The use of computed tomography in the diagnosis of abdominal cocoon. Clin Imaging. 2018;50:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Singhal M, Krishna S, Lal A, Narayanasamy S, Bal A, Yadav TD, Kochhar R, Sinha SK, Khandelwal N, Sheikh AM. Encapsulating Peritoneal Sclerosis: The Abdominal Cocoon. Radiographics. 2019;39:62-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Hassine HB, Chaouch MA, Touir W, Jabra SB, Zouari K, Noomen F. A case report of idiopathic sclerosing encapsulating peritonitis causing an acute bowel occlusion in adult. Int J Surg Case Rep. 2024;115:109249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Bouardi NE, Lamrani MYA, Haloua M, Alami B, Boubou M, Maaroufi M. [Idiopathic sclerosing encapsulating peritonitis: a case report]. Pan Afr Med J. 2021;38:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Kaur R, Chauhan D, Dalal U, Khurana U. Abdominal cocoon with small bowel obstruction: two case reports. Abdom Imaging. 2012;37:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Bright B, Salam R, Moorthy S. A Case Series and Brief Review of Literature on Encapsulating Peritoneal Sclerosis: Unveiling the Cocoon. Cureus. 2024;16:e73802. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Sharma V, Moinuddin Z, Summers A, Shenoy M, Plant N, Vranic S, Prytula A, Zvizdic Z, Karava V, Printza N, Vlot J, van Dellen D, Augustine T. Surgical management of Encapsulating Peritoneal Sclerosis (EPS) in children: international case series and literature review. Pediatr Nephrol. 2022;37:643-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Vidal E, Edefonti A, Puteo F, Chimenz R, Gianoglio B, Lavoratti G, Leozappa G, Maringhini S, Mencarelli F, Pecoraro C, Ratsch IM, Cannavò R, De Palo T, Testa S, Murer L, Verrina E; Italian Registry of Pediatric Chronic Dialysis. Encapsulating peritoneal sclerosis in paediatric peritoneal dialysis patients: the experience of the Italian Registry of Pediatric Chronic Dialysis. Nephrol Dial Transplant. 2013;28:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Wu Z, Wang S, Yuan S, Lin M. Clinical efficacy and safety of somatostatin in the treatment of early postoperative inflammatory small bowel obstruction: A protocol for systematic review and meta analysis. Medicine (Baltimore). 2020;99:e20288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |