Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.103642
Revised: January 28, 2025
Accepted: March 27, 2025
Published online: July 26, 2025
Processing time: 152 Days and 16.4 Hours
Hypophosphatasia (HPP) is a rare metabolic disorder caused by low tissue-nonspecific alkaline phosphatase (ALP) activity, presenting symptoms from bone demineralization to tooth loss. It affects multiple systems and is diagnosed based on clinical symptoms, radiological findings, and lab tests. This case report empha
We present a case of a 65-year-old female patient who was referred to our endoc
Recognizing HPP is crucial, as early diagnosis and treatment can significantly improve patient outcomes and prevent complications.
Core Tip: This case report highlights the importance of considering hypophosphatasia (HPP) in patients with unexplained bone pain and low alkaline phosphatase levels, especially in the presence of osteopenia or osteoporosis. It underscores the role of genetic testing and counseling in diagnosing and managing HPP, emphasizing early identification and treatment to improve patient outcomes. The report also illustrates how HPP can explain multiple symptoms often misdiagnosed as conditions like fibromyalgia. Additionally, it aims to raise awareness about enzyme replacement therapies that can significantly improve quality of life and reduce complications for HPP patients.
- Citation: Gill AS, Sharma P, Nassar M, Marte E. Hypophosphatasia: A case report. World J Clin Cases 2025; 13(21): 103642
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/103642.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.103642
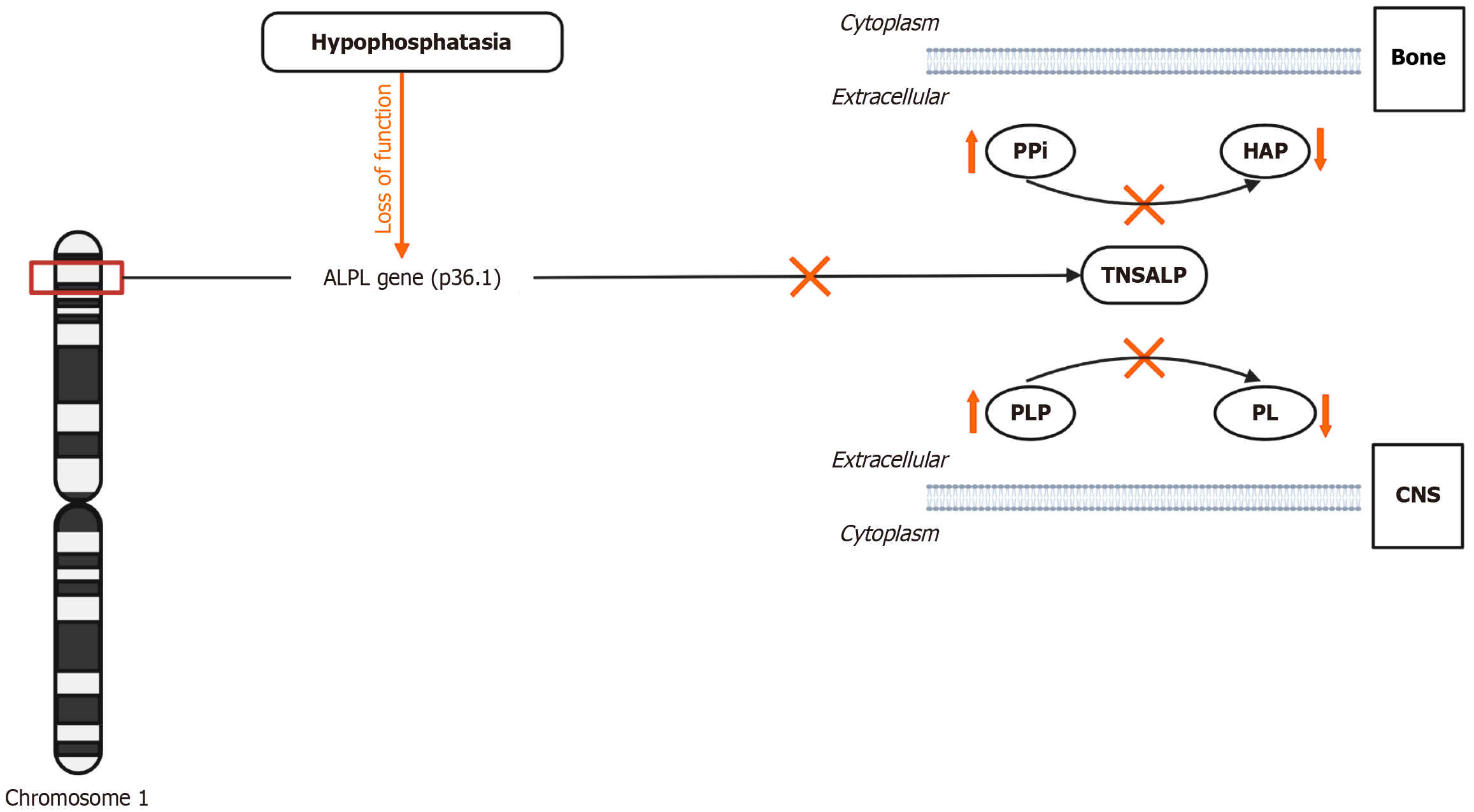
Hypophosphatasia (HPP) is an inborn error of metabolism caused by low activity of tissue-nonspecific alkaline phosphatase (TNSALP) (Figure 1). HPP prevalence varies globally, with Canada having the highest rate[1]. Severe forms of the disorder occur in approximately 1 in 100000 to 1 in 300000 live births in France and Northern Europe[2,3]. In contrast, moderate forms, including odontohypophosphatasia (odonto-HPP) and adult-onset variants, are more common, with an estimated prevalence of 1 in 6370 in European populations[2]. In the United States, around 500–600 individuals are clinically diagnosed with HPP; however, the actual prevalence is likely higher as milder forms are often underdiagnosed[4]. Over 400 ALPL gene variants cause HPP, with symptoms ranging from severe bone demineralization to tooth loss depending on the degree of suppression of the TNSALP enzyme[1,5]. While earlier studies have established the enzymatic and genetic underpinnings of HPP, recent advancements have focused on improving diagnostic accuracy and expanding therapeutic options[6,7].
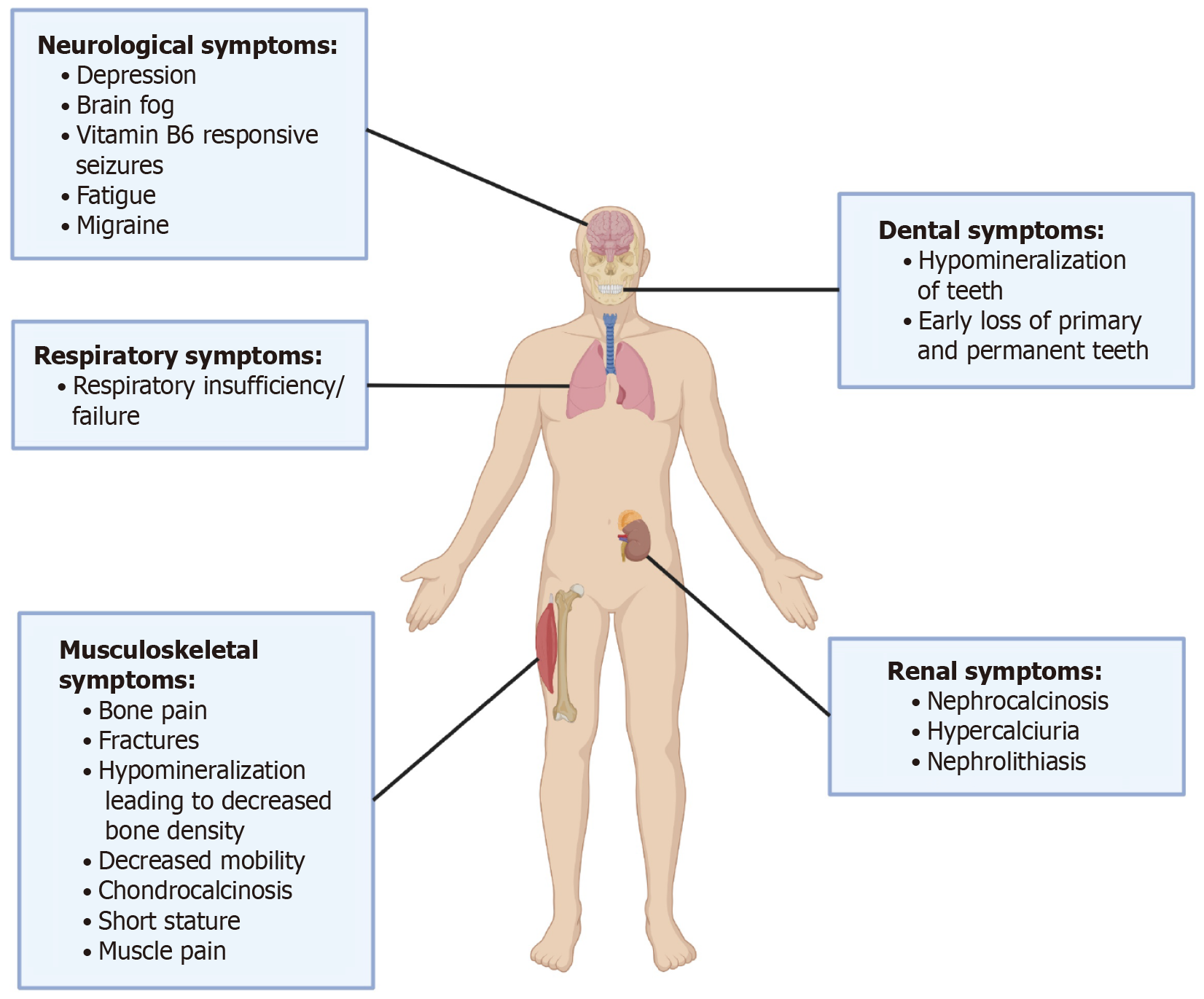
HPP manifests in diverse forms, each with distinct symptoms and severity (Figure 2). Perinatal (lethal) HPP, the most severe, causes stillbirth or early infant death due to severe skeletal hypomineralization and respiratory failure[2]. Perinatal benign HPP, while initially similar, generally improves over time without enduring complications[8]. Infantile HPP manifests in the first six months with severe rickets, respiratory issues, and failure to thrive, often requiring urgent treatment[9]. Childhood HPP causes short stature, bone pain, fractures, and early tooth loss, with varying degrees of severity[8]. Adult HPP typically remains undetected until later life, characterized by stress and recurrent fractures, osteomalacia, and chronic pain[4]. The mildest variant, odonto-HPP, mainly impacts dental health, leading to premature tooth loss without significant skeletal issues[10]. Recent studies continue to refine our understanding of the clinical presentation and management of HPP in diverse populations[6,11].
In addition to the musculoskeletal system, HPP does affect multiple other organ systems of the body. HPP can cause renal issues like nephrocalcinosis and impair kidney function. Altered vitamin B6 metabolism may cause neurological symptoms like seizures in infants and neuropsychiatric symptoms in adults[5]. A combination of foundational research and recent advancements has improved the clinical and diagnostic criteria for HPP, with newer genetic insights broadening our understanding of its variability[7,12].
The diagnosis of HPP is based on clinical symptoms, radiological findings, and laboratory tests, including low serum ALP activity and elevated plasma vitamin B6 and urinary phosphoethanolamine (PEA) levels.
Genetic testing is crucial for confirmation and family counseling. Radiographic abnormalities in HPP include flared metaphyses, bone hypomineralization, and pseudofractures[5,7]. Enzyme replacement therapy is the primary treatment for HPP, improving bone health and reducing complications. Off-label treatments like teriparatide and anti-sclerostin antibodies have shown promise in adults with HPP[7,11]. Here we present an interesting case of an adult female diagnosed with HPP in her 60s.
A 65-year-old female presented with generalized bone pain, a history of osteopenia, and hypothyroidism.
The patient was referred to our endocrinology office by her primary care provider due to a history of osteopenia and hypothyroidism. During her initial evaluation, a Dual X-ray Absorptiometry (DXA) scan revealed osteopenia, with a Fracture Risk Assessment (FRAX) score that did not necessitate immediate treatment. She was managing her condition conservatively with calcium and vitamin D supplementation. During follow-up visits, the patient reported experiencing generalized bone pain.
The patient had a history of scoliosis, brain fog, dental issues including caries and cavities, and premature loss of most of her maxillary teeth in her 30 s. She also had a history of bronchitis as a child, muscle weakness, fatigue, and muscle and joint pain since before the age of 18. Diagnosed with fibromyalgia at around 13 years old, she reported persistent fatigue. She had undergone multiple surgeries for bilateral carpal tunnel syndrome.
The patient consumed alcohol socially and had smoked tobacco in her early 20 s, about 5-6 cigarettes a day for 7-8 years. Her mother had lost all her teeth by the age of 20. The patient herself had a history of dental issues throughout her life.
Upon physical examination, the patient appeared well-nourished and in no acute distress. Her vital signs were stable. Musculoskeletal examination revealed generalized tenderness over the bones, particularly in the lower extremities. A dental examination showed multiple missing teeth and poor dental health.
Laboratory tests showed a low ALP level of 36 U/L (normal range: 37–153), prompting further investigation for HPP. Repeat tests confirmed persistently low ALP at 35 U/L, with bone isoenzymes at 59% (28–66) and liver isoenzymes at 41% (25–69). Additionally, her vitamin B6 levels were elevated at 69.9 ng/mL (2.1–21.7), and her phosphate level was high at 5.1 mg/dL (2.1–4.3). Her calcium, vitamin D 25-hydroxy, and Parathyroid Hormone (PTH) levels were within normal ranges.
DXA scan: Revealed osteopenia with the lowest T-Score of -2.4 in her right proximal femur. The FRAX score indicated a 10.8% probability of a major osteoporotic fracture and a 1.7% probability of a hip fracture within the next 10 years.
Genetic testing: Identified a pathogenic variant in the ALPL gene: c.119C>T (p.Ala40Val), heterozygous.
The patient was diagnosed with HPP based on clinical symptoms, laboratory findings, and genetic testing.
Plans are underway to initiate enzyme replacement therapy with asfotase alfa to improve bone health and reduce fracture risk.
Schedule to follow up after starting enzyme replacement therapy with asfotase alfa.
ALP is crucial for bone mineralization, vitamin B6 metabolism, and tooth development. ALP dephosphorylates inorganic phosphate (PPi), an inhibitor of hydroxyapatite formation, promoting calcium and phosphate deposition in the extracellular matrix for proper bone mineralization[2]. ALP converts pyridoxal 5'-phosphate (PLP), the active form of vitamin B6, into pyridoxal, allowing cellular uptake and utilization in metabolic and neurological functions[3]. ALP is also vital in dentin mineralization in teeth, where its deficiency can lead to defective cementum formation and subsequent premature tooth loss[10].
HPP is a rare genetic disorder arising from mutations in the ALPL gene, which encodes the enzyme TNSALP. These mutations impair the enzyme's ability to function properly, leading to decreased activity and subsequent substrate accumulation. TNSALP is essential for dephosphorylating various substrates including PPi, PLP, and PEA, with its deficiency resulting in their buildup in tissues and serum, just like the elevation of vitamin B6 in our case (Figure 1). This accumulation causes several pathophysiological consequences: PPi, a natural inhibitor of hydroxyapatite formation, accumulates and disrupts bone mineralization, leading to conditions such as rickets in children and osteomalacia in adults[2,13]. Our patient has had osteopenia for several years, and may be related to this. Elevated levels of PLP, the active form of vitamin B6, can lead to neurological issues like seizures due to its failure to be dephosphorylated by TNSALP[3]. Although our patient did not have severe neurological symptoms, but she was diagnosed with fibromyalgia given her prolonged fatigue, and did report having brain fog since her childhood. Additionally, the disrupted breakdown of PPi severely affects bone and tooth mineralization, resulting in skeletal hypomineralization and dental findings like premature tooth loss and defects in dentin and enamel[10]. This was reflected in our patient losing all her maxillary teeth in her 30s and further her mother losing all her teeth. The severity of HPP symptoms varies according to the specific ALPL mutations, with homozygous or compound heterozygous mutations typically presenting severe, early-onset forms of the disease, while heterozygous mutations are associated with milder, adult-onset forms or (odonto-HPP)[8]. This may explain why our patient having heterozygous mutation had milder symptoms.
Treatment options for HPP are diverse, addressing both the underlying causes and the symptoms of the disease. A key treatment is enzyme replacement therapy, specifically using asfotase alfa (Strensiq™). This recombinant, bone-targeted form of TNSALP is crucial for patients, especially those with perinatal and infantile forms of HPP, as it has significantly improved outcomes in skeletal mineralization, pain reduction, overall survival and quality of life[13,14]. It is also approved for use in juvenile-onset and adult HPP patients, where it effectively manages symptoms such as fractures, pain, and impaired mobility, underscoring its broad applicability across different age groups and severities of the disease. Our patient is being planned for treatment with asfotase alfa and we hope to help her improve her symptoms and her quality of life. Another experimental treatment is bone marrow transplantation, which has shown promise in providing donor osteoblasts that improve bone mineralization, particularly in severe infantile HPP[9].
Gene therapy, involving techniques like lentivirally transduced bone marrow cells to deliver functional TNSALP, represents a potential future treatment option[15]. Supportive treatments include pain management with nonsteroidal anti-inflammatory drugs (NSAIDs) for chronic bone and muscle pain, and dietary adjustments like calcium supplementation and low-phosphate diets to manage metabolic imbalances[16,17]. Physical therapy is also recommended to enhance mobility and address musculoskeletal issues, particularly in children with delayed motor skills due to HPP[18). Additionally, ongoing experimental research, such as trials exploring modified TNSALP molecules for better skeletal targeting, continues to push the boundaries of treatment possibilities[19].
Recent clinical trials and studies over the last five years have expanded the understanding and treatment of HPP through various innovative approaches. Notable among these is a 7-year follow-up study on asfotase alfa, which has demonstrated sustained improvements in skeletal mineralization, respiratory function, growth, and cognitive development in infants and young children with life-threatening perinatal or infantile HPP, with most adverse events being mild to moderate[6]. Another study focused on the therapeutic efficacy of asfotase alfa in pediatric-onset HPP, revealing significant advancements in bone mineralization, motor function, and survival rates in severe cases[20]. The anti-sclerostin monoclonal antibody BPS804 was examined in a Phase 2 trial, showing good tolerance and promising increases in bone formation biomarkers and bone mineral density among adults with HPP[21]. Additionally, bone marrow transplantation has been explored experimentally in infants with severe HPP, indicating potential benefits in skeletal mineralization, though it faces challenges like immune compatibility and graft failure[9]. Furthermore, a 5-year study on the use of asfotase alfa in adults and adolescents reported significant improvements in functional abilities, normalization of TNSALP substrates, and enhanced quality of life[22]. Lastly, a retrospective study assessing the natural progression of untreated perinatal and infantile HPP highlighted the high mortality rates without intervention and identified vitamin B6-dependent seizures as a critical prognostic marker[6]. These studies collectively underscore the ongoing efforts and progress in combating this challenging condition.
Novel therapies and off-label treatments for HPP are expanding, offering new hope for managing this challenging condition. Gene therapy shows promise, with ARU-2801, a novel agent targeting the ALPL gene, demonstrating sustained enzyme activity and safety in non-human primate studies, suggesting it could be a single-dose solution for HPP[23]. Additionally, in-utero gene therapy using adeno-associated virus to deliver TNSALP has been effective in murine models, potentially offering early intervention for severe cases[24]. Bone marrow transplantation has been explored experimentally in infants with severe HPP, where transplanted mesenchymal stem cells or osteoblasts have improved skeletal mineralization[9]. Teriparatide, an off-label use of recombinant PTH 1-34, has benefited adults with HPP, particularly those previously on bisphosphonates, by enhancing bone formation and reducing fractures[23]. NSAIDs have been crucial in managing severe limb pain and enhancing the quality of life in pediatric HPP patients[16]. Lastly, advances in enzyme replacement therapy, like enhanced formulations of asfotase alfa and ex vivo lentiviral transduction therapy, have shown significant improvements in bone mineralization and survival in experimental models[14,15].
This case highlights the importance of considering HPP in patients with unexplained bone pain and low ALP levels, especially in the presence of skeletal pathology like osteopenia or osteoporosis. The patient's history of dental issues, including early tooth loss, and family history of similar dental problems were crucial in guiding the diagnosis. Genetic testing confirmed the presence of a pathogenic ALPL gene variant, underscoring the role of genetic counseling in managing HPP. Early identification and appropriate treatment, such as enzyme replacement therapy with asfotase alfa, are essential to improve outcomes and prevent complications in HPP patients. This case emphasizes the need for a multidisciplinary approach to effectively diagnose and manage this rare metabolic disorder.
| 1. | Bertoldo F, Tripepi G, Zaninotto M, Plebani M, Scillitani A, Varenna M, Crotti C, Cipriani C, Pepe J, Minisola S, Pugliese F, Guarnieri V, Baffa V, Torres MO, Zanchetta F, Fusaro M, Rossini M, Brandi ML, Egan CG, Simioni P, Arcidiacono GP, Sella S, Giannini S. Possible role of bone turnover markers in the diagnosis of adult hypophosphatasia. J Bone Miner Res. 2024;40:79-86. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Mornet E. Genetics of hypophosphatasia. Arch Pediatr. 2017;24:5S51-5S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Shapiro JR, Lewiecki EM. Hypophosphatasia in Adults: Clinical Assessment and Treatment Considerations. J Bone Miner Res. 2017;32:1977-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Khan AA, Brandi ML, Rush ET, Ali DS, Al-Alwani H, Almonaei K, Alsarraf F, Bacrot S, Dahir KM, Dandurand K, Deal C, Ferrari SL, Giusti F, Guyatt G, Hatcher E, Ing SW, Javaid MK, Khan S, Kocijan R, Linglart A, M'Hiri I, Marini F, Nunes ME, Rockman-Greenberg C, Roux C, Seefried L, Simmons JH, Starling SR, Ward LM, Yao L, Brignardello-Petersen R, Lewiecki EM. Hypophosphatasia diagnosis: current state of the art and proposed diagnostic criteria for children and adults. Osteoporos Int. 2024;35:431-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 6. | Whyte MP, Simmons JH, Moseley S, Fujita KP, Bishop N, Salman NJ, Taylor J, Phillips D, McGinn M, McAlister WH. Asfotase alfa for infants and young children with hypophosphatasia: 7 year outcomes of a single-arm, open-label, phase 2 extension trial. Lancet Diabetes Endocrinol. 2019;7:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Reis FS, Lazaretti-Castro M. Hypophosphatasia: from birth to adulthood. Arch Endocrinol Metab. 2023;67:e000626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Whyte MP, Zhang F, Wenkert D, McAlister WH, Mack KE, Benigno MC, Coburn SP, Wagy S, Griffin DM, Ericson KL, Mumm S. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone. 2015;75:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 9. | Cahill RA, Wenkert D, Perlman SA, Steele A, Coburn SP, McAlister WH, Mumm S, Whyte MP. Infantile hypophosphatasia: transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab. 2007;92:2923-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Bloch-Zupan A. Hypophosphatasia: diagnosis and clinical signs - a dental surgeon perspective. Int J Paediatr Dent. 2016;26:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Shirinezhad A, Esmaeili S, Azarboo A, Tavakoli Y, Hoveidaei AH, Zareshahi N, Ghaseminejad-Raeini A. Efficacy and safety of asfotase alfa in patients with hypophosphatasia: A systematic review. Bone. 2024;188:117219. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Seefried L, Kishnani PS, Moseley S, Denker AE, Watsky E, Whyte MP, Dahir KM. Pharmacodynamics of asfotase alfa in adults with pediatric-onset hypophosphatasia. Bone. 2021;142:115664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, Van Sickle BJ, Simmons JH, Edgar TS, Bauer ML, Hamdan MA, Bishop N, Lutz RE, McGinn M, Craig S, Moore JN, Taylor JW, Cleveland RH, Cranley WR, Lim R, Thacher TD, Mayhew JE, Downs M, Millán JL, Skrinar AM, Crine P, Landy H. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Whyte MP, Rockman-Greenberg C, Ozono K, Riese R, Moseley S, Melian A, Thompson DD, Bishop N, Hofmann C. Asfotase Alfa Treatment Improves Survival for Perinatal and Infantile Hypophosphatasia. J Clin Endocrinol Metab. 2016;101:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Iijima O, Miyake K, Watanabe A, Miyake N, Igarashi T, Kanokoda C, Nakamura-Takahashi A, Kinoshita H, Noguchi T, Abe S, Narisawa S, Millán JL, Okada T, Shimada T. Prevention of Lethal Murine Hypophosphatasia by Neonatal Ex Vivo Gene Therapy Using Lentivirally Transduced Bone Marrow Cells. Hum Gene Ther. 2015;26:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Girschick HJ, Seyberth HW, Huppertz HI. Treatment of childhood hypophosphatasia with nonsteroidal antiinflammatory drugs. Bone. 1999;25:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Bianchi ML. Hypophosphatasia: an overview of the disease and its treatment. Osteoporos Int. 2015;26:2743-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Phillips D, Case LE, Griffin D, Hamilton K, Lara SL, Leiro B, Monfreda J, Westlake E, Kishnani PS. Physical therapy management of infants and children with hypophosphatasia. Mol Genet Metab. 2016;119:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Hofmann C, Jakob F, Seefried L, Mentrup B, Graser S, Plotkin H, Girschick HJ, Liese J. Recombinant Enzyme Replacement Therapy in Hypophosphatasia. Subcell Biochem. 2015;76:323-341. [PubMed] [DOI] [Full Text] |
| 20. | Bowden SA, Foster BL. Profile of asfotase alfa in the treatment of hypophosphatasia: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:3147-3161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Seefried L, Baumann J, Hemsley S, Hofmann C, Kunstmann E, Kiese B, Huang Y, Chivers S, Valentin MA, Borah B, Roubenoff R, Junker U, Jakob F. Efficacy of anti-sclerostin monoclonal antibody BPS804 in adult patients with hypophosphatasia. J Clin Invest. 2017;127:2148-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Kishnani PS, Rockman-Greenberg C, Rauch F, Bhatti MT, Moseley S, Denker AE, Watsky E, Whyte MP. Five-year efficacy and safety of asfotase alfa therapy for adults and adolescents with hypophosphatasia. Bone. 2019;121:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 23. | Doshi KB, Hamrahian AH, Licata AA. Teriparatide treatment in adult hypophosphatasia in a patient exposed to bisphosphonate: a case report. Clin Cases Miner Bone Metab. 2009;6:266-269. [PubMed] [DOI] [Full Text] |
| 24. | Sugano H, Matsumoto T, Miyake K, Watanabe A, Iijima O, Migita M, Narisawa S, Millán JL, Fukunaga Y, Shimada T. Successful gene therapy in utero for lethal murine hypophosphatasia. Hum Gene Ther. 2012;23:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |