Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.103105
Revised: March 12, 2025
Accepted: April 9, 2025
Published online: July 26, 2025
Processing time: 169 Days and 17.1 Hours
Diagnosing posterior inferior cerebellar artery dissection (PICAD) using radio
A 39-year-old man was admitted for dizziness and unstable gait for two days. Ph
Since MRA and CTA may fail to identify PICAD, HR-VW-MRI is key in diagnosis and follow-up evaluation. Aggressive medication may be effective and safe for treating PICAD.
Core Tip: Diagnosis of posterior inferior cerebellar artery dissection (PICAD) can be challenging with conventional imaging methods like magnetic resonance angiography or computed tomographic angiography. High-resolution vessel wall magnetic resonance imaging is crucial for accurate diagnosis and follow-up. Aggressive conservative treatment may be effective and safe for managing PICAD, with significant recovery observed in the patient within 3 months.
- Citation: Huang XM, Liao YQ, Cao LM. Massive cerebellar infarction caused by spontaneously isolated posterior inferior cerebellar artery dissection: A case report. World J Clin Cases 2025; 13(21): 103105
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/103105.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.103105
Cerebral artery dissection (AD) refers to a condition that causes blood to enter the blood vessel wall through a damaged tunica intima, resulting in dissection and intramural hematomas, arterial stenosis or occlusion, and even false aneurysm[1]. Cerebral AD can be spontaneous or traumatic. Neck trauma, yoga, severe cough, cervical massage[2], and golf can cause traumatic cerebral AD[3]. Spontaneous AD may be associated with congenital vascular wall structural abnor
A 39-year-old man was admitted for dizziness and an unstable gait, which had been persisting for two days, and right limb weakness that was first noticed 10 hours prior in June 2021.
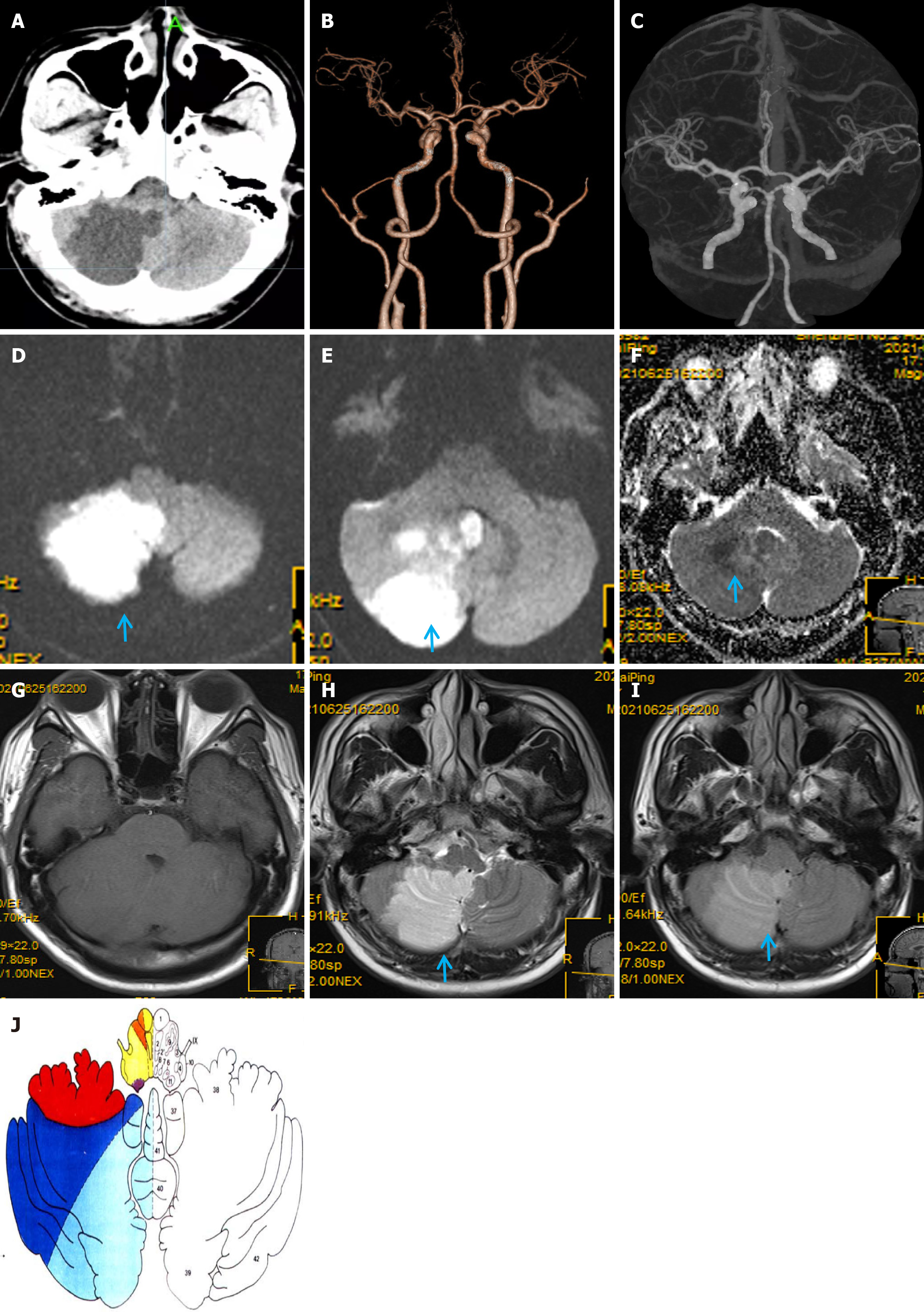
Massive right cerebellar infarction was observed on the head computed tomography (CT; Figure 1A) with no obvious abnormalities on craniocervical CT angiography (CTA; Figure 1B and C).
The patient had a history of alcoholic liver disease and smoking (averaging 10 cigarettes per day), but had no history of significant alcohol consumption.
Additionally, he had no history of hereditary disease.
Physical examination revealed decreased muscle strength in the right limb (5-/5), a positive finger-to-nose test, rapid alternating hand movement, heel-knee-tibia test on the right side, and Romberg’s sign.
Laboratory analysis showed weakly positive fecal occult blood test results and elevated levels of gamma-glutamyl transpeptidase (138 U/L), ferritin (380.0 ng/mL), and protein C (131.00%).
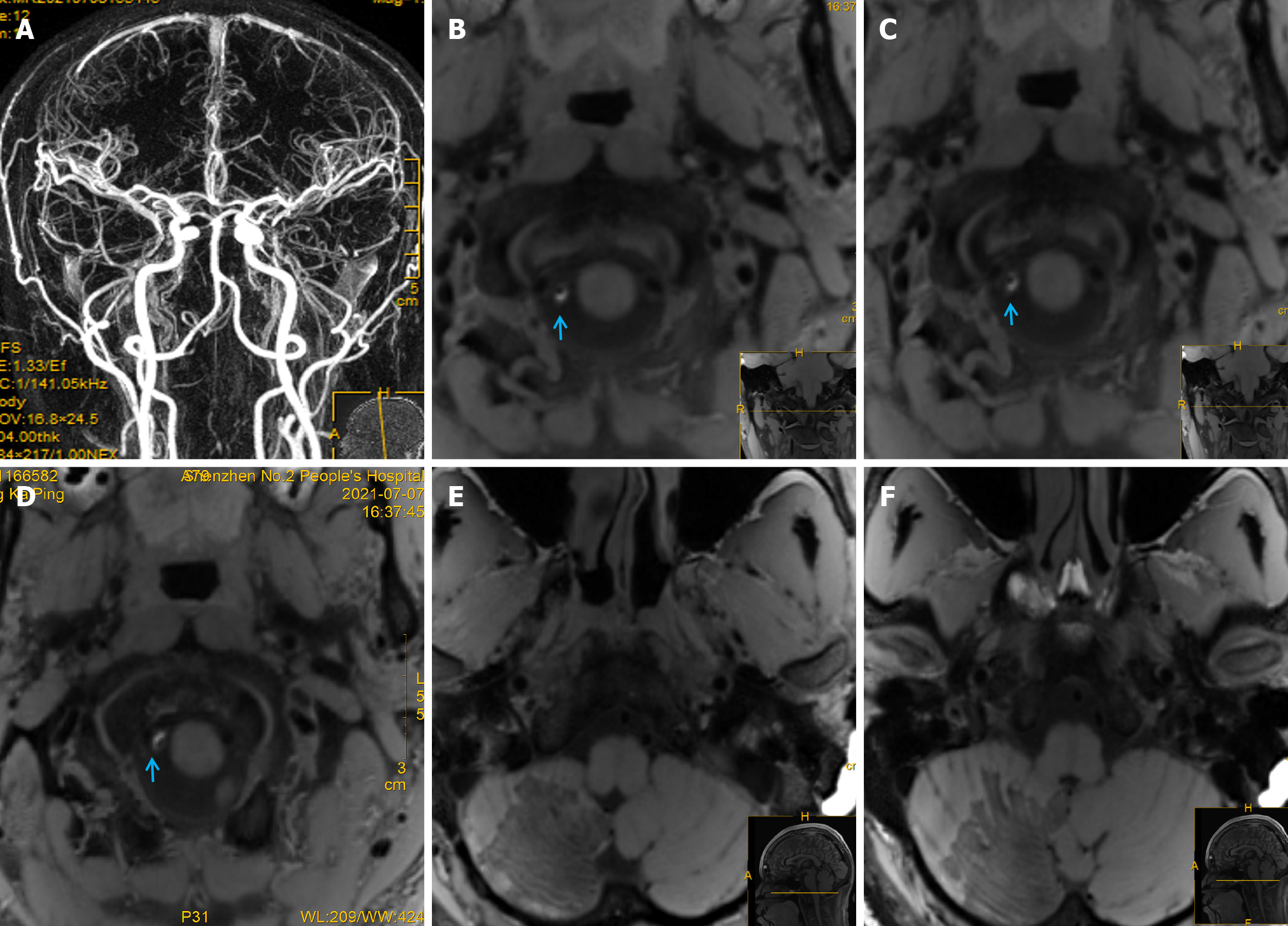
Chest CT showed scattered exudative lesions in both lungs, and bilateral lower lobes were clearly visible. Abdominal CT showed mild fatty liver, and echocardiography, contrast transcranial Doppler, contrast echocardiography, and 24-hour dynamic electrocardiography revealed obvious abnormalities. Furthermore, 1.5-T magnetic resonance imaging (MRI) showed a massive acute right cerebellar infarction (Figure 1D-I) in the area supplied by the PICA (Figure 1J)[7], with no obvious abnormalities on gadolinium-contrast magnetic resonance angiography (MRA) (Figure 2A). Moreover, 3.0-T high-resolution vessel wall (HR-VW) MRI showed right PICAD (Figure 2B-D).
A massive cerebellar infarction caused by spontaneously isolated PICAD.
Active conservative treatment was initiated after surgery was ruled out, which included the administration of intravenous Ginkgo biloba extract (GBE). Despite slight weakness, the patient was discharged on day 16 with clopidogrel 75 mg/day for 14 days, GBE tablets for one month, aspirin 0.1 g/day for 1.5 months, and citicoline capsules and atorvastatin for 1.5 months.
A follow-up HR-VW MRI at 1.5 months showed that the initial PICAD had disappeared (Figure 2E and F). At the three-month follow-up, the patient experienced slight weakness in the right limb while walking quickly.
Here, we report a rare case of a patient with a massive cerebellar infarction caused by spontaneously isolated PICAD. There were no obvious precipitating factors such as trauma, neck massage, or vigorous exercise, and no warning signs of AD such as head or neck pain. Notably, PICAD-related stroke is difficult to diagnose and can easily be misdiagnosed using conventional MRA or CTA.
Occipital headache, headache, or nuchal rigidity are common warning signs of dissection[2,8-11]; however, approximately 20% of patients have no warning symptoms before stroke[1]. Our patient experienced sudden vertigo, which was possibly related to posterior circulation ischemia or cerebellar infarction. PICAD can manifest with other symptoms, such as nausea, vomiting, confusion[2], seizure[10], postural imbalance, and Wallenberg syndrome[12], depending on the extent and location of the infarction or hemorrhage. The clinical manifestations of spontaneously isolated PICAD are similar to those of intracranial vertebral AD. The clinical course of PICAD is relatively stable and benign[12]. Only a few reports of isolated PICAD have been published (Table 1). Most cases of PICAD present with secondary SAH or intraventricular hemorrhage[2,8,10]; there are few reports of ischemic stroke in cases of PICAD[10,11], and fewer cases of massive cerebellar infarction. Edema secondary to massive cerebellar infarction can compress the fourth ventricle and cause acute obstructive hydrocephalus, resulting in intracranial hypertension, cerebral hernia, and even death. Effective and timely treatment can help control these symptoms.
| Patient | Age (years) and sex | Predisposing causes and main symptoms | Methods of diagnostic imaging | Types of stroke caused by PICAD | Main treatment | Outcome and follow-up |
| Patient 1[2] | 40-year-old woman | Sudden headache during sexual intercourse, followed by a loss of consciousness | DSA revealed right PICAD | CT showed SAH | Endovascular treatment | No neurological symptoms at discharge; 3-year DSA showed complete occlusion of the AD |
| Patient 2[2] | 66-year-old man | History of hypertension and development of a severe headache accompanied by vomiting and confusion | DSA demonstrated right PICAD | CT showed diffuse SAH with ventricular enlargement | Injection of a mixture of histoacryl and lipiodol | Neurological examination 3 years later was normal, and DSA showed that the AD was completely occluded |
| Patient 3[2] | 46-year-old woman | Vertigo and diplopia after a cervical manipulation, followed by a headache with nuchal rigidity | DSA showed right PICAD | CT and MRI revealed an SAH with cerebral ischemia in the territory of the PICA | Non-surgical treatment | Diplopia at discharge. DSA at 4 months displayed unchanged PICAD. No further symptoms observed at the 2-year follow-up |
| Patient 4[2] | 71-year-old woman | Sudden unconsciousness | DSA revealed left PICAD | CT showed SAH and intraventricular hemorrhage | Occlusion of the pseudoaneurysm was performed with coils | Intense weakness, diplopia, and cognitive impairment 3 years after therapy |
| Patient 5[2] | 42-year-old woman | Intense posterior headache, dizziness, and vomiting after cervical manipulation | DSA revealed right PICAD | CT revealed right cerebellar infarction | Intravenous heparin for 2 weeks | Clinical state improved considerably. The patient was free of symptoms after 2 years |
| Patient 6[8] | 63-year-old man | Severe acute occipital headaches and vomiting | DSA revealed left PICAD | CT showed a massive SAH in the posterior fossa | Aneurysmorrhaphy and a wrapping procedure | Symptoms resolved after surgery |
| Patient 7[8] | 28-year-old man | Sudden onset of vertigo and numbness of the left hemiface | DSA revealed left PICAD | Not mentioned | Conservative treatment, followed by endovascular treatment | Freedom from neurological symptoms at the 5-month follow up |
| Patient 8[8] | 33-year -old woman | Sudden severe headaches after transient unconsciousness | DSA showed a left PICAD | CT revealed SAH | PICAD was trapped and resected | At 5 months, neurological examination revealed slightly weak voice |
| Patient 9[9] | 48-year-old man | Severe, throbbing headache | MRI revealed a right PICAD | No infarctions or hemorrhages | Conservative therapy | Headache rapidly improved. MRI showed almost-resolved PICAD 4 months later |
| Patient 10[10] | 52-year-old woman | Nausea, vomiting, vertigo, gait instability, and headaches for 1 day | CTA showed focal stenosis in the left PICAD | CT and MRI showed an extensive left cerebellar hemisphere infarct | Aspirin, statin, and antihypertensive medication | Full recovery within 3 months; 7-, 12- and 18- month CTA showed the beaded appearance of the left PICA |
| Patient 11[10] | 60-year-old woman | Acute headaches, nausea, vomiting, loss of consciousness, and seizure | CTA and DSA showed a left PICAD | CT revealed SAH, intraventricular hemorrhage, and hydrocephalus | Endovascular treatment for PICAD | 1-year follow-up CTA showed minimal decrease in size of PICAD, accompanied by mild cognitive impairment |
| Patient 12[11] | 41-year-old man | Mild left occipital headache, followed by dizziness, nausea, and vomiting | DSA detected a PICAD | MRI revealed infarction in the left cerebellar hemisphere | Intravenous edaravone and oral ibudilast | 10-week follow-up MRA indicated resolution of the PICAD, and symptoms improved |
Diagnosing PICAD is often difficult and requires close and repeated imaging evaluations. Here, PICAD was identified by its characteristic origin from the distal vertebral artery (VA) and its unique trajectory around the medulla oblongata, to supply the inferior cerebellum (Figure 1J). In contrast, the VA primarily supplies the medulla oblongata, pons, and dorsal portions of the cerebellum. On HR-VW MRI, the PICA can be distinguished by its smaller caliber and distinct course compared with the larger VA. Therefore, differentiation is primarily based on the anatomical relationships, blood supply regions, and specific imaging features. PICAD should be considered a differential diagnosis in patients with of PICA territory infarction (Figure 2).
Visible signs of PICAD on CTA or MRA are rare, as in our patient, which implies that such findings may relate to the imaging processing technology or occur coincidentally. The PICA is the largest intracranial branch of the VA; however, owing to its small diameter and curvature, conventional MRI cannot easily detect intramural hematomas (IMH). HR-VW MRI plays a key role in the diagnosis and subsequent evaluation of curative effects. T1-weighted and dark-blood HR-VW MRI can be used to identify micro-IMHs in the PICA, thus making these techniques important for the diagnosis of AD in young adults. Furthermore, MRI has the advantage of soft-tissue contrast, which is helpful for showing a crescent-shaped IMH in the false lumen[13]. HR-VW MRI can detect IMH and improve the non-invasive diagnostic accuracy of isolated PICAD[13]. HR-VW MRI is a high-resolution, multiparametric MRI sequence that directly visualizes the intracranial artery wall and its pathological changes, allowing for better pathology characterization[14].
HR-VW MRI is valuable in distinguishing between atherosclerotic and non-atherosclerotic intracranial vascular lesions. Wall hematoma is the most common direct sign on HR-VW MRI in patients with craniocervical AD, whereas intimal flap or double-lumen signs are the least common. The detection rates of HR-VW MRI for IMH, aneurysmal dilatation, and intimal flaps (double-lumen sign) are 86%, 71%, and 47%, respectively[15]. In this patient, a crescent-shaped IMH was detected, but the double-lumen sign was not obvious. Furthermore, double-lumen sign, aneurysm dilatation, and IMH can gradually disappear during recovery, and the HR-VW-MRI findings of PICAD can show dilated, thickened, or string-of-beads changes in the outer diameter of blood vessels with IMH, which indicate obvious enhancement[16].
Owing to the unique signs of AD on digital subtraction angiography (DSA), including aneurysmal dilatation or the string-of-beads sign, the double-lumen sign, or an intimal flap[17], DSA is the gold standard for diagnosing AD[10]. However, DSA cannot display the vascular wall directly, which limits its use for vascular-wall dissection diagnosis[13]. Less than 10% of patients show double-lumen signs on DSA, and AD commonly presents with non-specific lumen stenosis with or without pseudoaneurysms, making the diagnosis of dissection difficult[18].
In conclusion, PICAD should be considered in patients with acute cerebellar infarction in the blood supply area of the PICA, even when no clear precipitating factors or warning signs are present. HR-VW MRI is crucial for diagnosis and follow-up evaluation. The pathogenesis (particularly congenital vascular wall structural abnormalities or hereditary abnormalities of the elastic tissue and collagen) and appropriate treatment of PICAD require further investigation.
We would like to thank the “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) for its support.
| 1. | Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 970] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Sedat J, Chau Y, Mahagne MH, Bourg V, Lonjon M, Paquis P. Dissection of the posteroinferior cerebellar artery: clinical characteristics and long-term follow-up in five cases. Cerebrovasc Dis. 2007;24:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Maroon JC, Gardner P, Abla AA, El-Kadi H, Bost J. "Golfer's stroke": golf-induced stroke from vertebral artery dissection. Surg Neurol. 2007;67:163-8; discussion 168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Matsumoto J, Ogata T, Abe H, Higashi T, Takano K, Inoue T. Do characteristics of dissection differ between the posterior inferior cerebellar artery and the vertebral artery? J Stroke Cerebrovasc Dis. 2014;23:2857-2861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tawk RG, Bendok BR, Qureshi AI, Getch CC, Srinivasan J, Alberts M, Russell EJ, Batjer HH. Isolated dissections and dissecting aneurysms of the posterior inferior cerebellar artery: topic and literature review. Neurosurg Rev. 2003;26:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Yamaguchi M, Kim K, Mizunari T, Umeoka K, Koketsu K, Isayama K, Morita A. Formation of a Large Fusiform Aneurysm near a Medullary Infarction Caused by Dissection of the Posterior Inferior Cerebellar Artery. J Nippon Med Sch. 2024;91:129-133. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: brainstem and cerebellum. Neurology. 1996;47:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 306] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Dinichert A, Rüfenacht DA, Tribolet N. Dissecting aneurysms of the posterior inferior cerebellar artery: report of four cases and review of the literature. J Clin Neurosci. 2000;7:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kishi Y. Spontaneous healing of an isolated posterior inferior cerebellar artery dissection without stroke: a case report. BMC Neurol. 2019;19:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Mayeku J, Schlaug G, Ogilvy CS, Thomas AJ. Clinical and neuroradiological characteristics of ischemic stroke and subarachnoid hemorrhage in isolated posterior inferior cerebellar artery dissection: Literature review and report of 2 cases. Interdiscip Neurosurg. 2019;18:100521. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Korematsu K, Yoshioka S, Abe E, Nagai Y, Kai Y, Morioka M, Kuratsu J. Spontaneous resolution of isolated dissecting aneurysm on the posterior inferior cerebellar artery. Acta Neurochir (Wien). 2008;150:77-81; discussion 81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Park MG, Choi JH, Yang TI, Oh SJ, Baik SK, Park KP. Spontaneous isolated posterior inferior cerebellar artery dissection: rare but underdiagnosed cause of ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kano Y, Inui S, Oguri T, Kato H, Yuasa H, Morimoto S, Sakurai K. Utility of T2-weighted high-resolution vessel wall imaging for the diagnosis of isolated posterior inferior cerebellar artery dissection at acute and early subacute stages. J Neurol Sci. 2020;411:116693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Mazzacane F, Mazzoleni V, Scola E, Mancini S, Lombardo I, Busto G, Rognone E, Pichiecchio A, Padovani A, Morotti A, Fainardi E. Vessel Wall Magnetic Resonance Imaging in Cerebrovascular Diseases. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Cho SJ, Choi BS, Bae YJ, Baik SH, Sunwoo L, Kim JH. Image Findings of Acute to Subacute Craniocervical Arterial Dissection on Magnetic Resonance Vessel Wall Imaging: A Systematic Review and Proportion Meta-Analysis. Front Neurol. 2021;12:586735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Bagh I, Olin JW, Froehlich JB, Kline-Rogers E, Gray B, Kim ESH, Sharma A, Weinberg I, Wells BJ, Gu X, Gornik HL. Association of Multifocal Fibromuscular Dysplasia in Elderly Patients With a More Benign Clinical Phenotype: Data From the US Registry for Fibromuscular Dysplasia. JAMA Cardiol. 2018;3:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Obusez EC, Jones SE, Hui F. Vessel wall MRI for suspected isolated basilar artery dissection. J Clin Neurosci. 2016;27:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Ahn SS, Kim BM, Suh SH, Kim DJ, Kim DI, Shin YS, Ha SY, Kwon YS. Spontaneous symptomatic intracranial vertebrobasilar dissection: initial and follow-up imaging findings. Radiology. 2012;264:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |