Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.104062
Revised: March 4, 2025
Accepted: April 3, 2025
Published online: July 26, 2025
Processing time: 134 Days and 17.4 Hours
Congenital renal arteriovenous fistula (RAVF) is a clinically rare condition and frequently missed and misdiagnosed. Multimodal imaging techniques can pro
A 69-year-old female patient presented with gross hematuria that had persisted for 10 days. The patient underwent ultrasound examinations of the kidneys and renal blood vessels, enhanced computed tomography, three-dimensional com
Multimodal imaging techniques complement each other when diagnosing RAVF, providing detailed diagnostic information that can aid in accurate diagnosis and treatment. In addition, this case reminds the sonographer to pay more attention to the color doppler flow imaging and blood flow spectrum when examining the kidney, so as to avoid misdiagnosis of renal cystic lesions as renal cysts and missed diagnosis of RAVF.
Core Tip: Congenital renal arteriovenous fistula (RAVF) is a clinically rare disease that is often missed and misdiagnosed. We report the case of a patient with congenital left RAVF diagnosed through combined multimodal imaging techniques, including ultrasound, enhanced computed tomography, three-dimensional computed tomography angiography, and digital subtraction angiography of the renal arteries. The imaging techniques provide complementary, detailed diagnostic information and play a critical role in improving our understanding of RAVFs, providing a reliable basis for clinical treatment.
- Citation: Lv SP, Qin LL, Mou H, Huang T, Wang KQ. Multimodal imaging techniques for the diagnosis of congenital left renal arteriovenous fistula: A case report. World J Clin Cases 2025; 13(21): 104062
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/104062.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.104062
Renal arteriovenous fistula (RAVF) is an abnormal arteriovenous shunt that forms between the renal artery and vein through an abnormal channel, bypassing the capillary network and directly connecting the artery and vein[1]. RAVF can be categorized as acquired, idiopathic, or congenital, among which congenital RAVF has a low prevalence of approximately 0.04%. The clinical manifestations are primarily gross hematuria and hypertension[2]. Due to its nonspecific clinical manifestations and low degree of clinical recognition and systematic summarization, congenital RAVF is easily missed and misdiagnosed. This report presents a case of congenital left RAVF diagnosed using multimodal imaging techniques. The aim is to enhance understanding of the disease, reduce the risk of missed and misdiagnosis, provide a more detailed and reliable basis for clinical diagnosis, and timely treatment planning.
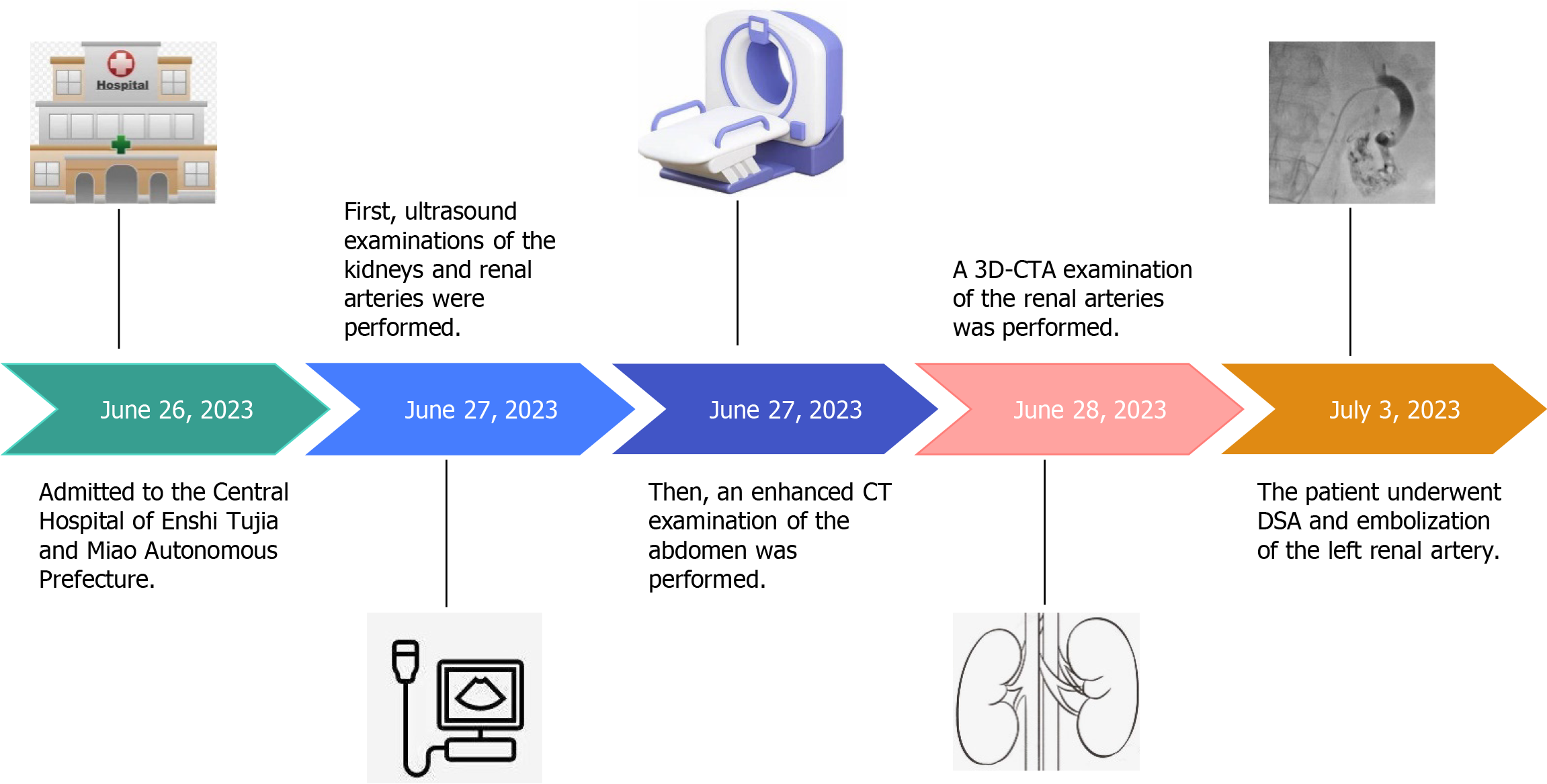
On June 26, 2023, a 69-year-old female patient was admitted to the hospital with gross hematuria that had persisted for 10 days.
Ten days prior to admission, the patient developed hematuria without an apparent cause, accompanied by blood clots, frequent urination, and nocturia 2-3 times.
The patient had a history of hypertension, taking amlodipine benzenesulfonate tablets to control her blood pressure, and had a history of heart failure.
There was no family history of kidney disease.
On physical examination, the patient’s vital signs were as follows: Body temperature, 36.6 °C; pulse, 72 beats/minute; respiration, 19/minute; and blood pressure, 126/80 mmHg. There was no tenderness or percussion pain in the bilateral kidney areas.
The laboratory test results upon admission were as follows: White blood cell count (6.03 × 109/L; normal range: 4.0-10.0 × 109/L), red blood cell count (5.26 × 1012/L; normal range: 3.5-5.0 × 1012/L), platelet count (187 × 109/L; normal range: 90-300 × 109/L), and hemoglobin level (106 g/L; normal range: 110-150 g/L). Routine urine examination revealed increased red blood cells to 28 cells/µL, positive occult blood, and normal renal and coagulation functions.
After admission, the patient underwent ultrasound, enhanced computed tomography (CT), three-dimensional CT angio
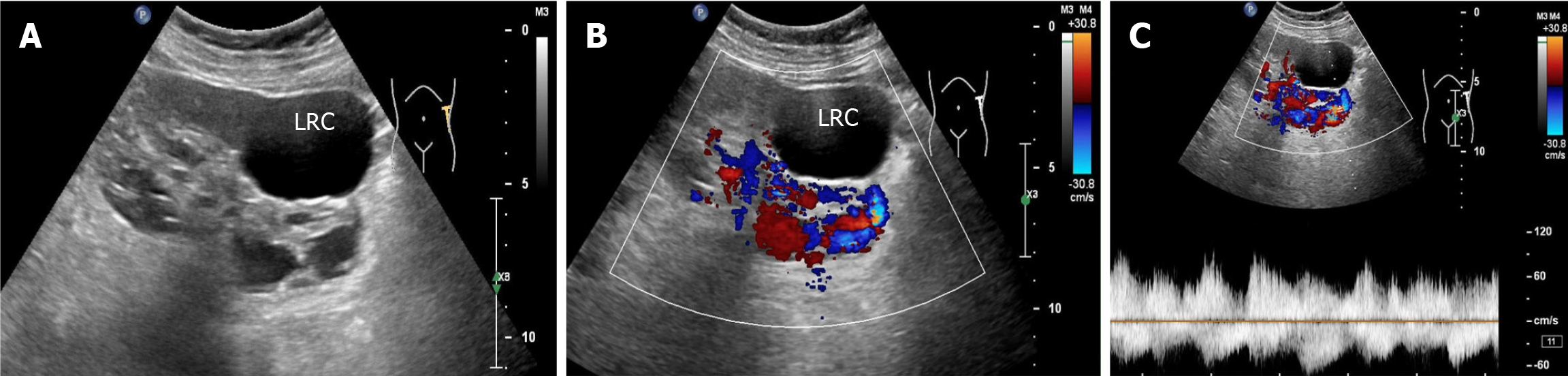
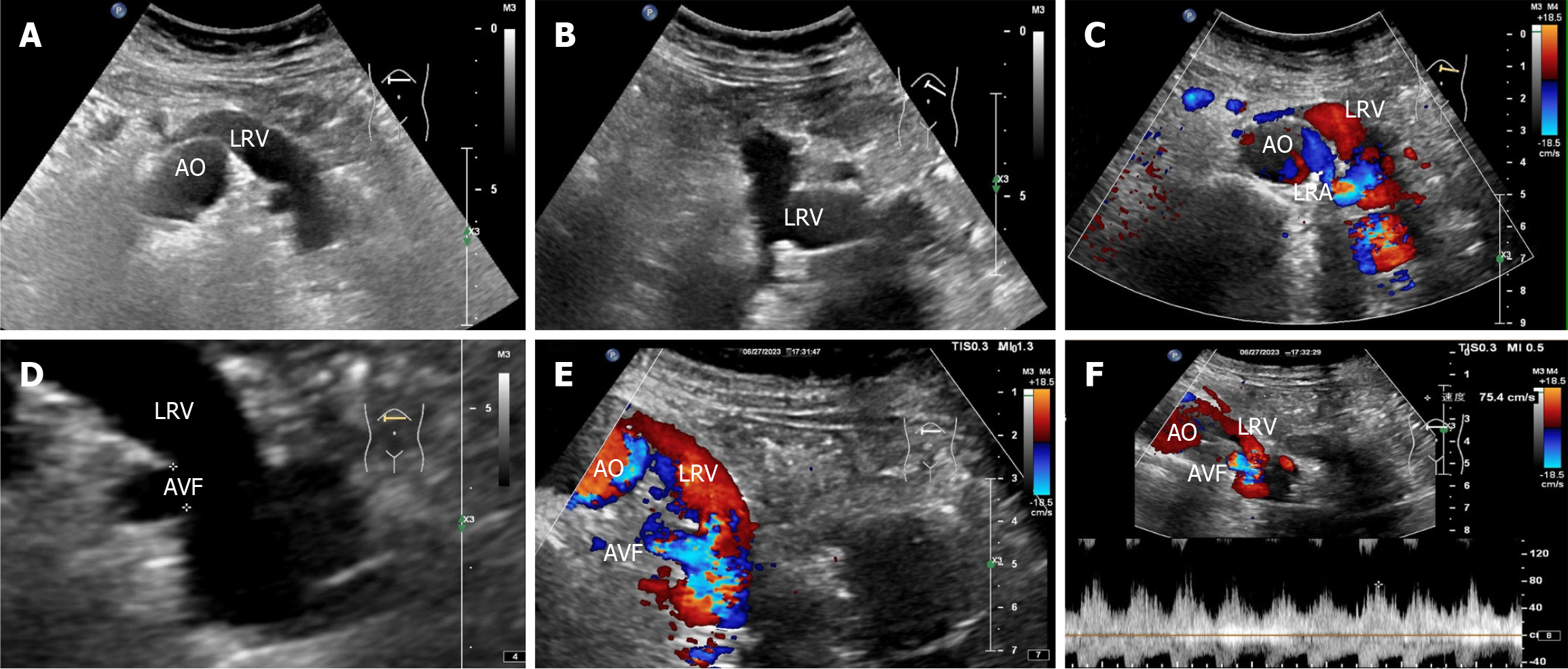
Renal ultrasonography revealed the possible presence of left RAVF in the kidney. To confirm the existence of RAVF outside the kidney, renal vascular ultrasonography was performed. 2D images revealed that the left renal vein was widened and uneven in thickness, with an internal diameter of 1.8 cm at the widest point (Figure 3A and B). CDFI re
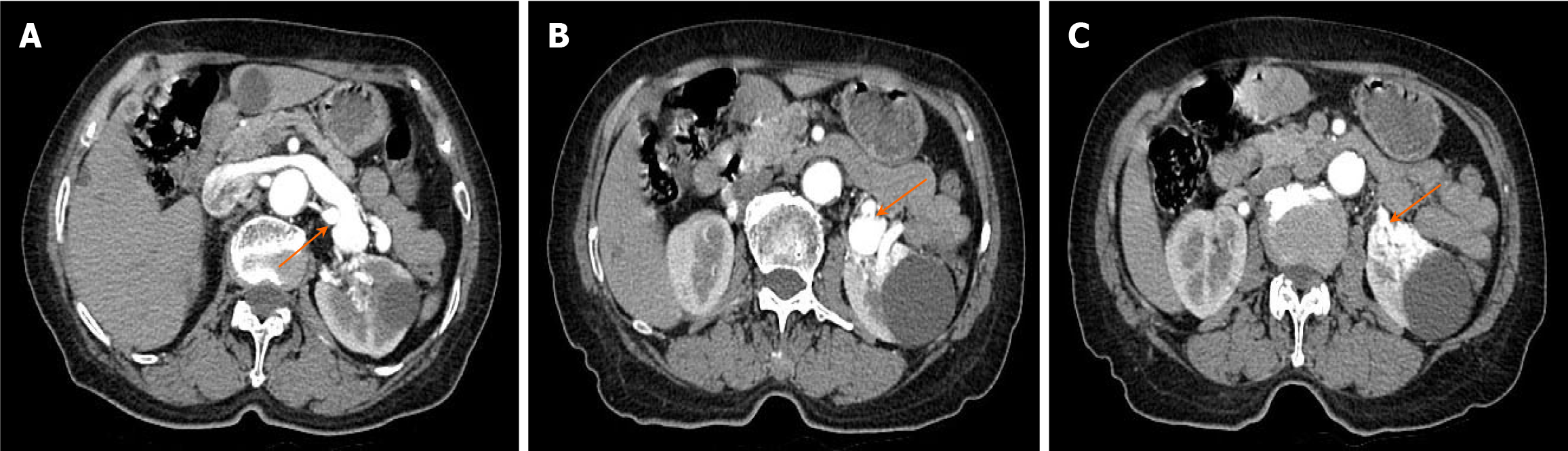
Ultrasound definitely diagnosed the presence of RAVF between the main renal artery and vein. In order to further clarify the presence or absence of RAVF in the tortuous vascular mass of the kidney, more objectively understand the location of the lesion and its anatomic relationship with the surrounding tissues and organs, exclude and identify RAVF caused by other diseases such as renal space-occupying lesions, an enhanced CT examination of the abdomen was performed on June 27, 2023. The scan revealed early visualization of the left renal vein in the arterial phase, with the left renal artery as the blood-supplying artery. The left renal vein was markedly thickened, with localized verrucous dilatation (Figure 4A). The branches of the left renal artery were in communication with the left renal vein, and a rounded low-density shadow was observed in the middle part of the left kidney with a maximum diameter of approximately 4.2 cm, which did not show noticeable enhancement (Figure 4B). A nodular isointense shadow was observed in the middle and lower poles of the left kidney, with a maximum cross-section of 2.9 cm × 2.7 cm. This area showed markedly uneven strengthening, and the left renal artery branch was observed to be in communication with the left renal vein (Figure 4C). Enhanced CT revealed left renal occupancy, with tumor-like dilatation of the adjacent left renal vein, leading to a diagnosis of possible left RAVF.
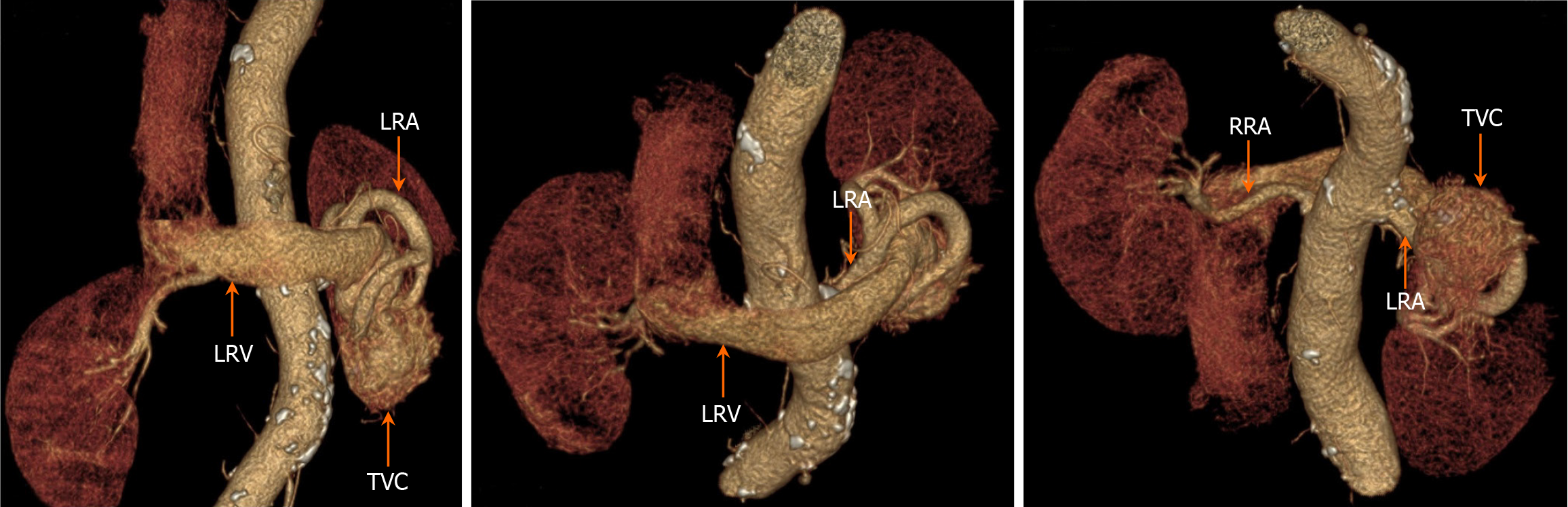
The results of enhanced CT showed the left renal artery was the feeding artery and the left renal vein was the draining vein, thus inferring several obvious fistula positions. In addition, renal neoplastic lesions were excluded, but the smaller fistula could not be clearly identified. 3D-CTA focuses on blood vessels, which can form clear, multi-plane and multi-angle 3D reconstructed images to provide more comprehensive morphological information of blood vessels. This helps doctors comprehensively understand the condition and provides accurate information for interventional treatment. On June 28, 2023, a 3D-CTA examination of the renal arteries revealed thickening of the left renal artery, localized communication with the left renal vein, aneurysmal dilatation of the left renal vein, and irregularly massed vascular shadows with vasculature-like enhancement in the lower pole of the left kidney (Figure 5). The results of 3D-CTA examination suggested that the left renal vascular tortuosity and tumor-like extension of the adjacent left renal vein might be considered as a diagnosis of RAVF.
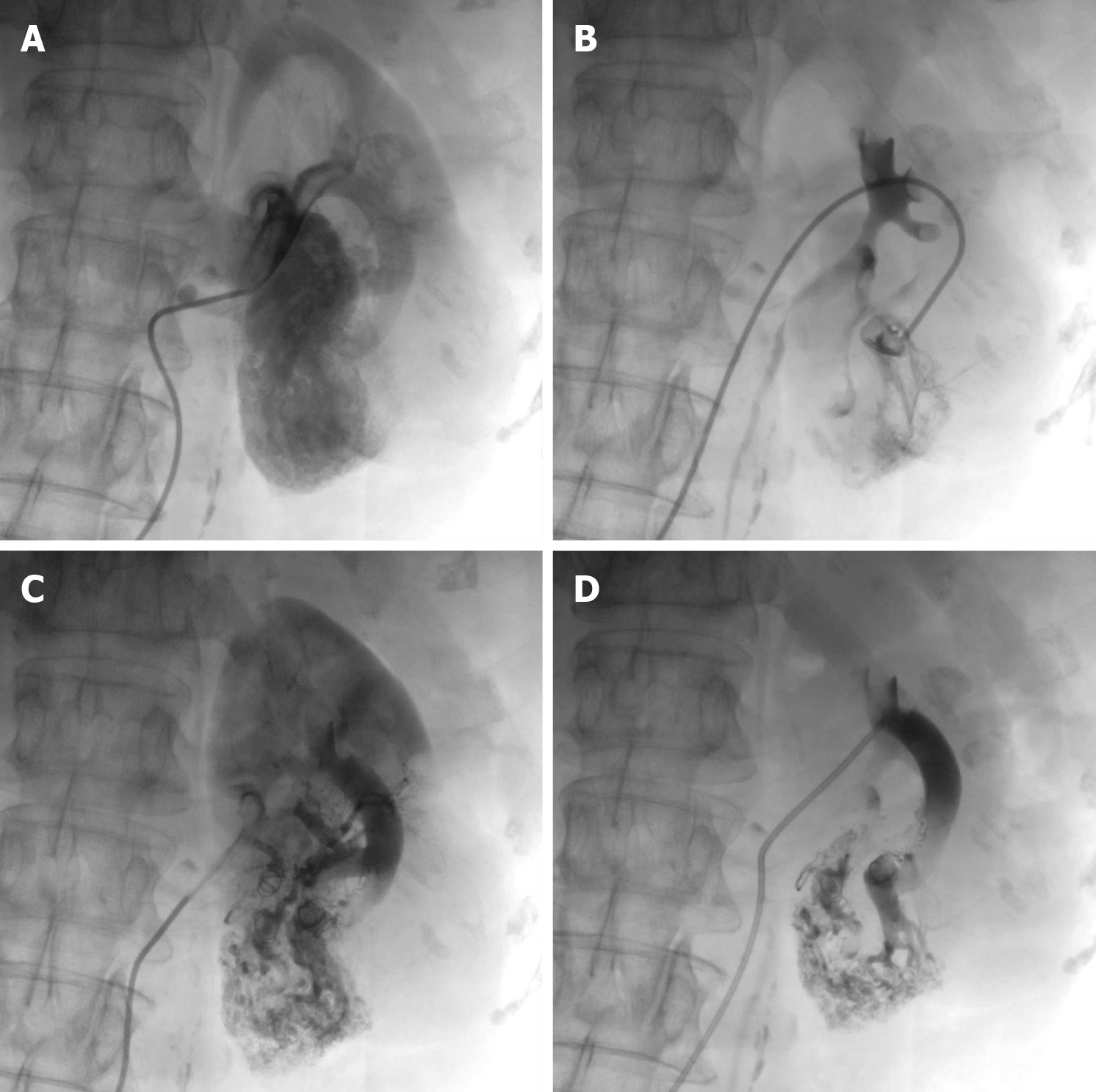
In addition to being the gold standard for diagnosing RAVF, DSA also has a therapeutic role. Because DSA is an invasive procedure, if the family agrees to the treatment, DSA can play a dual advantage in the diagnosis and treatment of RAVF. On July 3, 2023, after the patient and his family signed an informed consent form, the patient underwent DSA of the left renal artery under local anesthesia. RAVF formation was observed in four branches of the left renal artery with contrast medium extravasation, and significant lamellar vascular malformations were observed in the lower pole of the left kidney (Figure 6A and B).
The patient was diagnosed with a left RAVF.
The patient underwent left renal artery embolization immediately after renal arteriography. The diseased branches of the left renal artery were embolized with five spring coils. Postoperative imaging confirmed complete embolization of the blood-supplying vessels of the lesion (Figure 6C and D).
The patient’s hematuria resolved after left renal artery embolization without significant bleeding or other complications. She recovered well after one year of postoperative follow-up.
RAVF is a relatively rare disease in clinical practice. In terms of etiology, RAVF can be divided into three categories: Acquired, idiopathic, and congenital. Congenital RAVF is a malformation caused by congenital abnormalities of vascular development. This condition is also called congenital renal arteriovenous malformation and accounts for approximately 25% of RAVF, with a prevalence of approximately 0.04%[3-5]. The exact etiology of RAVF is believed to involve the residual anastomotic branches between the arteries and veins at birth. The typical renal arteriographic presentation of congenital RAVF is a varicose vascular mass with multiple arteriovenous fistulas[6,7]. In this case, there were no apparent predisposing factors, such as renal surgery, trauma, inflammation, or tumors, and there was no history of pseudoaneurysm. Therefore, combined with renal arteriographic features, the patient was diagnosed with congenital RAVF.
The clinical manifestations of RAVF are dominated by urinary and circulatory symptoms, mainly hematuria and hypertension, which may be accompanied by cardiac enlargement and congestive heart failure[2,8]. The primary cause of hematuria in congenital RAVF is that the lesion is generally located in the submucosal layer of the collecting system, where the vascular smooth muscle is thin and prone to rupture and hemorrhage when the venous pressure increases. In a recent case report, a patient with left renal arteriovenous malformation presented with symptoms of lumbar abdominal pain, hematuria, and no history of hypertension[9]. In this case, the patient had gross hematuria which was thought to be caused by the left RAF. Hypertension due to RAVF is primarily due to a large amount of blood shunt that reduces renal parenchyma perfusion, which in turn results in the secretion of a large amount of renin by the renal tissues distal to the arteries of the blood supply due to ischemia and atrophy, causing renin-dependent hypertension. In this case, the patient had a history of hypertension, cardiac manifestations of ascending aortic dilatation and biatrial enlargement, and a history of heart failure. These changes may be related to RAVF.
With the improvement and development of imaging equipment, various imaging techniques have been developed and applied to diagnose RAVF. Current diagnostic methods for RAVF include ultrasonography, CT, magnetic resonance angiography (MRA), and DSA. Each examination technique has its characteristics, and they complement each other. Ultrasound has the advantages of being noninvasive, convenient, real-time, and inexpensive. Color doppler ultrasound is sensitive to changes in blood flow and can detect hemodynamic changes produced by RAVF. For this reason, ultrasound is currently considered a better screening tool for diagnosing RAVF.
When the RAVF is relatively small, there is no obvious manifestation on 2D ultrasound images. However, when the fistula is large, arterialization of the affected veins can cause the renal veins to become locally tortuous and dilated on 2D sonograms. The intrarenal veins appear spongy and show localized anechoic areas. In addition, the location and size of the fistula can be observed in 2D images. RAVF color doppler ultrasound reveals abnormal blood flow signals with high speed and turbulence in tortuous and dilated blood vessels. RAVF spectral doppler of blood flow has the characteristics of high speed, low resistance, burrs, and no empty window. This is because the arterial blood flow is shunted through the fistula and directly into the vein, which results in arterialization of venous blood flow.
An arterial-like flow spectrum is detected in the draining vein, which is a characteristic manifestation of ultrasound in diagnosing RAVF[10]. In this case, the patient’s 2D ultrasound images, color doppler, and spectral doppler ultrasound features were consistent with the ultrasound manifestations of RAVF, which supported the diagnosis of left RAVF. However, ultrasound revealed only one more obvious fistula on the outside of one kidney, without revealing the exact size of the fistula within the intrarenal pedunculated vascular mass. Notably, this patient had not only RAVF but also renal cysts in the left kidney. When the 2D ultrasound examination revealed several anechoic areas within or around the kidney, it was impossible to consider it a simple renal cyst based on conventional thinking. In such cases, color doppler flow should be activated. When blood flow signals are found in anechoic areas, spectral doppler should be used to observe the blood flow spectral characteristics to distinguish it from renal cysts. Color doppler ultrasound can easily distinguish RAVF from renal cysts. For anechoic lesions near the renal hilum in the central region of the kidney, especially small asymptomatic lesions, ultrasound may present a risk of missed diagnosis. Second, for patients with hematuria, in addition to considering common stones and tumors, ultrasound physicians should also consider renal vascular abnormalities as one of the rare causes of hematuria. In particular, when 2D sonography shows cystic lesions in the kidney, CDFI should be initiated to identify vascular abnormalities. The hematuria in this patient was caused by left RAVF, not by a left renal cyst.
Ultrasonic diagnosis of RAVF depends on the ultrasonic instrument and the expertise of the ultrasonic doctor. However, CT imaging is more objective and provides images that can be viewed directly from a computer or film. This is conducive to allowing multiple doctors to discuss the case simultaneously. Plain CT is insufficient for diagnosing RAVF, but enhanced CT can detect RAVF well[11]. Enhanced CT is to observe the changes of strengthened blood supply of the lesions, more objectively understand the location of the lesion and its anatomic relationship with surrounding tissues and organs, and exclude and identify RAVF caused by other space-occupying lesions. In this case, the feeding arteries and draining veins of RAVF could be observed with enhanced CT, thus inferring several obvious fistula locations, but smaller fistulas could not be clearly identified. With the continuous advancements in CT technology, especially the wide app
From an objective point of view, enhanced CT and 3D-CTA are superior to color ultrasound, but they complement each other in the diagnosis of RAVF, providing richer reference information for renal arteriography. DSA can identify small RAVFs, compensating for the shortcomings of ultrasound, enhanced CT, and 3D-CTA. Renal arteriography remains the gold standard for diagnosing RAVF, as it can clarify the shape, course, distribution, and pathological changes of blood vessels. Compared with CT and 3D-CTA, DSA allows real-time dynamic viewing. In particular, with DSA technology, the images are clearer, small arteriovenous fistulas can be detected, and RAVF can be treated during diagnosis[15]. Therefore, despite the high cost, the capabilities are still irreplaceable. According to the performance characteristics of renal artery DSA, RAVFs are classified into varicose and aneurysm-like types. Studies report that congenital RAVFs are primarily of the varicose type and partly of the aneurysm-like type[16]. Typical angiographic manifestations of congenital RAVF include dilatation and distortion of small arteries, dilatation and thickening of draining veins, and simultaneous visualization of the draining veins with the blood-supplying arteries; the inferior vena cava may also be visualized earlier. Multiple arteriovenous fistulas are often present in congenital RAVFs[17]. In this case, the DSA features were consistent with a varicose vein type of congenital RAVF.
The treatment principles for congenital RAVF include relieving symptoms, minimizing renal parenchymal impairment, and preventing and ameliorating a range of associated complications caused by vascular malformations[18]. A smaller RAVF without clinical symptoms can be managed through observation and follow-up, while RAVFs with clinical symp
In summary, the patient was diagnosed with a left RAVF using combined multimodal imaging techniques, including ultrasound, enhanced CT, 3D-CTA, and DSA. These techniques complement each other in diagnosing RAVF and can provide more detailed diagnostic information, assisting doctors in diagnosing and treating RAVF more accurately. In addition, this case reminds the sonographer to pay more attention to the CDFI and blood flow spectrum when examining the kidney, so as to avoid misdiagnosis of renal cystic lesions as renal cysts and missed diagnosis of RAVF.
We thank the patient reported in this paper, and her family for the assistance.
| 1. | Jia ZY, Zhou CG, Xia JG, Zhao LB, Zhang W, Liu S, Shi HB. Endovascular Treatment of 12 Cases of Renal Arteriovenous Malformations: The Experience of 1 Center and an Overview of the Literature. Vasc Endovascular Surg. 2018;52:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Zhou F, Cui Y, Zhao Q, Xu H, Zu M, Xu W. Hypertension Caused by Renal Arteriovenous Fistula with Multiple Renal Artery Aneurysms. Ann Vasc Surg. 2021;70:565.e11-565.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Khawaja AT, McLean GK, Srinivasan V. Successful intervention for high-output cardiac failure caused by massive renal arteriovenous fistula-a case report. Angiology. 2004;55:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cai J, Ding L, Xie Y, Wang Y. Congenital renal arteriovenous malformation with cirsoid and cavernosal-type characteristics: a case report. J Int Med Res. 2021;49:3000605211016381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Banthia R, Kumar A, Prasad R, Lal H. Congenital renal arteriovenous malformation: a rare cause of visible haematuria. BMJ Case Rep. 2021;14:e242347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Cho KJ, Stanley JC. Non-neoplastic congenital and acquired renal arteriovenous malformations and fistulas. Radiology. 1978;129:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Melo NC, Mundim JS, Costalonga EC, Lucon AM, Santello JL, Praxedes JN. Three cases of hypertension and renal arteriovenous fistula with a de novo fistula. Arq Bras Cardiol. 2009;92:e36-e38, e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Albak LJ, Shah AH, Tam JW. Cardiac failure and pulmonary hypertension secondary to renal arteriovenous malformation: a case report. J Med Case Rep. 2021;15:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Wang X, Zhao Z. Renal arteriovenous malformation causing hematuria: Case report and review of the literature. Medicine (Baltimore). 2023;102:e34547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Takebayashi S, Aida N, Matsui K. Arteriovenous malformations of the kidneys: diagnosis and follow-up with color Doppler sonography in six patients. AJR Am J Roentgenol. 1991;157:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Honda H, Onitsuka H, Naitou S, Hasuo K, Kamoi I, Hanada K, Kumazawa J, Masuda K. Renal arteriovenous malformations: CT features. J Comput Assist Tomogr. 1991;15:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Abdel-Gawad EA, Housseini AM, Cherry KJ, Bonatti H, Maged IM, Norton PT, Hagspiel KD. CT angiography of renal arteriovenous fistulae: a report of two cases. Vasc Endovascular Surg. 2009;43:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ishikawa T, Fujisawa M, Kawabata G, Kamidono S. Assessment of availability of magnetic resonance angiography (MRA) in renal arteriovenous fistula. Urol Res. 2004;32:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Bagga H, Bis KG. Contrast-enhanced MR angiography in the assessment of arteriovenous fistula after renal transplant biopsy. AJR Am J Roentgenol. 1999;172:1509-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Choi SK, Min GE, Lee DG. Congenital Renal Arteriovenous Malformation: Diagnostic Clues and Methods. Medicina (Kaunas). 2021;57:1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 16. | King BF, Hattery RR. Case of the day. Ultrasound. Congenital cirsoid renal arteriovenous malformation (AVM) involving the lower pole of the right kidney. Radiographics. 1990;10:1101-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Murata S, Onozawa S, Nakazawa K, Akiba A, Mine T, Ueda T, Yasui D, Sugihara F, Kondoh Y, Kumita S. Endovascular embolization strategy for renal arteriovenous malformations. Acta Radiol. 2014;55:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Kuklik E, Sojka M, Karska K, Szajner M. Endovascular Treatment of Renal Arteriovenous Fistula with N-Butyl Cyanoacrylate (NBCA). Pol J Radiol. 2017;82:304-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zhang Z, Yang M, Song L, Tong X, Zou Y. Endovascular treatment of renal artery aneurysms and renal arteriovenous fistulas. J Vasc Surg. 2013;57:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Hongsakul K, Bannangkoon K, Boonsrirat U, Kritpracha B. Transarterial Embolization of a Renal Artery Aneurysm Concomitant With Renal Arteriovenous Fistula. Vasc Endovascular Surg. 2018;52:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Oyama M, Tamura H, Hidaka Y, Furuie K, Kuraoka S. Renal arteriovenous fistula discovered ~2 years after renal biopsy: A case report. Clin Case Rep. 2023;11:e7538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Duc VT, Duong N, Phong NT, Nam NH, Quoc DA, Cuong T, Huy NH, Duy TL, Chien PC. Large renal arteriovenous fistula treated by embolization: a case report. Radiol Case Rep. 2021;16:2289-2294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |