Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.105911
Revised: March 13, 2025
Accepted: March 20, 2025
Published online: July 26, 2025
Processing time: 76 Days and 15.6 Hours
Lower salivary uric acid concentrations, the most abundant antioxidant agent in saliva, have been observed in patients with periodontitis compared to individuals with periodontal health. However, the independent association of salivary uric acid levels with periodontitis, accounting for other risk factors, as well as its association with periodontitis severity, has not been investigated.
To the independent association of salivary uric acid levels with periodontitis and the association of salivary uric acid levels with the severity of periodontitis.
This observational and prospective study measured salivary uric acid levels in subjects with periodontitis (characterized by periodontal loss of tissue) and in subjects without periodontitis (periodontal health or localized gingivitis in < 30% of sites). Multivariate regression analysis was performed to determine independent factors associated with periodontitis. Spearman’s rho correlation coefficient was used to assess the relationship between salivary uric acid levels and periodontitis severity. A receiver operating characteristic analysis was carried out to evaluate the diagnostic performance of salivary uric acid levels in periodontitis, reporting the area under curve (AUC) and its 95%CI.
We included 121 subjects, 61 of them with periodontitis and 60 without periodontitis (39 with periodontal health and 21 with local gingivitis). Subjects with periodontitis compared to those without periodontitis were older (P < 0.001), had higher salivary uric acid levels (P = 0.002), higher rate of arterial hypertension history (P = 0.001) and higher rate of never-smoker history (P < 0.001). The AUC for periodontitis diagnosis by salivary uric acid levels was 66% (95%CI: 57%-75%; P < 0.001) and the better cut-off point was 111 nmol/mL. Multiple logistic regression analysis showed an independent association of salivary uric acid levels < 111 nmol/mL (OR = 6.14; 95%CI: 2.015-18.721; P = 0.001) with periodontitis after controlling for age, never-smoker history and arterial hypertension. A negative correlation of salivary uric acid levels and periodontitis severity was observed (rho = -0.32; P < 0.001).
The two novel findings of our research were, first, that low salivary uric acid concentrations are independently associated with periodontitis, even after accounting for established risk factors. Second, salivary uric acid levels show a negative correlation with periodontitis severity.
Core Tip: Lower salivary uric acid concentrations, the most abundant antioxidant agent in saliva, have been observed in patients with periodontitis compared to individuals with periodontal health. The two novel findings of our research were, first, that low salivary uric acid concentrations are independently associated with periodontitis, even after accounting for established risk factors. Second, salivary uric acid levels show a negative correlation with periodontitis severity.
- Citation: Lorente L, Hernández Marrero E, Abreu-Gonzalez P, Lorente Martín AD, González-Rivero AF, Marrero González MJ, Hernández Marrero C, Hernández Marrero O, Jiménez A, Hernández Padilla CM. Low salivary uric acid levels are independently associated with periodontitis. World J Clin Cases 2025; 13(21): 105911
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/105911.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.105911
Periodontitis is a chronic inflammatory disease characterized by microorganism invasion of the periodontium. It has a high prevalence, affecting 50% of individuals in its mild forms and 7% in advanced stages and has important economic consequences, with an estimated $79 billion annually worldwide[1].
Several periodontitis risk factors have been described, such as diabetes mellitus[2], systemic lupus erythematosus and rheumatoid arthritis[3], sex[4], arterial hypertension[5], age[6], obesity[7], oral cancer, dental hygiene, immunosuppression and consumption of toxic substances (drugs, tobacco, tea, alcohol, coffee)[8].
In periodontitis, the destruction of the periodontium occurs due to the activation of pathophysiological pathways such inflammation[9,10], oxidation[11-14] and programmed cell[15,16].
Free radicals, highly reactive compounds, can cause damage to nucleic acids, proteins and lipids. Different types of free radicals are involved in periodontitis[11-14]: (1) Reactive nitrogen species (nitric oxide, nitrite and nitrate) that cause nitrosative stress; and (2) Reactive oxygen species (ROS) (hydroxyl radical, peroxyl radical and superoxide anion radical) that cause oxidative stress.
Different types of antioxidant agents exist to prevent damage from free radicals which can be categorized into enzymatic and non-enzymatic antioxidant defense system[11-14]. Oxidative damage will depend on the balance of free radical production and antioxidant systems activity. The antioxidant enzyme system includes glutathione peroxidase, superoxide dismutase, thioredoxin, catalase and glutathione reductase. The antioxidant non-enzymatic system includes albumin, melatonin, glutathione, vitamin E, bilirubin, vitamin C and uric acid. Uric acid accounts for approximately 70% of the total antioxidative capacity of saliva[17,18].
Two meta-analyses have analyzed the relationship between saliva and blood concentrations of uric acid and chronic periodontitis[19,20]. Both concluded that patients with chronic periodontitis compared to healthy subjects had lower uric acid saliva concentrations and higher concentrations of uric acid in blood. In the meta-analysis published by Uppin et al[19] in 2022 8 studies were included with 159 periodontitis patients and 149 controls, and in the meta-analysis published by Ye et al[20] in 2023 9 studies with 233 periodontitis patients and 174 controls were included. However, the association of salivary uric acid levels with periodontitis, after adjusting for risk factors, and its association with severity of periodontitis have not been reported. Therefore, the novel objectives of our study were to determine whether these associations exist.
This study was initiated after obtaining approval from the Clinical Research Ethics Committee of the Hospital Universitario de Canarias. Prior to inclusion, all participants provided written informed consent. This prospective and observational study was conducted at Clínica Dental Cándido (La Laguna, Tenerife, Canary Islands, Spain). Patients with periodontitis (charachterized by periodontal tissue loss) and subjects without periodontitis (including those with localized gingivitis in < 30% sites and those with periodontal health) were included. Diagnosis of periodontitis and classification of its severity were based on current internationally accepted criteria[21]. Subjects younger than 18 years were excluded.
Clinical periodontal health was established when the subject showed no bleeding on probing or showed bleeding in less than 10% of locations and showed no clinical interproximal attachment loss or bone loss. Localized gingivitis was established when the subject showed bleeding in 10%-30% of locations, and didn’t present clinical interproximal attachment loss or bone loss. Periodontitis was established when the subject showed bone loss or clinical interproximal attachment loss. Periodontitis severity classification was assessed using the following 3 criteria: (1) Clinical interproximal attachment loss: Stage I with < 3 mm, Stage II with 3 a 4 mm, Stage III or IV with ≥ 5 mm; (2) Radiographic bone loss: Stage I with coronal third < 15%, Stage II with coronal third 15%-33%, Stage III or IV with middle or apical third; (3) Tooth loss: Stage I or II with none, Stage III with ≤ 4 teeth, Stage III with ≥ 5 teeth.
We recorded the following variables: Age, salivary uric acid levels, body mass index (BMI) (kg/m²), sex, arterial hypertension, diabetes mellitus, obesity (BMI≥ 30 kg/m²), never smoker, coffee, tea, alcohol, rheumatoid arthritis, immunesupressive therapy, radiotherapy, methotrexate, drugs consumption, oral cancer, systemic lupus erythematosus, chemotherapy and dental hygiene with toothbrushing.
We used the Navazesh technique to obtain whole unstimulated saliva samples[22]. Saliva collection took place in the morning (from 8 to 10 a.m.) to reduce possible influences by circadian rhythm on salivary biomarker concentrations. Participants did not drink, smoke, or brush their teeth for at least two hours prior to sample collection. At the Dental Clinic, subjects rinsed their mouths with 10 mL. of deionized water 3 times. They then sat comfortably for 30 minutes with their heads slightly tilted forward, eyes open, and avoiding oro-facial movements. During this period, they refrained from swallowing saliva, allowing it to accumulate in the mouth before expelling it into a collection bottle. Saliva samples were centrifuged for 10 minutes at 3000 rpm at 24 °C to remove debris and cells. The supernatants were then pipetted into Eppendorf tubes and stored at -80ºC for subsequent salivary concentration determinations. Some subjects included in this study had also been included in a previous publication of our team, in which salivary nitrite levels were reported[23]. In the present study, salivary uric acid levels were assessed.
Salivary uric acid levels were measured using the Randox kit reagents (Randox Laboratories, Country Antrim, United Kingdom), based on the method developed by Fossati et al[24]. Uric acid is converted by uricase into allantoin and H2O2, which, under the catalytic influence of peroxidase, oxidizes 3,5-dichloro-2-hydroxybenzenesulfonic acid and 4-amono
We used Kruskal Wallis test to determine whether had significant differences existed between subject groups in salivary uric acid concentrations. Continuous variables were compared between subject groups using the Mann-Whitney U-test, Mann-Whitney U-test, while categorical variables were analyzed using the χ2 test. We analyzed the association between salivary uric acid and periodontitis severity using Spearman’s rho correlation coefficient. We carried out a receiver operating characteristic analysis to evaluate the diagnostic performance of salivary uric acid levels for periodontitis, reporting the area under curve (AUC) and its 95%CI. We selected the point of salivary uric acid levels < 111 nmol/mL based on the Youden[25]. We performed a multivariate regression analysis to determine whether an independent asso
A total of 121 subjects were included, 61 with periodontitis and 60 without periodontitis (39 with periodontal health and 21 with local gingivitis). The number of subjects in each periodontal state and their salivary uric acid concentrations are showed in Table 1. We found statistically significant differences between subject groups in salivary uric acid concentrations (P = 0.01).
| Total | Subjects without periodontitis | Subjects with periodontitis | Uric acid (nmol/mL) | P value | |
| 0.01 | |||||
| Periodontal health | 39 (32.2) | 39 (65.0) | 0 | 126 (79-203) | |
| Localized gingivitis | 21 (17.4) | 21 (35.0) | 0 | 112 (86-142) | |
| Periodontitis stage I | 17 (14.0) | 0 | 17 (27.9) | 102 (53-137) | |
| Periodontitis stage II | 24 (19.8) | 0 | 24 (39.3) | 93 (62-171) | |
| Periodontitis stage III | 13 (10.7) | 0 | 13 (21.3) | 86 (53-111) | |
| Periodontitis stage IV | 7 (5.8) | 0 | 7 (11.5) | 61 (21-94) |
Subjects with periodontitis compared to those without periodontitis were older (P < 0.001), had higher salivary uric acid levels (P = 0.002), higher rate of arterial hypertension history (P = 0.001) and higher rate of never-smoker history (P < 0.001). In our series, no subjects had with a history of drug consumption, oral cancer, systemic lupus erythematosus, chemotherapy or dental hygiene without toothbrushing. Statistically significant differences were not found in BMI, sex, diabetes mellitus, obesity, coffee, tea, alcohol, rheumatoid arthritis, immunosupressive therapy, radiotherapy and metrotexate between subjects suffering or not periodontitis (Table 2).
| Subjects without periodontitis (n = 60) | Subjects with periodontitis (n = 61) | P value | |
| Age (years) | 41 (34-49) | 60 (52-68) | < 0.001 |
| Salivary uric acid (nmol/mL) | 112 (82-176) | 89 (54-119) | 0.002 |
| Body mass index (kg/m²) | 24.8 (22.9-26.7) | 24.8 (22.6-28.4) | 0.81 |
| Sex female | 40 (66.7) | 38 (62.3) | 0.71 |
| Arterial hypertension | 3 (5.0) | 19 (31.1) | 0.001 |
| Diabetes mellitus | 0 | 5 (8.2) | 0.06 |
| Obesity | 7 (11.9) | 9 (14.8) | 0.79 |
| Never smoker | 47 (78.3) | 26 (42.6) | < 0.001 |
| Coffee | 51 (85.0) | 54 (88.5) | 0.60 |
| Tea | 5 (8.3) | 3 (4.9) | 0.49 |
| Alcohol | 27 (45.0) | 31 (50.8) | 0.59 |
| Rheumatoid arthritis | 1 (1.7) | 4 (6.6) | 0.37 |
| Immunosupressive therapy | 1 (1.7) | 3 (4.9) | 0.62 |
| Radiotherapy | 0 | 3 (4.9) | 0.24 |
| Metrotexate | 1 (1.7) | 0 | 0.50 |
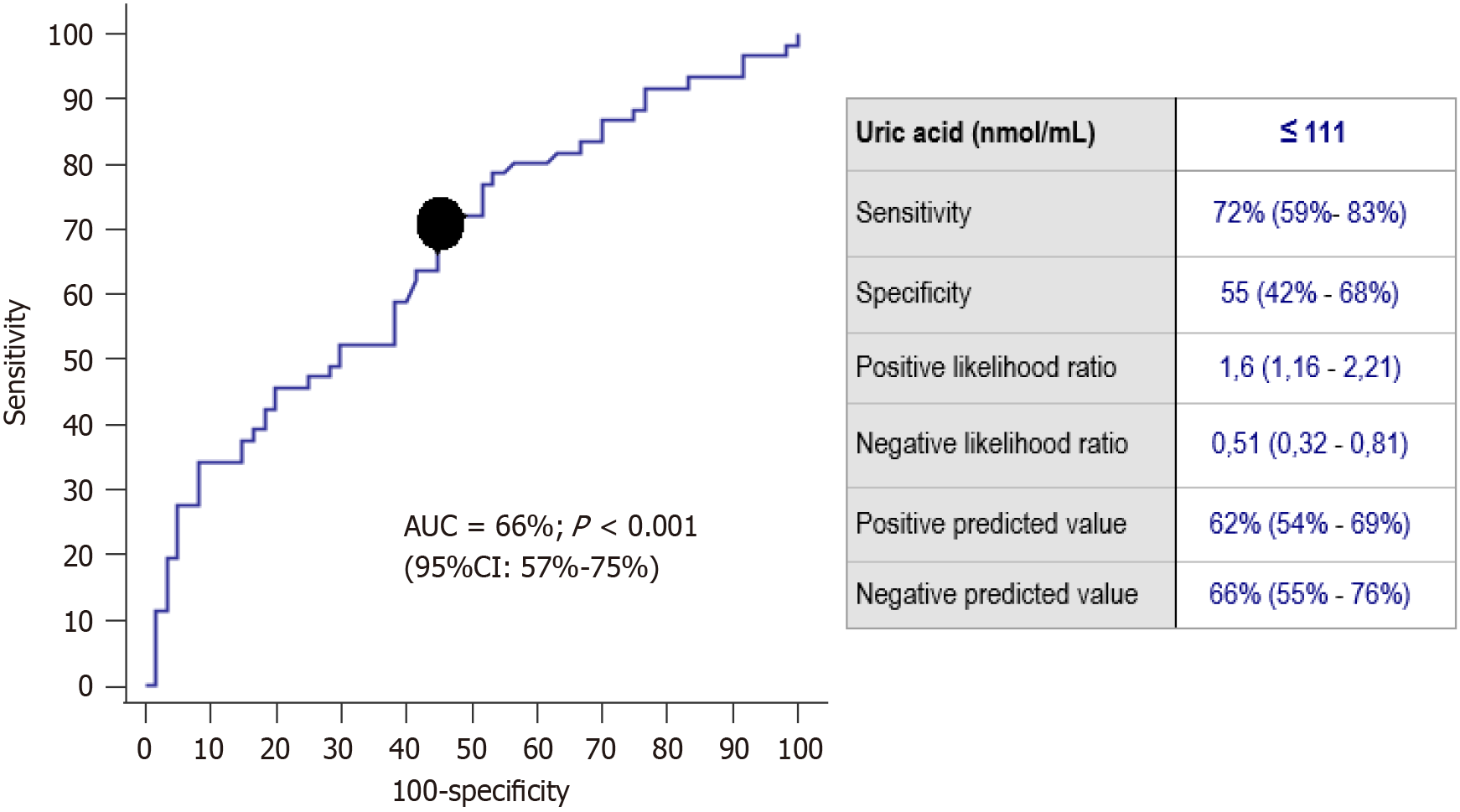
The AUC for periodontitis diagnosis by salivary uric acid levels was 66% (95%CI: 57%-75%; P < 0.001) and the better cut-off point was 111 nmol/mL (Figure 1). Multiple logistic regression analysis showed an independent association of salivary uric acid levels < 111 nmol/mL (OR = 6.14; 95%CI: 2.015-18.721; P = 0.001) with periodontitis after controlling for age, never-smoker history and arterial hypertension (Table 3). A negative correlation of salivary uric acid levels and periodontitis severity was observed (rho = -0.32; P < 0.001).
| Odds ratio | 95%CI | P value | |
| Salivary uric acid levels < 111 nmol/mL (yes vs non) | 6.14 | 2.015-18.721 | 0.001 |
| Age (years) | 1.13 | 1.073-1.197 | < 0.001 |
| Never smoker (yes vs non) | 0.44 | 0.154-1.254 | 0.12 |
| Arterial hypertension (yes vs non) | 1.49 | 0.289-7.661 | 0.63 |
We found that subjects with periodontitis, compared to those without periodontitis, had lower salivary uric acid levels, which is consistent with the findings of two previous meta-analyses[19,20]. In addition, a novel finding of our study was the independent association between low salivary uric acid concentrations and periodontitis after adjusting for other risk factors. Another novel and interesting result was the negative association between salivary uric acid levels and the severity of periodontitis.
We also identified two other variables independently associated with periodontitis: Never-smoking history and age. These findings are in agreement with the results of previous studies[6,8]. However, no other variables were associated with periodontitis in our series, and the relatively low sample size may have influenced this outcome.
Conflicting results have been reported regarding uric acid concentrations in saliva and blood in patients with and without periodontitis. Two meta-analyses concluded that patients with periodontitis, compared to those with periodontal health, had lower salivary concentrations and higher blood concentrations of uric acid[19,20]. Several potential explanations exist for the lower salivary uric acid concentrations observed in patients with periodontitis. One possibility is increased consumption of salivary uric acid due to elevated oxidative stress during periodontal infection, where uric acid can be oxidized by ROS into allantoin. Another explanation is that salivary uric acid may serve as a substrate for microorganism synthesis in dental plaque[26]. However, the exact mechanisms remain unclear, and further investigation is needed.
We acknowledge that our study had some limitations. A larger sample size would have allowed us to include only those with periodontal health in the group of subjects without periodontitis, excluding those with localized gingivitis. Additionally, we did not determine uric acid levels in other samples (gingival crevicular fluid or blood). We did not calculate the sample size because it was incidental, aiming to explore an issue that had not been previously addressed. However, the sample size was sufficient to report the association of salivary uric acid concentrations with periodontitis and its severity. We excluded subjects under 18 years of age because they are often excluded in research studies. How
We believe that the most important point of our study is not that salivary uric acid levels can help in diagnosing periodontitis but that they could influence its development and severity. The diagnosis and classification of periodontitis severity are straightforward using clinical periodontal examination and X-rays, eliminating the need for saliva extraction. However, determining salivary uric acid levels could help assess the antioxidant status of saliva in patients with perio
Another important aspect could be whether the deviation of salivary uric acid levels in the mouths of patients with gingivitis and normal patients could predict the likelihood of their subsequent development of periodontitis in the future, and this could be another potential line of investigation.
Our modest study only attempts to put a small stone in the knowledge of periodontitis reporting for the first time an independent association between salivary uric acid levels and periodontitis and an association between salivary uric acid levels and severity of periodontitis.
In conclusion, the two novel findings of our research were the independent association of low salivary uric acid concentrations and periodontitis, regardless of other risk factors, and the negative association between salivary uric acid levels and periodontitis severity. Second, salivary uric acid levels show a negative correlation with periodontitis severity.
Clínica Dental Cándido (Laguna. Tenerife. Spain) clinic has paid the costs of consumables and reagents for the determinations.
| 1. | Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47 Suppl 22:4-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 941] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 2. | Enteghad S, Shirban F, Nikbakht MH, Bagherniya M, Sahebkar A. Relationship Between Diabetes Mellitus and Periodontal/Peri-Implant Disease: A Contemporaneous Review. Int Dent J. 2024;74:426-445. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Tan PR, Lee AJL, Zhao JJ, Chan YH, Fu JH, Ma M, Tay SH. Higher odds of periodontitis in systemic lupus erythematosus compared to controls and rheumatoid arthritis: a systematic review, meta-analysis and network meta-analysis. Front Immunol. 2024;15:1356714. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Shiau HJ, Reynolds MA. Sex differences in destructive periodontal disease: a systematic review. J Periodontol. 2010;81:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Tada A, Tano R, Miura H. The relationship between tooth loss and hypertension: a systematic review and meta-analysis. Sci Rep. 2022;12:13311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Clin Periodontol. 2018;45 Suppl 20:S130-S148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Adam M. Obesity as a risk factor for periodontitis - does it really matter? Evid Based Dent. 2023;24:48-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Salvi GE, Roccuzzo A, Imber JC, Stähli A, Klinge B, Lang NP. Clinical periodontal diagnosis. Periodontol 2000. 2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 9. | Viglianisi G, Santonocito S, Lupi SM, Amato M, Spagnuolo G, Pesce P, Isola G. Impact of local drug delivery and natural agents as new target strategies against periodontitis: new challenges for personalized therapeutic approach. Ther Adv Chronic Dis. 2023;14:20406223231191043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Gomes PR, Rocha MD, Lira JA, Coelho FA, Alves EH, Nascimento HM, Oliveira SM, Carmo RR, Araújo HT, Silva FR, Vasconcelos DF. Salivary biomarkers present in patients with periodontitis without clinical distinction: findings from a meta-analysis. Med Oral Patol Oral Cir Bucal. 2023;28:e457-e466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Andrukhov O, Rausch-Fan X. Oxidative Stress and Antioxidant System in Periodontitis. Front Physiol. 2017;8:910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 12. | Toczewska J, Konopka T. Activity of enzymatic antioxidants in periodontitis: A systematic overview of the literature. Dent Med Probl. 2019;56:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, Sun X, Mao Y, He B, Hou Y, Zhou Y, Zhou Q, Ma J, Huang S. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J Clin Periodontol. 2019;46:608-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 14. | Vo TTT, Chu PM, Tuan VP, Te JS, Lee IT. The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Zhang A, Zhang C, Zhang Y, Hu T, Cheng R. PANoptosis is a compound death in periodontitis: A systematic review of ex vivo and in vivo studies. Oral Dis. 2024;30:1828-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | He W, Fu Y, Yao S, Huang L. Programmed cell death of periodontal ligament cells. J Cell Physiol. 2023;238:1768-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res. 1994;21:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Nagler RM, Klein I, Zarzhevsky N, Drigues N, Reznick AZ. Characterization of the differentiated antioxidant profile of human saliva. Free Radic Biol Med. 2002;32:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Uppin RB, Varghese SS. Estimation of Serum, Salivary, and Gingival Crevicular Uric Acid of Individuals With and Without Periodontal Disease: A Systematic Review and Meta-analysis. J Int Soc Prev Community Dent. 2022;12:393-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Ye LW, Zhao L, Mei ZS, Zhou YH, Yu T. Association between periodontitis and uric acid levels in blood and oral fluids: a systematic review and meta-analysis. BMC Oral Health. 2023;23:178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45 Suppl 20:S149-S161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 460] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 22. | Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 898] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 23. | Lorente L, Hernández Marrero E, Abreu González P, Lorente Martín AD, González-Rivero AF, Marrero González MJ, Hernández Marrero C, Hernández Marrero O, Jiménez A, Hernández Padilla CM. Observational prospective study to determine the association and diagnostic utility of salivary nitrite levels in periodontitis. Quintessence Int. 2025;56:100-107. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980;26:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 26. | Barnes VM, Teles R, Trivedi HM, Devizio W, Xu T, Mitchell MW, Milburn MV, Guo L. Acceleration of purine degradation by periodontal diseases. J Dent Res. 2009;88:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |