Published online Aug 6, 2025. doi: 10.12998/wjcc.v13.i22.105596
Revised: March 29, 2025
Accepted: April 11, 2025
Published online: August 6, 2025
Processing time: 105 Days and 16.1 Hours
Mediastinal lymph nodes (MLNs) can be enlarged due to various benign or malignant causes. Endoscopic ultrasound (EUS) is often employed for the acquisition of tissue specimens of the enlarged MLN (EMLN).
To determine the causes, document the symptoms, and determine factors predicting good yield of EUS-guided EMLN biopsy.
All patients having EMLN (> 10 mm) on thoracic imaging and referred for EUS-guided biopsy were included in this retrospective observational study. Adequacy of the tissue specimen was assessed by the endoscopist with macroscopic on-site evaluation (MOSE) and then sent to a histopathologist for final diagnosis. Analysis for factors predicting good biopsy yield was then performed.
Of the total 243 patients with EMLN, 131 (53.9%) were males. The mean age was 47.6 (± 14.7) and range 14-86 years. Commonest causes of EMLN were tuber
Tuberculosis, anthracosis and metastatic disease were the commonest causes of EMLN. More than half the cases with EMLN had no chest-related symptoms. Large MLN size and satisfactory MOSE observation predicted a good biopsy yield.
Core Tip: Endoscopic ultrasound effectively diagnoses causes of enlarged mediastinal lymph nodes (MLNs), including tuberculosis, anthracosis, and metastatic diseases, with over half of cases lacking chest-related symptoms. Factors predicting good diagnostic yield include MLN size > 12 mm, subcarinal location, use of fine-needle biopsy over fine-needle aspiration, and satisfactory macroscopic on-site evaluation (MOSE). Larger MLNs and optimal MOSE were the strongest predictors on multivariate analysis, emphasizing the importance of accurate imaging and procedural technique in achieving reliable diagnostic outcomes.
- Citation: Tasneem AA, Luck NH, Mubarak M. Mediastinal lymphadenopathy: Causes, symptoms and factors predicting good yield of endoscopic ultrasound-guided biopsy. World J Clin Cases 2025; 13(22): 105596
- URL: https://www.wjgnet.com/2307-8960/full/v13/i22/105596.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i22.105596
Mediastinal lymphadenopathy (ML) or enlarged mediastinal lymph nodes (EMLNs) can be due to various benign and malignant causes[1]. Benign causes include various chronic infections (tuberculosis, fungal infections), multi-systemic disorders (sarcoidosis), long-standing inflammatory disorders (pneumoconiosis, interstitial pneumonitis), or autoimmune disorders. Malignant causes can be either primary (lymphoproliferative disorder) or due to metastasis from organs like the lung, breast, esophagus, or other body organs. Many patients with EMLNs are symptomatic due to ongoing diseases of the respiratory tract or thoracic organs, while others may be asymptomatic and diagnosed incidentally while undergoing full-body imaging for evaluation of chronic fever, unexplained weight loss, or staging of malignancies arising from either side of the diaphragm[2].
Evaluation of the cause of ML has largely been a domain of the pulmonologist or the general physician. Initially, it involves a computed tomography (CT) scan of the chest or bronchoscopy. However, acquisition of histopathologic specimens of EMLNs is essential to make a diagnosis, and this is made possible either through mediastinoscopy, endobronchial ultrasound (EBUS), or endoscopic ultrasound (EUS). Lymph nodes that lie in the superior and anterior mediastinum can be approached through cervical or transthoracic mediastinoscopy, while those close to the respiratory tract can be accessed via EBUS. However, those EMLNs that lie in the posterior mediastinum and are in close proximity to the esophagus can be biopsied during EUS by gastroenterologists who are trained in this field[3]. The main drawback with EUS is that it does not allow visualization of the respiratory tract. The various MLN that are approachable from the esophagus include subcarinal, right hilar, left hilar, posterior para-tracheal, lower para-esophageal regions, and from the aortopulmonary (AP) window[4]. Ultrasound Doppler guidance is utilized while taking a biopsy to avoid injury to the great vessels arising from the heart.
The causes of ML can vary depending on the geographic location. For instance, ML due to various kinds of respiratory infections are more common in overcrowded rural regions, while pneumoconiosis is more common in areas with different types of atmospheric pollution. It is, therefore, imperative upon us to identify the commonest causes of this condition in our part of the world.
The aim of this research was to identify the various causes of EMLNs in our setup, study the associated symptoms, and detect factors predictive of a good yield of EUS-guided biopsy.
This retrospective observational study was performed in the department of gastroenterology, Sindh Institute of Urology and Transplantation, from October 2020 to June 2024. Patients included were of any gender and age above 14 years who were found to have EMLNs and referred to us for EUS-guided biopsy. A diameter of more than 10mm of any of the MLNs was considered enlarged lymph nodes. All patients participating in this study had provided informed consent. The study was performed in accordance with the Helsinki Declaration of 1975, as revised in 2000.
The biodata of all the patients included in the study were recorded in the prestructured proforma along with the indication of the procedure. History, clinical examination, and pertinent laboratory tests were documented. EUS of the patients was performed as a day-care procedure in our endoscopy unit. The procedure was done under conscious sedation in the majority of cases, while general anaesthesia was administered only to children below the age of 16 years.
Pentax (linear) and Olympus echoendoscopes were used to perform the EUS. Characteristics of the EMLN, including their size and shape, were noted. The bore, and type of needle used, and the number of passes performed were mentioned in the proforma. Fine needle aspiration (FNA) and fine needle biopsy (FNB) (Boston Scientific and Cook) were employed to obtain the biopsy specimen. Needles having bores of 22 G or 25 G were utilized. Macroscopic on-site evaluation (MOSE) was performed by the endoscopist at the time of biopsy taking as the facility of rapid on-site evaluation (ROSE) was not available. MOSE was considered to be satisfactory if, macroscopically, at least two worm-like, continuous tissue core fragments each 1 inch long, were obtained. The secured tissue specimen was put in formalin and sent to the histopathologist for analysis. To identify the type of neoplasm, primary or metastatic, different specialized tumor markers were used. The final histopathological report showing the diagnosis was recorded. If the biopsy specimen was found to be enough for establishing the diagnosis on histopathologic analysis, it was considered as good yield.1
SPSS, version 26.0, was used for analysis. To describe the clinical characteristics, mean and standard deviation were used for quantitative variables like age, and frequencies or percentages for categorical variables like gender. χ² and Fisher's exact test were employed to determine the predictors of good yield of biopsy. Univariate analysis was followed by multivariate analysis to adjust for the covariates. A P-value of less than 0.05 was considered to be statistically significant.
A total of 243 patients with EMLN were included in the study. Of these, males were 131 (53.9%) and females 112 (46.1%). The age range was 14 to 86 years with a mean age of 47.6 (SD ± 14.7). The sources from which the patients were referred to us are shown in Table 1. The majority of the patients were referred to us by general physicians who had discovered ML on imaging for evaluation of various medical conditions. Others were referred by oncologists who wanted evaluation of the ML while staging of cancers or during surveillance after surgery or chemotherapy. The remaining patients were referred by the nephrology team, which included those who took care of renal donors, hemodialysis-dependent, and post-renal transplant patients.
| Referral source | n (%) |
| General physicians/pulmonologists | 101 (41.6) |
| Oncologist | 90 (37.0) |
| Nephrologist/Urologist | 52 (21.4) |
| Hemodialysis dependent patients | 29 |
| Potential renal donors | 12 |
| Renal transplanted patients | 11 |
The sizes of the MLN were: Mean size of the largest diameter (length) 21.2 ± 11.8 mm, while width was 11.8 ± 7.7 mm. The locations of the lymph nodes in the mediastinum that were targeted for biopsy are shown in Table 2. A summary of the size of the needles used for biopsy, the number of passes made, and the diagnostic yield is shown in Table 3. In the majority of the cases, the 22 G needles were used and the number of passes in most cases was two or more. The diagnostic yield was found by the histopathologist to be good in 89.7% of the cases.
| Mediastinal location | n (%) |
| Subcarinal/Precarinal | 196 (80.7) |
| Right hilar | 24 (9.9) |
| Left hilar | 10 (4.1) |
| Aortopulmonary window | 7 (2.9) |
| Lower para-esophageal | 3 (1.2) |
| Upper para-esophageal/Para-tracheal | 2 (0.8) |
| Characteristics of needles and histopathology specimen | n (%) |
| Size of needle | |
| 22 G | 236 (97.1) |
| 25 G | 7 (2.9) |
| Number of passes | |
| 2 or more passes | 222 (91.4) |
| 1 pass only | 21 (8.6) |
| Macroscopic onsite evaluation | |
| Satisfactory | 211 (86.8) |
| Unsatisfactory | 32 (13.2) |
| Yield on histopathology | |
| Good | 218 (89.7) |
| Poor | 25 (10.3) |
The causes of the ML that we found in our study are shown in Table 4. The commonest cause was tuberculosis (accounting for one-third of all cases of ML) followed by anthracosis, neoplastic disease, sarcoidosis and others. The miscellaneous group consisted of epithelioid cell collection in 5, chemotherapy induced changes in 2, necrotic tissue in 2, keratin slough in 2, and granulomatous inflammation of unknown cause in 1 patient. Normal lymphoid tissue was found in 23 (9.5%) patients and suboptimal in 16 (6.6%) patients. The specific histologic features that were looked for by the histopathologist and the various tumor markers employed to make the diagnosis are shown in Table 5.
| Causes of mediastinal lymphadenopathy | n (%) |
| Tuberculosis | 82 (33.7%) |
| Anthracosis | 53 (21.8) |
| Minimal to mild | 32 |
| Moderate | 14 |
| Severe | 7 |
| Neoplastic disease | 43 (17.7) |
| Metastatic | |
| Breast cancer | 9 |
| Lung cancer | 7 |
| Pancreatobiliary cancer | 6 |
| Esophageal cancer | 5 |
| Renal cancer | 5 |
| Hepatocellular cancer | 2 |
| Neuroendocrine cancer | 2 |
| Gastrointestinal stromal tumor | 1 |
| Undetermined origin | 3 |
| Primary | |
| Lymphoproliferative disorder | 2 |
| Kaposi’s sarcoma | 1 |
| Sarcoidosis | 14 (5.8) |
| Miscellaneous | 12 (4.9) |
| Epithelioid cell collection | 5 |
| Post chemotherapy changes | 2 |
| Necrotic tissue | 2 |
| Keratin slough | 2 |
| Granulomatous inflammation | 1 |
| Normal histology | 23 (9.5) |
| Suboptimal tissue | 16 (6.6) |
| Diagnosis | Histological features |
| Tuberculosis | Granulomas with caseating necrosis; Epithelioid granulomas |
| Sarcoidosis | Granulomas without caseating necrosis |
| Anthracosis | CLM |
| Occasional CLMs: Minimal to mild anthracosis | |
| Significant CLMs: Moderate anthracosis | |
| Abundant CLMs: Severe anthracosis | |
| Metastatic disease | Abnormal cells with large nuclei, nucleoli and scanty cytoplasm |
| Immunohistochemical markers used | |
| CK AE1/3: Esophageal cancer | |
| GATA-3, Mammaglobin: Breast cancer | |
| TTF-1: Lung adenocarcinoma | |
| Hep par, Alfafetoprotein: Hepatocellular cancer | |
| PAX-8: Renal cell carcinoma | |
| CK7, CK19: Pancreatobiliary cancer | |
| CD31, CD34: Kaposi’s sarcoma | |
| CD15, CD20, CD30: Hodgkin’s lymphoma |
A significant number of cases in our study had no chest-related symptoms like cough, breathlessness, chest pain, fever, etc. The frequency of patients with absent chest-related symptoms among the major causes of ML is shown in Table 6. More than half the cases with tuberculosis, anthracosis, and malignant disease had no chest-related symptoms. Among patients found to have histological evidence of anthracosis (i.e., having carbon-laden macrophages), nearly half of them, 28/53 (i.e., 52.8%), were evaluated for reasons other than chest complaints. Most of the patients found to have sarcoidosis had chest-related symptoms like dry cough and other complaints, too, like joint pain and abdominal pain due to arthritis and nephrocalcinosis, respectively.
| Total number of patients (n) | Patients with absent chest related symptoms (n) | Percentage | |
| Tuberculosis | 82 | 51 | 62.2% |
| Anthracosis | 53 | 31 | 58.5% |
| Malignant disease | 43 | 28 | 65.1% |
| Sarcoidosis | 14 | 5 | 35.7% |
| Others | 12 | 9 | 75.0% |
The results of the statistical analysis to identify the factors associated with good yield of EUS-guided biopsy are shown in Table 7. Univariate analysis showed that factors predicting good yield of biopsy specimen included subcarinal location of MLN (P = 0.026), MLN size > 12 mm (P < 0.0001), use of FNB (vs FNA) needles (P = 0.049) and satisfactory macroscopic onsite evaluation (MOSE) (P < 0.0001). Multivariate analysis showed that MLN size (length) ≥ 12 mm (P = 0.005) and satisfactory MOSE (P < 0.0001) were predictors of good biopsy yield.
| Clinical variable | Univariate analysis | Multivariate analysis | |||||
| Good yield | Poor yield | Odds Ratio | Confidence interval | P value | |||
| Age | < 40 | 68 | 11 | 0.57 | 0.249–1.337 | 0.195 | |
| > 40 | 150 | 14 | |||||
| Gender | Male | 115 | 16 | 0.63 | 0.266–1.482 | 0.285 | |
| Female | 103 | 9 | |||||
| Location of node | Subcarinal | 180 | 16 | 2.66 | 1.096–6.478 | 0.026 | |
| Others | 38 | 9 | |||||
| Size of LN | ≥ 12 mm | 200 | 13 | 10.25 | 4.083–25.762 | < 0.0001 | 0.005 |
| < 12 mm | 18 | 12 | |||||
| Bore of the needle used | 22G | 211 | 25 | 0.89 | 0.856–0.934 | 1.000 | |
| 25G | 7 | 0 | |||||
| Type of needle | FNB | 202 | 20 | 3.16 | 1.046–9.523 | 0.049 | |
| FNA | 16 | 5 | |||||
| Number of passes | ≥ 2 | 201 | 21 | 2.25 | 0.693–7.317 | 0.247 | |
| < 2 | 17 | 4 | |||||
| MOSE | Satisfactory | 202 | 9 | 22.44 | 8.574–58.753 | < 0.0001 | < 0.0001 |
| Unsatisfactory | 16 | 16 | |||||
Literature review shows that most of the research evaluating the causes of ML employed EBUS and transbronchial needle aspiration. Our study was unique because it is among the very few that have studied this aspect through use of transesophageal EUS. We noted that the commonest cause of EMLN was tuberculosis, followed by anthracosis and metastatic disease. It is not surprising that tuberculosis is quite common in Pakistan and can lead to EMLN. An Indian study by Dhamija et al[5] also showed that tuberculosis was the commonest cause of ML, as evaluated by EBUS. These findings are similar to ours, possibly because India and Pakistan are neighboring countries that share somewhat similar geographic and socioeconomic characteristics. Also, a Polish study by Dabrowska et al[6] showed that lung malignancy and sar
In our study, anthracosis was the second most common cause of EMLN, indicating the effect of air pollution due to the increasing number of automobiles, biomass degradation, and active or passive smoking. Although the majority of the patients had only mild anthracosis, it is important to take adequate steps on the governmental level to curb this menace. Our third common cause was metastatic disease, with breast and lung cancers being the commonest causes of primary cancer, and pancreatobiliary, esophageal, kidney, and liver cancers being less common but important causes of metastases to the mediastinum. A study by Fiterman et al[9] from Israel showed that the commonest causes of metastatic lymphadenopathy were breast, hematological, and lung cancers[9]. Besides, Dabrowska et al[6] showed that lung cancers were the commonest primary of metastatic ML. We too observed that breast and lung cancers were the commonest cause of mediastinal metastases. However, a significant number of mediastinal metastases that we studied were gastrointestinal or renal in origin. This is possibly because we received a significant amount of referrals from other gastroenterologists who were treating gastrointestinal tract tumors, including those arising from the esophagus, liver (hepatocellular cancer), pancreas, and biliary tree (gall bladder cancer and cholangiocarcinoma). We also observed that the mediastinum is an important site of metastasis from kidney cancers (particularly the clear cell renal cell carcinoma variant) and from neuroendocrine cancers originating from gastrointestinal or urinary tracts.
An interesting but worrisome fact demonstrated in our study was that more than half of the patients with EMLNs had no chest-related symptoms and were referred for biopsy when found to have ML on CT scan of the chest. Examples of such patients were those undergoing donor nephrectomy or end stage renal disease patients being evaluated for renal transplantation. Various studies performed around the world have shown that tuberculous ML is usually associated with pulmonary symptoms like cough, breathlessness, fever, chest pain, etc., due to simultaneous involvement of the lung parenchyma. However, many case reports have been published that show a lack of symptoms among patients with EMLN. For instance, Pirina et al[10] reported a case of tuberculous ML who had no evidence of compromised immunity or chest-related symptoms. Similarly, Cong et al[11] from Vietnam reported a case of otherwise asymptomatic adult primary tuberculosis that was incidentally detected in a hemodialysis-dependent patient undergoing evaluation for renal transplantation. Furthermore, cases of tuberculous mediastinal lymphadenitis have also been reported in whom uncommon symptoms like dysphagia and insidious back pain had led to thoracic imaging that revealed ML[12,13]. Besides, studies have shown that even metastatic ML can present without symptoms[14]. A study done by Catalano et al[15] from the United States has also shown that asymptomatic patients may harbor EMLN from benign or malignant causes[15]. Potential explanations for the high number of asymptomatic nature of EMLN include firstly, the high prevalence of early stage tuberculosis among apparently healthy individuals in the Indo-Pak Asian region; secondly, increasing utilization of cross-sectional imaging for screening (among transplant donors) and staging (of cancer patients) purposes; and thirdly, increasing referrals for biopsy of MLN because of growing awareness among the medical fraternity regarding the benefits of EUS compared to yesteryears. In this context, we recommend regular use of screening chest radiographs in high tuberculosis-prevalent areas and yearly or two-yearly CT scans of the chest among high-risk populations (transplant candidates, patients on immunosuppression) for early identification and prevention of the spread of tuberculosis.
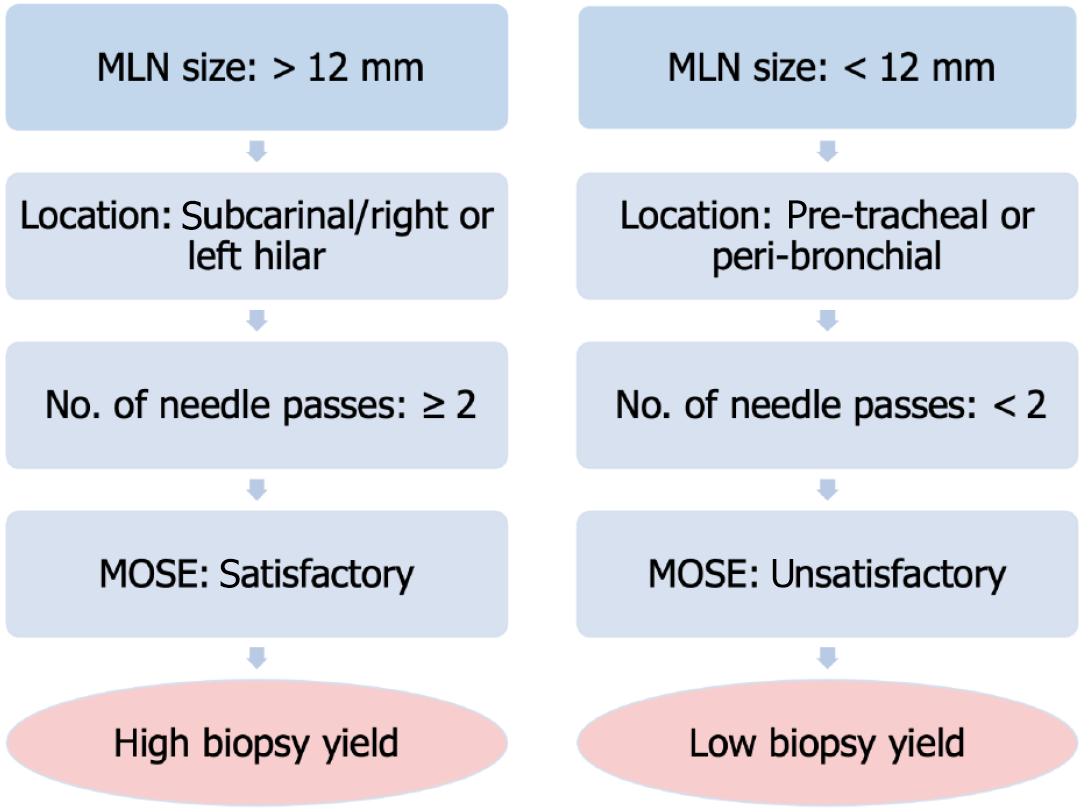
Univariate analysis, in our study, showed that a large size of MLN (i.e., ≥ 12 mm), subcarinal location of MLN, use of FNB needles, and a satisfactory MOSE were associated with good biopsy yield. This suggests that a thicker lymph node provides better yield compared to a thinner one. Fujii et al[16] from Japan also showed that a size of > 16 mm of mediastinal or abdominal lymph nodes was associated with an accurate diagnosis. Furthermore, Kennedy et al[17] from Ireland showed that the sampling accuracy of EBUS decreased with lymph node size ≤ 5 mm and with paratracheal location. In our study, biopsies were obtained with an EUS endoscope from the esophagus with subcarinal lymph nodes, providing the best diagnostic yield. This is possibly because the subcarinal location is easily accessible with the EUS scope, which lies straight and allows a good handling of the needle while taking the biopsy. In contrast, other locations like the right hilar, left hilar, or AP window may require slight scope angulation and hence affect the amount of tissue acquired. In support of this finding, a research by Bonifazi et al[18] from Italy, which studied transbronchial biopsies in more than 8000 patients, also showed that a lymph node size (short axis length ≥ 2 cm), subcarinal and paratracheal location, and the use of histological needle by an experienced bronchoscopist were associated with positive yield. However, in our study, the multivariate analysis showed that MOSE was associated with a good biopsy yield. The criteria we used for a satisfactory MOSE were the procurement of at least two worm-like, continuous tissue core fragments, each at least 1 inch long. We think that in those parts of the world where ROSE is not available, the endoscopist can utilize this valuable piece of information to ensure that the secured biopsy specimen is enough for diagnosis making and hence avoid the need for repeated EUS procedures. We propose that when ROSE is not available, an algorithm incorporating four factors may help in predicting a good yield of EUS-guided biopsy: MLN size > 12 mm, easily approachable location of LN (subcarinal/right or left hilar), ≥ 2 needle passes, and satisfactory MOSE (Figure 1).
The strengths of our study are a good sample size and its uniqueness in the utilization of EUS rather than EBUS or mediastinoscopy for evaluation of ML. The limitations of our study include single-center nature, retrospective design, inability to utilize the standardized ROSE protocol, potential sampling errors in challenging locations (non-subcarinal locations like pre-tracheal and peribronchial), and lack of longitudinal follow-up for assessment of false negatives. In the future, multi-center prospective studies using standardized ROSE protocols may help to further validate our findings.
Tuberculosis is a very common cause of ML in South Asia. Also, such patients may not have any chest-related symptoms and can be potential carriers of the bacterium, with the potential of spread to other community members. Screening of such patients is essential through basic imaging tests like chest radiograph and other specialized tests that employ detection of mycobacterial DNA. Anthracosis is also quite common in this part of the world, and taking adequate steps on governmental level to minimize atmospheric pollution and discouraging smoking is necessary. Although the role of EBUS is well recognized in tissue sampling of MLN, EUS from the esophagus is equally useful, for that matter, if the EMLN are approachable. Generally, the use of ROSE is helpful in assessing the adequacy of the sampled tissue; however, in resource-poor setups where this facility is not available, a good biopsy yield can be ensured by the endosonologist if the criterion for satisfactory MOSE is utilized at the time of securing the biopsy specimen.
| 1. | Iyer H, Anand A, Sryma PB, Gupta K, Naranje P, Damle N, Mittal S, Madan NK, Mohan A, Hadda V, Tiwari P, Guleria R, Madan K. Mediastinal lymphadenopathy: a practical approach. Expert Rev Respir Med. 2021;15:1317-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Day JC. Mediastinal and Hilar Lymphadenopathy. Principles and Practice of Pediatric Infectious Diseases. Philadelphia: Elsevier, 2023: 154-161. [DOI] [Full Text] |
| 3. | Kwong WT, Savides TJ. Endoscopic Ultrasonography in the Evaluation of Posterior Mediastinal Lesions. Philadelphia: Elsevier, 2019: 100-108. [DOI] [Full Text] |
| 4. | Okasha HH, El-Meligui A, Pawlak KM, Żorniak M, Atalla H, Abou-Elmagd A, Abou-Elenen S, El-Husseiny R, Alzamzamy A. Practical approach to linear EUS examination of the mediastinum. Endosc Ultrasound. 2021;10:406-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Dhamija A, Basu A, Sharma V, Bakshi P, Verma K. Mediastinal Adenopathy in India: Through the Eyes of Endobranchial Ultrasound. J Assoc Physicians India. 2015;63:15-18. [PubMed] |
| 6. | Dabrowska M, Faber K, Tandejko-burdyna M, Korczynski P, Krenke R. Etiology of mediastinal lymph node enlargement in patients who underwent EBUS-TBNA. Eur Respir J. 2019;54 (suppl 63):PA3095. [DOI] [Full Text] |
| 7. | Evison M, Crosbie PA, Morris J, Martin J, Barber PV, Booton R. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA. BMJ Open Respir Res. 2014;1:e000040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Aksoy Y. Causes of Isolated Mediastinal Lymphadenopathy: Analysis of 348 Patients Undergoing Cervical Mediastinoscopy. Erciyes Med J. 2022;44:581-586. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Fiterman N, Berkman N, Kuint R. Predictors of malignant lymph node involvement in patients with mediastinal lymphadenopathy and previous cancer: A cohort study. Thorac Cancer. 2022;13:631-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Pirina P, Spada V, Santoru L, Polo MF, Molicotti P, Marras V, Cossu Rocca P, Canu S, Zanetti S, Fois AG. Chest tuberculosis with mediastinal asymptomatic lymphadenitis without lung involvement in an immunocompetent patient. J Infect Dev Ctries. 2013;7:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Cong CV, Ly TT, Anh PQ, Duc NM. Primary mediastinal lymph node tuberculosis diagnosed using endobronchial ultrasound-guided transbronchial needle aspiration: Literature review and case report. Radiol Case Rep. 2022;17:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Xiong L, Mao X, Li C, Liu Z, Zhang Z. Posterior mediastinal tuberculous lymphadenitis with dysphagia as the main symptom: a case report and literature review. J Thorac Dis. 2013;5:E189-E194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Ren X, Li K, Li L, Zhao G. Mediastinal tuberculous lymphadenitis presenting with insidious back pain in a male adult: a case report and review of the literature. J Int Med Res. 2021;49:300060520987102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Chalian H, McAdams HP, Lee Y, Duan F, Wu Y, Khoshpouri P, Patz EF Jr. Mediastinal Lymphadenopathy in the National Lung Screening Trial (NLST) Is Associated with Interval Lung Cancer. Radiology. 2022;302:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Catalano MF, Nayar R, Gress F, Scheiman J, Wassef W, Rosenblatt ML, Kochman M. EUS-guided fine needle aspiration in mediastinal lymphadenopathy of unknown etiology. Gastrointest Endosc. 2002;55:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Fujii Y, Kanno Y, Koshita S, Ogawa T, Kusunose H, Masu K, Sakai T, Yonamine K, Kawakami Y, Murabayashi T, Kozakai F, Noda Y, Okada H, Ito K. Predictive Factors for Inaccurate Diagnosis of Swollen Lymph Nodes in Endoscopic Ultrasound-Guided Fine Needle Aspiration. Clin Endosc. 2019;52:152-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kennedy MP, Jimenez CA, Morice RC, Sarkiss M, Lei X, Rice D, Eapen GA. Factors Influencing the Diagnostic Yield of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration. J Bronchology Interv Pulmonol. 2010;17:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Bonifazi M, Zuccatosta L, Trisolini R, Moja L, Gasparini S. Transbronchial needle aspiration: a systematic review on predictors of a successful aspirate. Respiration. 2013;86:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |