Published online Aug 6, 2025. doi: 10.12998/wjcc.v13.i22.104283
Revised: March 28, 2025
Accepted: April 14, 2025
Published online: August 6, 2025
Processing time: 148 Days and 3.9 Hours
Autoimmune myocarditis (AM) associated with autoimmune diseases can cause complete atrioventricular block (CAVB), but the related autoantigens and the underlying mechanisms are unclear. Anti-SSA/Ro antibodies may play an important role in this process, but cases of AM with positive anti-SSA/Ro antibodies are rare. In addition, arrhythmias, such as atrioventricular block, are very common in patients with autoimmune diseases, but severe atrioventricular block requiring permanent pacemaker implantation is extremely rare.
The patient in this case had AM with anti-SSA/Ro antibody positivity, which was associated with connective tissue disease, and the patient subsequently developed CAVB. After intensive immunosuppressive therapy, the antibody test results became negative, and pulmonary hypertension significantly improved. However, the outcome of permanent pacemaker implantation did not change.
In clinical practice, the awareness of adult AM associated with autoimmune diseases combined with CAVB should be strengthened in clinicians, and anti-SSA/Ro antibodies may play a role in this process. Therefore, improving the detection of antibodies and early intervention, such as active immunosuppression therapy, may be very important for improving disease prognosis. For patients who do not respond to immunosuppressive therapy, implantation of a permanent pacemaker may become an essential treatment option.
Core Tip: Anti-SSA/Ro antibodies may affect the conduction system in adults, leading to atrioventricular block, even complete atrioventricular block (CAVB). Anti-SSA/Ro antibody-positive autoimmune myocarditis (AM) complicated with CAVB is rarely reported, and the pathogenesis is not clear. Cases requiring permanent pacemaker implantation are extremely rare. This paper reports a patient who had anti-SSA/Ro antibody-positive AM complicated with CAVB, and immunosuppressive therapy did not change the outcome of permanent pacemaker implantation.
- Citation: Xiao PB, Yang XR. Anti-SSA/Ro antibody-positive autoimmune myocarditis combined with complete atrioventricular block requiring implantation with a permanent pacemaker: A case report. World J Clin Cases 2025; 13(22): 104283
- URL: https://www.wjgnet.com/2307-8960/full/v13/i22/104283.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i22.104283
Autoimmune myocarditis (AM) is an autoimmune-mediated inflammatory disease of the myocardium that can lead to complete atrioventricular block (CAVB). Viral infection is the most common etiology[1]. In addition, noninfectious factors, including autoimmune diseases, play a very important role in the pathogenesis of myocarditis[2]. Although there is a clear association between autoimmune factors and myocarditis, the relevant autoantigens and the underlying mechanisms are still not well understood.
Anti-SSA/Ro antibodies are common autoantibodies that can cause a variety of autoimmune diseases and may affect the adult cardiovascular system, leading to atrioventricular block (AVB) and even CAVB[3,4]. At present, cases of anti-SSA/Ro antibody-positive AM complicated with CAVB are rare, and cases requiring permanent pacemaker implantation are even rarer.
This paper reports a case of anti-SSA/Ro antibody-positive AM complicated with CAVB. The patient’s autoimmune status improved after immunosuppressive treatment, but the outcome of permanent pacemaker implantation did not change.
The patient was a 36-year-old Han woman. Her height was 164 cm, and her weight was 65 kg. She was admitted to the hospital on May 16, 2020 due to chest tightness and shortness of breath for 1 month.
The patient developed chest tightness, shortness of breath, and occasional dyspnea after fatigue 1 month prior, which were aggravated during activities and was not significantly relieved after rest. There was no rash, dry eyes, dry mouth, or repeated oral ulcers.
The patient was previously healthy.
The patient’s personal and family history was unremarkable.
The rhythm was normal, pulmonary second heart sound (P2) was hyperactive, and a grade 2/6 systolic murmur could be heard in the precordial area without a pericardial friction sound.
The results of laboratory examinations were as follows: Anti-nuclear antibody (ANA): 1:640 (+); 1:1000 (-), cytoplasmic granule type, anti-SSA/Ro60 antibody (+); N-terminal pro-brain natriuretic peptide: 594 pg/mL; immunoglobulin A: 2.25 g/L; immunoglobulin G: 7.08 g/L; immunoglobulin M: 0.87 g/L; complement 3: 1.30 g/L; complement 4: 0.21 g/L; erythrocyte sedimentation rate: 20 mm/h; C-reactive protein: 0.65 mg/L.
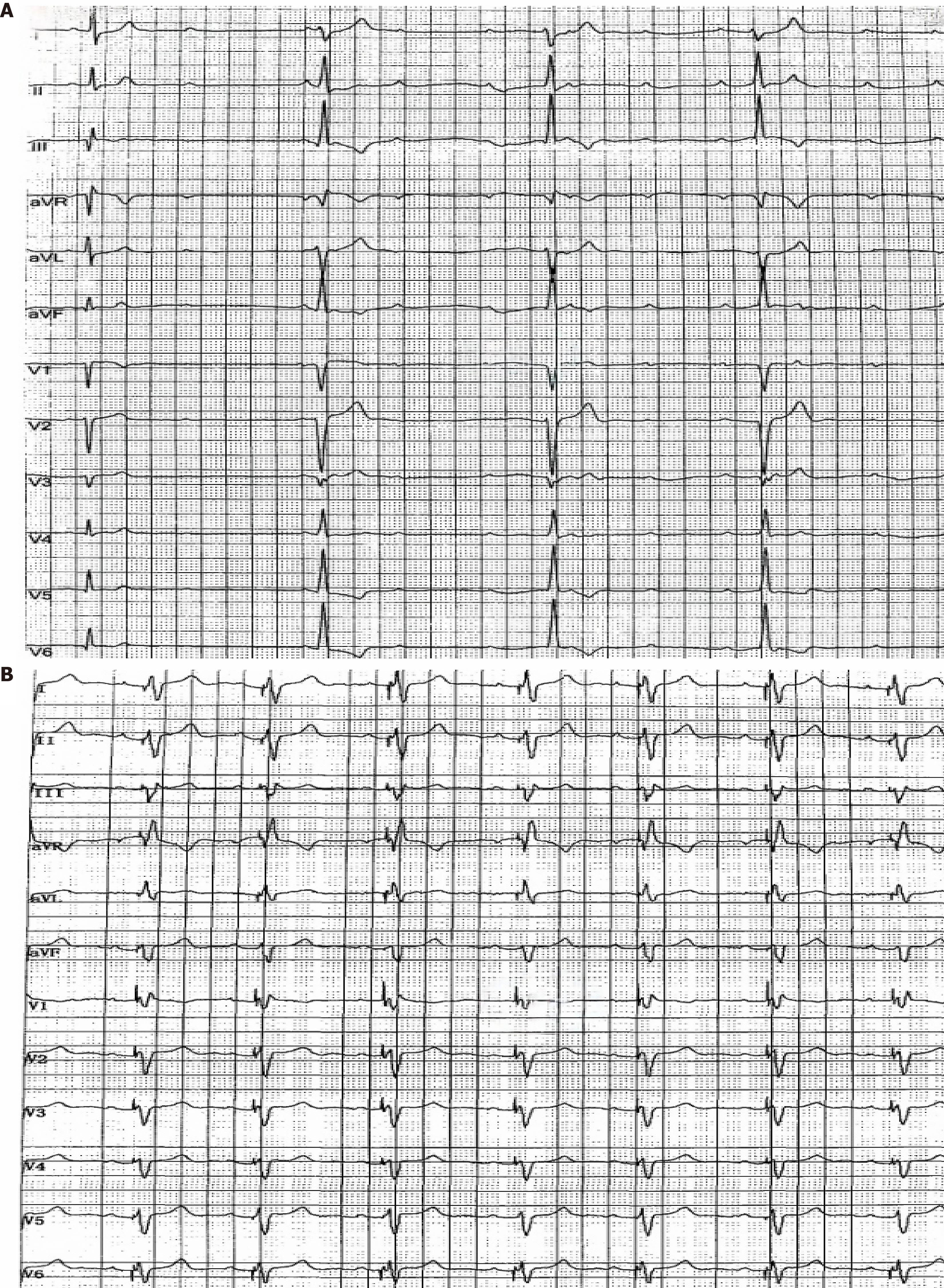
Chest computed tomography (CT) revealed local atelectasis in both lungs, with some exhibiting calcifications. Cardiac ultrasound revealed moderate pulmonary hypertension, a pulmonary systolic pressure of 74 mmHg, and slight pericardial effusion. Twenty-four-hour Holter electrocardiogram (ECG) revealed intermittent third-degree AVB (Figure 1A). Contrast enhanced cardiac magnetic resonance (MR) revealed mild to moderate aortic valve insufficiency with minimal myocardial fat infiltration and fibrosis. Pulmonary artery computed tomography angiography, head CT, and ultrasound of the carotid artery, thyroid, and abdomen were normal.
AM, connective tissue disease, third-degree AVB, and secondary pulmonary hypertension.
Methylprednisolone (200 mg qd for 3 days, followed by 40 mg qd), human immunoglobulins (25 g qd for 3 days), and cyclophosphamide (0.4 g/2 weeks) via intravenous drip were administered. On the second day of immunosuppressive treatment, the patient experienced dizziness and transient amaurosis, and electrocardiographic monitoring revealed a third-degree AVB. After temporary treatment with transthoracic cardiac pacing, atrioventricular conduction returned to normal. Multiple ECG reexaminations revealed normal atrioventricular conduction, considering that the patient had no indication for permanent pacemaker implantation, and reexamination by cardiac ultrasound revealed a significant decrease in pulmonary systolic pressure of 36 mmHg. Given that the immunosuppressive therapy was effective, methylprednisolone (40 mg qd for 1 month, then gradually reduced to 24 mg qd) and cyclophosphamide (0.4 g/2 weeks for a total of 1.2 g) were applied for maintenance therapy. During follow-up, the anti-SSA/Ro60 antibody test results were negative. However, after 2 months, the patient repeatedly experienced chest tightness, palpitations, and a slow heart rate, lasting for approximately 1-2 hours. Multiple electrocardiogram examinations revealed third-degree AVB. Considering that the degree third-degree AVB caused by autoimmune factors had no clear reversible signs after immunosuppression but had clear indications for permanent pacemaker implantation, permanent pacemaker implantation was performed after the exclusion of contraindications. There was no discomfort, such as dizziness or palpitations, after surgery, and 24-hour Holter ECG revealed no further third-degree AVB (Figure 1B). Multiple ECG examinations revealed normal atrioventricular conduction. In addition, reexamination of anti-SSA/Ro60 antibodies was negative, and cardiac ultrasound revealed that the pulmonary systolic pressure was 34 mmHg.
The patient was followed regularly after surgery. Cyclophosphamide (0.4 g/2 weeks for a total of 4 g) was stopped after 3 months because of the consideration of gonadal inhibition, and cyclosporin (50 mg bid) was initiated. At present, the patient is receiving methylprednisolone (4 mg qd) and cyclosporine (50 mg bid) for maintenance therapy. Regular review revealed that the patient was negative for anti-SSA/Ro60 antibodies. ECG examinations showed normal atrioventricular conduction, and cardiac ultrasound indicated normal pulmonary systolic pressure.
AM is a myocardial inflammatory disease mediated by autoimmunity that can lead to AVB, including CAVB (i.e., third-degree AVB), and can be caused by infectious and noninfectious factors[1]. Among the infectious factors, viral infection is the most common. An increasing number of studies have confirmed that autoimmune diseases, such as systemic lupus erythematosus (SLE), also play a very important role in the pathogenesis of myocarditis[2]. Despite a clear association between autoimmune factors and myocarditis, the relevant autoantigens and the underlying mechanisms remain unclear.
Anti-SSA/Ro antibodies are a class of autoantibodies that are commonly observed in systemic lupus erythematosus, cutaneous lupus erythematosus, Sjogren’s syndrome, scleroderma, and primary sclerosing cholangitis and play important roles in the diagnosis and prognosis of polymyositis/dermatomyositis. Studies have shown that anti-SSA/Ro antibodies can also be detected in healthy individuals, and they are the most common anti-nuclear antibodies in the general population, with a positive rate of approximately 0.5%-8%[5]. Maternal anti-SSA/Ro antibodies can be transmitted to the fetus across the placenta, resulting in congenital heart block, one of the most serious fetal complications. This consistent effect of anti-SSA/Ro antibodies on the fetal heart is well established, but whether the adult heart is an immune target for anti-SSA/Ro antibodies is unclear.
Currently, a growing number of studies suggest that anti-SSA/Ro antibodies may affect the conduction system in adults, leading to arrhythmias, especially AVB. Villuendas et al[3] conducted a retrospective study on 3586 patients who needed pacemaker implantation due to CAVB in their research center from 1987 to 2012. After excluding patients who died, were lost to follow-up, were older, and had organic heart disease or other known causes of AVB, 19 patients with unexplained CAVB aged 18–50 years were selected for ANA antibody detection; of these patients, 2 were positive for anti-SSA/Ro antibodies (10.5%). This study suggested that anti-SSA/Ro antibodies may play a role in the occurrence and development of CAVB in adults. Logar et al[4] reported that 36 anti-SSA/Ro antibody-positive SLE patients (22%) had a greater incidence of conduction abnormalities than 31 anti-SSA/Ro antibody-negative SLE patients (3%) and 50 healthy controls (2%) in the anti-SSA/Ro antibody-positive group. This study suggested that anti-SSA/Ro antibodies are potentially associated with myocarditis and abnormal cardiac conduction in SLE patients. Therefore, in adults with CAVB, the influence of autoimmune factors on the disease should be excluded, and the detection of anti-SSA/Ro antibodies may be very important for myocarditis-related AVB.
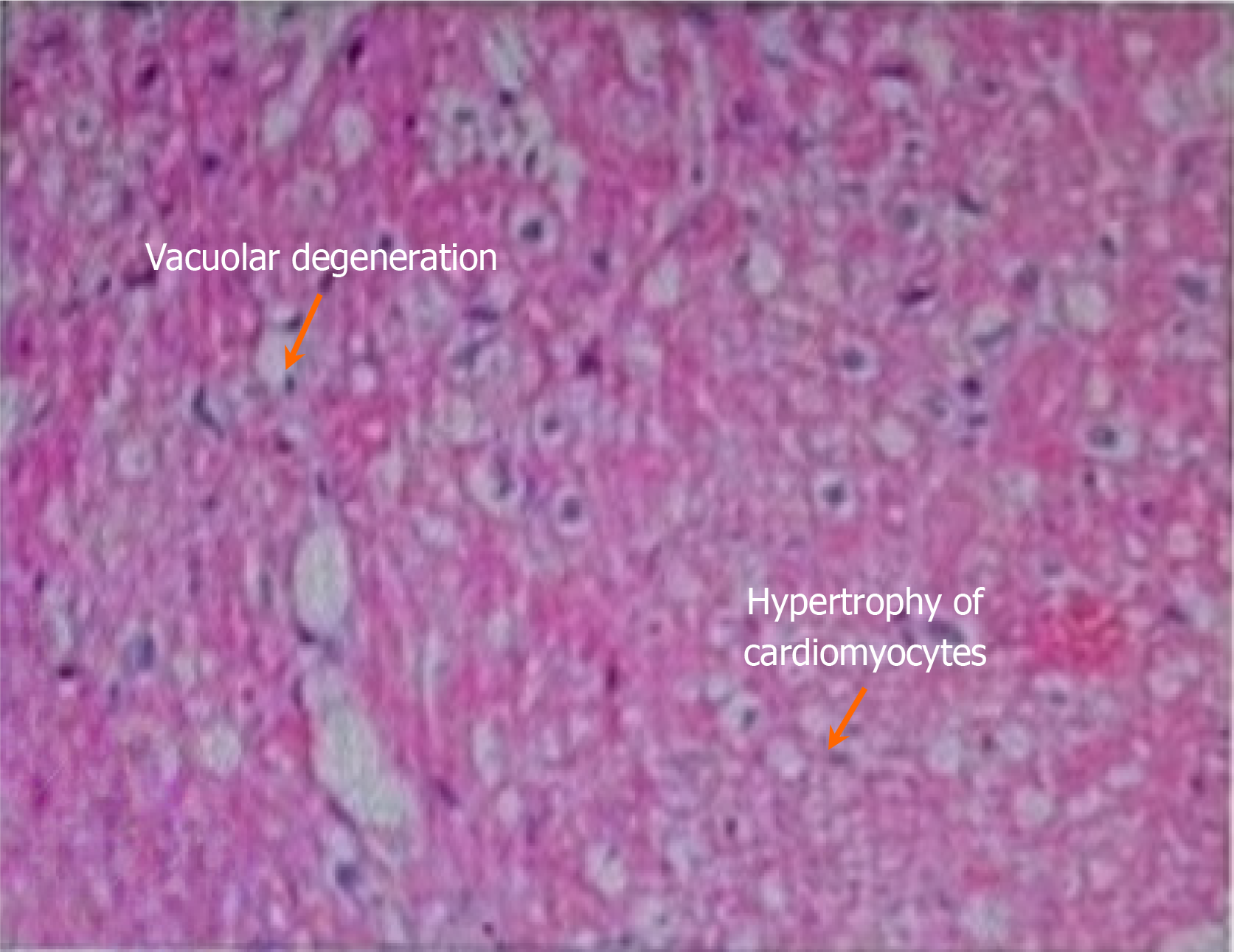
Although the relationship between anti-SSA/Ro antibodies and adult AVB has been increasingly studied, the specific pathogenic mechanism remains unclear. CAVB is the most serious pathological manifestation produced by anti-SSA/Ro antibodies in fetuses or newborns, and this consistent role of anti-SSA/Ro antibodies has long been recognized[6]. Congenital CAVB is typically irreversible due to fibrosis and calcification of the atrioventricular node, and reversal has not been reported. The specific mechanism is explained by two main hypotheses: The inflammation theory and the electrophysiological theory. In addition, Lazzerini et al[7] proposed that anti-SSA/Ro antibodies may significantly inhibit related currents by interfering with calcium ion channels (L and T types) in cardiomyocytes, thus leading to AVB. Compared with adult cardiomyocytes, fetal cardiomyocytes have less L-type calcium channel expression, and their sarcoplasmic reticulum is structurally and functionally underdeveloped, resulting in a limited calcium reserve. Thus, the incidence of anti-SSA/Ro antibody-induced conduction abnormalities is lower in adults compared with fetuses or neonates. This may also explain the possibility that CAVB in adults with anti-SSA/Ro antibodies can be reversed. Adult cardiomyocytes have increased calcium ion channels and calcium ion reserve, which can reduce the damage to cardiac conduction tissue caused by electrophysiological interference induced by anti-SSA/Ro antibodies, thereby reducing apoptosis and slowing the irreversible development of tissue damage[8]. In this case, the patient was positive for ANA and anti-SSA/Ro antibodies. In addition, CAVB and pulmonary hypertension were present at the same time, and myocardial biopsy pathology indicated AM. After methylprednisolone, human immunoglobulin, and cyclophosphamide immunosuppressive treatment, these antibodies were no longer detected in the patient, and pulmonary hypertension was significantly improved (Figure 2). Thus, the diagnosis of AM caused by connective tissue disease was clear. Anti-SSA/Ro antibodies may play a key role in pathogenesis. However, the pathology of this patient suggested myocardial fibrosis, so CAVB may have been irreversible (Figure 3).
Arrhythmias, such as AVB, are common in patients with autoimmune diseases, but severe AVB requiring pacemaker implantation is rare[3]. Santos-Pardo et al[9] reported a case of CAVB in an adult patient who was positive for anti-SSA/Ro antibodies. The patient’s symptoms were reversed after initial treatment with methylprednisolone at 1 mg/kg/d and subsequent maintenance treatment with methylprednisolone at 4 mg/d combined with azathioprine. The patient’s condition was stable. In addition, Lazzerini et al[10] reported two cases of CAVB in adults positive for anti-SSA/Ro antibodies. One case recovered after immunosuppressive treatment, and the other one was implanted with a permanent pacemaker. However, no immunosuppressant was administered before surgery, and only oral prednisone was administered after surgery.
In terms of treatment, the primary focus of AM secondary to connective tissue disease is to treat the primary autoimmune disease first. At present, there are few studies on CAVB caused by anti-SSA/Ro antibody-positive AM, and there is no consensus regarding its treatment. However, there is a general consensus on the treatment of congenital AVB, and it is possible that adult patients with anti-SSA/Ro antibody-mediated CAVB associated with AM can adopt the above treatment strategies. Hansahiranwadee et al[11] proposed that fetal second-degree AVB was likely to develop into CAVB. The intrauterine treatment of congenital AVB mentioned by these authors included glucocorticoids, intravenous immunoglobulins, and maternal plasma exchange, which have been used to treat fetal autoimmune AVB. Some researchers have proposed that hydroxychloroquine can prevent the recurrence of fetal congenital AVB in mothers positive for anti-SSA/Ro antibodies[12]. Lazzerini et al[7] conducted glucocorticoid tests on nine adult AVB patients positive for anti-SSA/Ro antibodies and confirmed the positive effect of immunomodulatory therapy in the short- and medium-term follow-up of these patients. Five of these patients received immunotherapy in addition to hormones (including azathioprine, high-dose immunoglobulin, and hydroxychloroquine), which effectively maintained AVB improvement. Cyclophosphamide is widely used to induce and treat unusual cardiac complications, including AVB, in SLE, ANCA-associated vasculitis, and other autoimmune diseases[13-16]. Based on the above studies, we induced re
The awarenss of adult AM and CAVB associated with autoimmune diseases should be strengthened in clinicians, and anti-SSA/Ro antibodies may play an important role in this process. Therefore, positive detection of antibodies and early intervention, such as active immunosuppressive therapy, are potentially very important for disease prognosis. For patients with such disease that cannot be reversed by immunosuppressive therapy, permanent pacemaker implantation may be inevitable.
| 1. | Won T, Song EJ, Kalinoski HM, Moslehi JJ, Čiháková D. Autoimmune Myocarditis, Old Dogs and New Tricks. Circ Res. 2024;134:1767-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Al-Nokhatha SA, Khogali HI, Al Shehhi MA, Jassim IT. Myocarditis as a lupus challenge: two case reports. J Med Case Rep. 2019;13:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Villuendas R, Olivé A, Juncà G, Salvador I, Martínez-Morillo M, Santos-Pardo I, Pereferrer D, Zamora E, Bayes-Genis A. Autoimmunity and atrioventricular block of unknown etiology in adults: the role of anti-Ro/SSA antibodies. J Am Coll Cardiol. 2014;63:1335-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Logar D, Kveder T, Rozman B, Dobovisek J. Possible association between anti-Ro antibodies and myocarditis or cardiac conduction defects in adults with systemic lupus erythematosus. Ann Rheum Dis. 1990;49:627-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Lazzerini PE, Cevenini G, Qu YS, Fabris F, El-Sherif N, Acampa M, Cartocci A, Laghi-Pasini F, Capecchi PL, Boutjdir M, Lazaro D. Risk of QTc Interval Prolongation Associated With Circulating Anti-Ro/SSA Antibodies Among US Veterans: An Observational Cohort Study. J Am Heart Assoc. 2021;10:e018735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Strasburger JF, Wacker-Gussmann A. Congenital Heart Block in Subsequent Pregnancies of SSA/Ro-Positive Mothers: Cutting Recurrence in Half. J Am Coll Cardiol. 2020;76:303-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lazzerini PE, Murthy Ginjupalli VK, Srivastava U, Bertolozzi I, Bacarelli MR, Verrengia D, Salvini V, Accioli R, Carbone SF, Santoro A, Cartocci A, Cevenini G, Cantara S, Cantore A, Bisogno S, Brucato A, Laghi-Pasini F, Acampa M, Capecchi PL, Boutjdir M. Anti-Ro/SSA Antibodies Blocking Calcium Channels as a Potentially Reversible Cause of Atrioventricular Block in Adults. JACC Clin Electrophysiol. 2023;9:1631-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Boutjdir M, Lazzerini PE, Capecchi PL, Laghi-Pasini F, El-Sherif N. Potassium Channel Block and Novel Autoimmune-Associated Long QT Syndrome. Card Electrophysiol Clin. 2016;8:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Santos-Pardo I, Martínez-Morillo M, Villuendas R, Bayes-Genis A. Anti-Ro antibodies and reversible atrioventricular block. N Engl J Med. 2013;368:2335-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Lazzerini PE, Brucato A, Capecchi PL, Baldi L, Bacarelli MR, Nucci C, Moscadelli V, Morozzi G, Boutjdir M, Laghi-Pasini F. Isolated atrioventricular block of unknown origin in the adult and autoimmunity: diagnostic and therapeutic considerations exemplified by 3 anti-Ro/SSA-associated cases. HeartRhythm Case Rep. 2015;1:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hansahiranwadee W. Diagnosis and Management of Fetal Autoimmune Atrioventricular Block. Int J Womens Health. 2020;12:633-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Richez C, Cordel N, Maillard H, Willems A, Chasset F, Belot A, Arnaud L, Lazaro E, Hachulla E, Costedoat-Chalumeau N. Practical management of patients on hydroxychloroquine. Joint Bone Spine. 2021;88:105316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Rogaczewska M, Puszczewicz M, Stopa M. Exclusively ocular and cardiac manifestation of granulomatosis with polyangiitis - a case report. BMC Ophthalmol. 2019;19:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Lai YW, Chua CG, Lim XR, Francis PJ, Xu C, Howe HS. Autoimmune Rheumatic Disease Flares with Myocarditis Following COVID-19 mRNA Vaccination: A Case-Based Review. Vaccines (Basel). 2022;10:1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Zagelbaum Ward NK, Linares-Koloffon C, Posligua A, Gandrabur L, Kim WY, Sperber K, Wasserman A, Ash J. Cardiac Manifestations of Systemic Lupus Erythematous: An Overview of the Incidence, Risk Factors, Diagnostic Criteria, Pathophysiology and Treatment Options. Cardiol Rev. 2022;30:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Thomas G, Cohen Aubart F, Chiche L, Haroche J, Hié M, Hervier B, Costedoat-Chalumeau N, Mazodier K, Ebbo M, Cluzel P, Cordel N, Ribes D, Chastre J, Schleinitz N, Veit V, Piette JC, Harlé JR, Combes A, Amoura Z. Lupus Myocarditis: Initial Presentation and Longterm Outcomes in a Multicentric Series of 29 Patients. J Rheumatol. 2017;44:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Tselios K, Deeb M, Gladman DD, Harvey P, Akhtari S, Mak S, Butany J, Urowitz MB. Antimalarial-induced Cardiomyopathy in Systemic Lupus Erythematosus: As Rare as Considered? J Rheumatol. 2019;46:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |