Published online Jul 16, 2025. doi: 10.12998/wjcc.v13.i20.102279
Revised: February 21, 2025
Accepted: March 8, 2025
Published online: July 16, 2025
Processing time: 177 Days and 16.1 Hours
Cerebrospinal fluid (CSF) leaks in the temporal bone arise from osteodural defects, resulting in an abnormal connection between the subarachnoid space and the adjacent tympanomastoid cavity, which often manifests as otorrhea. Patients typically exhibit symptoms such as headache, unilateral hearing impairment, aural fullness, or even meningitis. Imaging studies are critical for identifying and differentiating the location and characteristics of CSF leaks. However, when the leak's origin remains ambiguous, diagnostic surgery may be warranted to both confirm the diagnosis and facilitate treatment. This report discusses an uncom
The patient, a 58-year-old man, was admitted for evaluation of left ear fullness, hearing loss, and nasal discharge. Notably, when supine, clear fluid drained from the left nasal cavity, with improvement noted upon sitting. A nasal examination did not reveal significant findings, while the otologic evaluation indicated an intact periosteum; however, considerable fluid accumulation was identified within the left middle ear. Despite undergoing multiple periosteal punctures and conservative medical management, the middle ear effusion persisted. Imaging studies, including magnetic resonance imaging (MRI) and computed tomography, confirmed the presence of left-sided CSF otorrhea, and the head MRI indicated potential CSF rhinorrhea. This raised challenges in determining whether the CSF leak originated from the sphenoid sinus or the temporal bone. Given that CSF otorrhea may drain through the external auditory canal and CSF rhinorrhea from the sellar region can present as nasal leakage, differentiation proved complex. In this case, with an intact external auditory canal, CSF from the middle ear was observed to flow into the nasal cavity via the Eustachian tube. Therefore, leakage from both sites could be misconstrued as CSF rhinorrhea, complicating the diagnostic process. Consequently, an exploratory surgical procedure was performed, revealing an incomplete dura mater on the temporal aspect of the petrous bone, which was subsequently repaired.
Benign intracranial hypertension can result in meningeal protrusion or meningoencephalocele, which may lead to CSF leakage that generally responds favorably to mucosal repair. In instances where imaging fails to identify the source of the leak or when diagnostic options are limited, proactive exploratory surgery is advisable. Although surgical interventions carry inherent risks, the application of endoscopic techniques by experienced surgeons renders this approach a feasible choice for addressing both diagnostic and therapeutic challenges.
Core Tip: This case report details a 58-year-old male patient diagnosed with temporal bone cerebrospinal fluid leaks. The complexity of the case stemmed from the atypical configuration of the defect at the leakage site, compounded by difficulties in preoperative differential diagnosis due to the presence of an empty sella and a defect in the petrous bone identified on computed tomography and magnetic resonance imaging. Following extensive discussions, the patient underwent an exploratory procedure, successfully repairing the dura mater defect. The efficacy of mucosal repair under endoscopic guidance was highlighted as a promising approach in such clinical scenarios.
- Citation: He YS, Zheng Y. Exploratory operation in a patient with spontaneous temporal bone cerebrospinal fluid leaks: A case report. World J Clin Cases 2025; 13(20): 102279
- URL: https://www.wjgnet.com/2307-8960/full/v13/i20/102279.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i20.102279
Spontaneous cerebrospinal fluid (CSF) leaks occur in the absence of any identifiable pathologies or traumatic history[1]. When such leaks originate from the paranasal sinuses, they typically manifest as rhinorrhea, while those emanating from the temporal bone may lead to conductive hearing loss and an abnormal feeling of fullness in the ear. In instances where the tympanic membrane remains intact yet a defect exists in the petrous temporal bone, CSF may escape through the Eustachian tube into the nasopharynx, a condition clinically recognized as CSF leak[2,3]. Diagnosing these leaks presents significant challenges and often results in delays, particularly when they are intermittent, as they may be overlooked or misidentified as chronic nasal discharge[3]. Temporal bone CSF leaks, which frequently occur due to trauma or surgical interventions, may also be associated with congenital anomalies, neoplasms, or infections, attributable to their proximity to the subarachnoid space[4]. While the majority of CSF leaks are attributed to head trauma and surgical procedures, a spontaneous occurrence rate of 6%-24% has been documented, with recognized etiologies including empty sella syndrome, hydrocephalus, and meningoencephalocele[5]. Standard diagnostic modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and cisternography, serve as effective tools in localizing leaks; however, if the origin remains elusive, a thorough assessment based on the patient’s clinical presentation may be warranted. This could necessitate exploratory surgery for repair, which typically yields favorable outcomes.
A 58-year-old male presented with left ear fullness, hearing impairment for six months, and nasal discharge persisting for over a month.
The patient initially sought care at Shenzhen Bao'an Traditional Chinese Medicine Hospital Group six months prior, reporting left-sided ear fullness and hearing loss. He was diagnosed with left secretory otitis media, receiving treatment that included antibiotic therapy and tympanic membrane puncture with fluid aspiration; however, there was no improvement in symptoms. Over a month ago, he noted clear fluid flowing from the left nasal cavity when lying down, which subsided upon sitting. These symptoms exacerbated over time, significantly impacting his daily activities and work. Subsequently, he was admitted to our department for further evaluation and management. A paracentesis of the left tympanic membrane revealed persistent clear fluid within the middle ear.
The patient had a history of hypertension for 7 years, with well-controlled blood pressure.
He reported no history of smoking or alcohol consumption and denied any similar familial conditions.
Physical examination revealed clear secretion continues to overflow from the left ear. No positive findings were observed in other specialized examinations.
A lumbar puncture indicated an initial pressure of 170 mmH2O; routine biochemical analysis of the CSF demonstrated total protein levels at 1292 mg/L and chloride at 123.09 mmol/L.
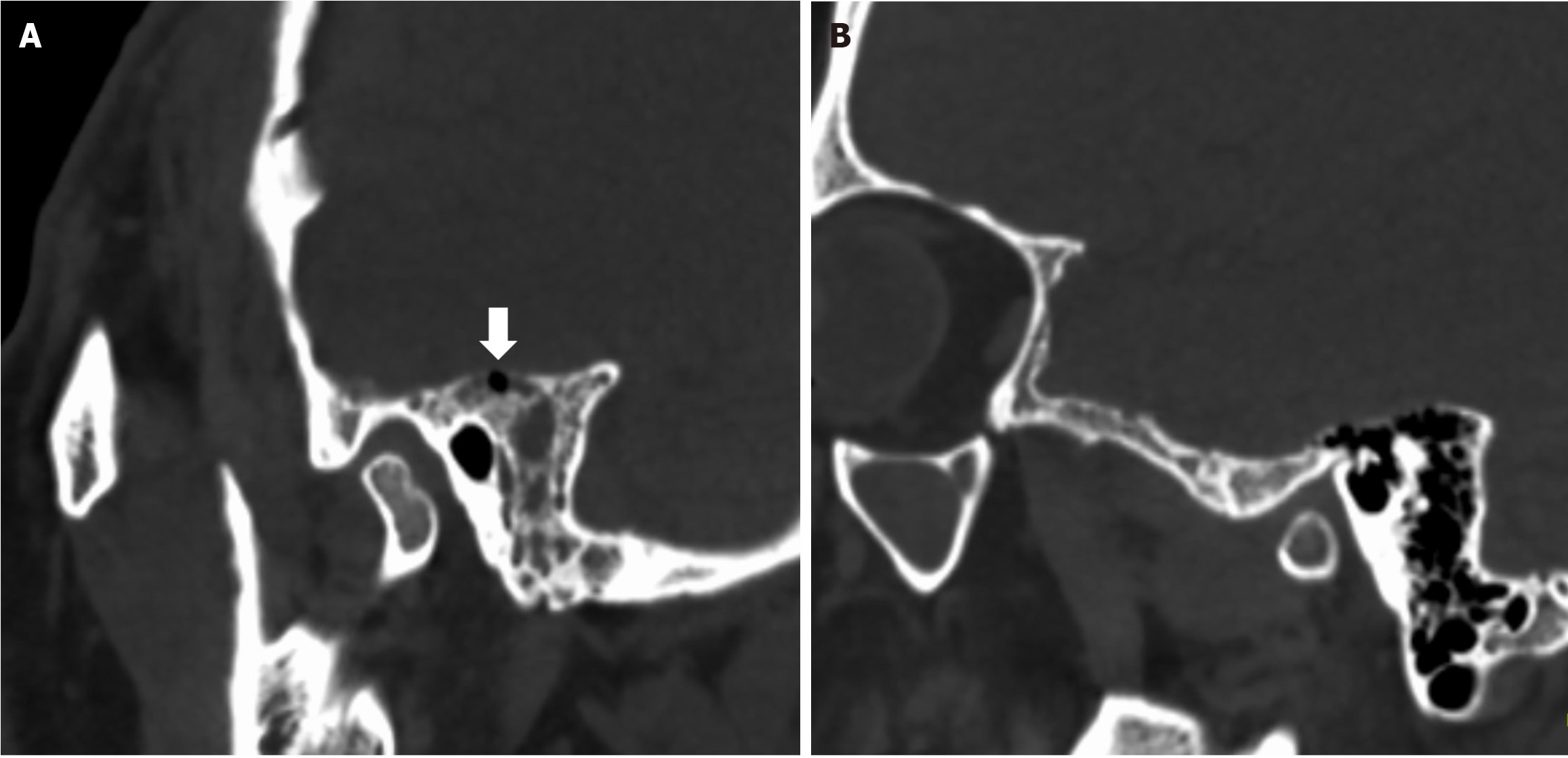
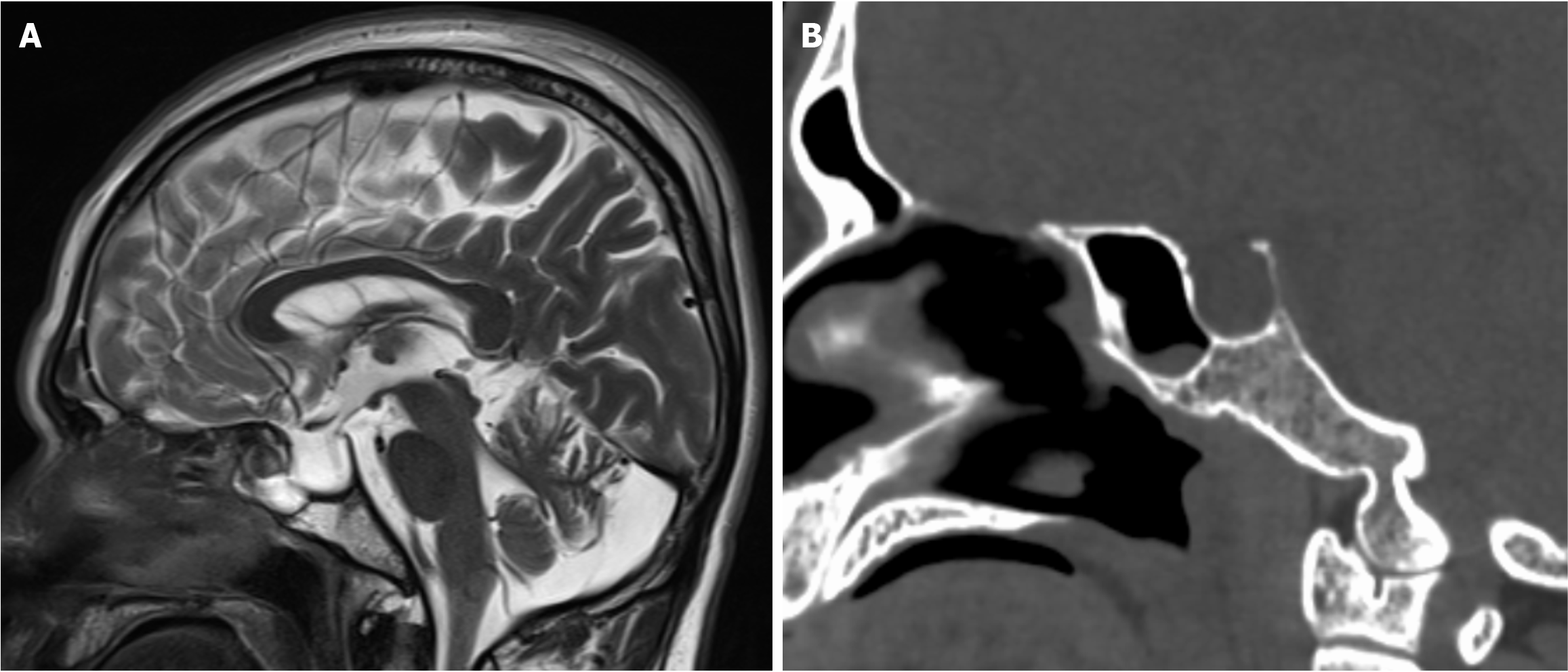
Head CT illustrated fluid accumulation within the petrous temporal bone, mastoid process, and middle ear, alongside a discontinuity in the petrous bone. Fluid was also noted in the left middle ear and external auditory canal (Figure 1). Head MRI revealed an enlarged sella turcica with posterior displacement of the compressed pituitary gland. Abnormal CSF signals were identified communicating in the sella region and sphenoid sinus. These imaging findings suggest a potential CSF leak in either the temporal bone or sella region.
Left spontaneous CSF leaks of the temporal bone.
Following conservative management, including the administration of ceftriaxone for infection and maintenance of a specific posture for over two weeks, it was concluded that the suspected leak site was not amenable to spontaneous healing. After careful deliberation, the patient agreed to proceed with exploratory surgery. Subsequently, owing to positive intraoperative observations, the patient underwent a surgical procedure to repair the CSF leaks utilizing mucosal tissue.
Symptoms such as ear fullness, auditory impairment, and rhinorrhea were completely alleviated within one week following the surgery. Throughout a follow-up duration of 12 months, there was no recurrence of symptoms, indicating a favorable recovery trajectory.
The patient presented with a prolonged history of hypertension, a risk factor that may contribute to the thinning of the calvarial (tegmental) bone and the subsequent weakening of the underlying dura mater[6]. Dong et al[7] conducted a case-control study revealing a prevalence of hypertension in patients experiencing CSF leaks reaching up to 34.35%, aligning with earlier research indicating a frequent coexistence of hypertension in this demographic. The pathological alterations associated with hypertension can ultimately lead to CSF leaks, posing significant diagnostic and therapeutic challenges. The treatment strategy is primarily influenced by the clinical presentation of the patient.
Spontaneous CSF leakage into the middle ear and surrounding areas is characterized as a pathological condition arising without an identifiable cause, distinct from traumatic head injuries, tumors, surgical interventions at the skull base, or congenital anomalies[8]. The precise localization of CSF leaks is crucial for improving surgical outcomes. Various imaging techniques such as high-resolutio CT, MRI, CT cisternography, MRI cisternography (MRC), intrathecal gadoli
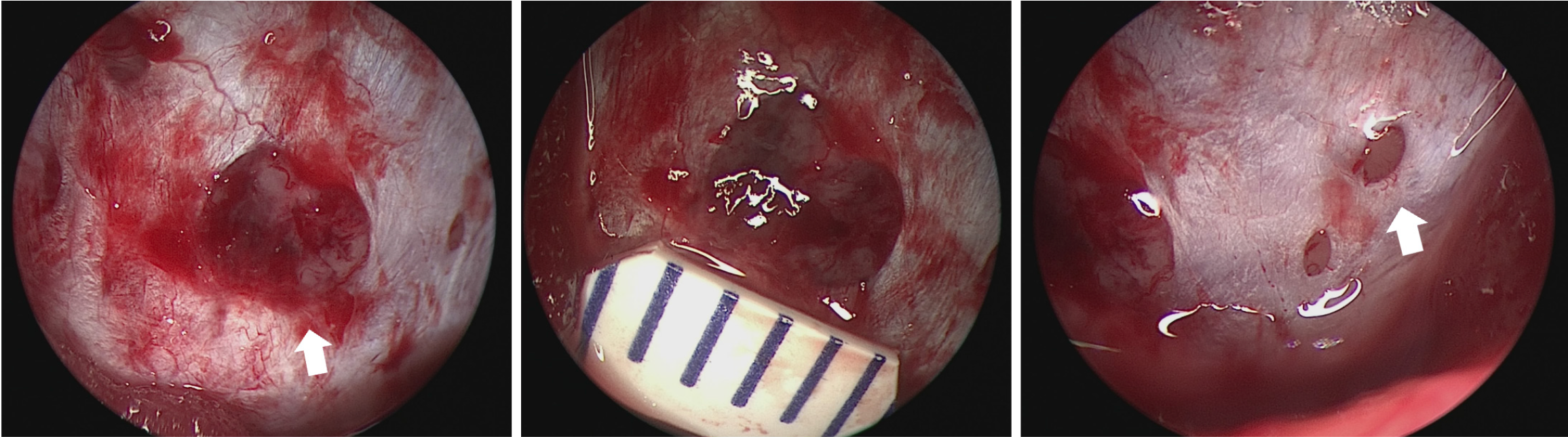
During the surgical procedure, it was observed that the temporal dura mater overlying the petrous bone was incomplete, exhibiting multiple circular defects within the otherwise intact dura mater of the skull base (Figure 3). These defects permitted the herniation of adjacent brain tissue and arachnoid structures. This observation elucidates the variability of the patient's symptoms in relation to changes in body position. The resolution of nasal leakage symptoms following surgery substantiates that CSF was indeed entering the nasal cavity via the Eustachian tube, thereby confirming the preoperative diagnosis of temporal bone CSF leaks.
Currently, various mechanisms have been suggested to explain the thinning of the tegmen bone, including congenital defects, intracranial hypertension, anomalous arachnoid granulations, cholesteatoma, and chronic middle ear disease leading to local ischemia and degradation of the dura mater[11]. High-resolution CT combined with MRI or MRC has emerged as complementary diagnostic modalities, facilitating the identification of meningocele and the assessment of bone defects in the skull base[12]. High-resolution CT scans have demonstrated sensitivity and specificity rates of 92% and 100%, respectively, in comparison to MRI, which has sensitivity and specificity rates of 87% and 100%[13]. Consequently, CT is recommended as the primary imaging technique for CSF rhinorrhea due to its diagnostic efficacy, cost-effectiveness, and non-invasive nature, whereas MRI serves as a secondary option. Despite its enhanced properties and elevated costs, the diagnostic process can be further supported by the identification of β-2-transferrin in nasal or tympanic secretions, which is recognized as the gold standard for diagnosing CSF leaks[14]. However, the application of β-2-transferrin testing is subject to debate, as many CSF leaks can be diagnosed solely through clinical history, physical examination, and imaging techniques. Reports indicate positivity rates as low as 55%-75%, and the high rates of false negatives are often attributed to the slow or intermittent nature of some leaks[15]. Consequently, numerous researchers, including the author of this study, have chosen not to routinely conduct β-2-transferrin tests when there is a significant suspicion based on clinical history and examination findings, suggesting that such testing may be unnecessary.
The diagnostic approach and subsequent management of the patient led to several important observations. Neuroendoscopic evaluation unveiled multiple circular defects on the dura mater located at the mid-skull base, with diameters ranging from 1-2 mm to 5 mm. Previous experience have classified dural defects encountered during surgery as slit-like, circular, or irregular in shape, with high-pressure-related defects typically exhibiting oval shapes, while low-pressure-related defects are more frequently observed as slit-like lesions. This supports the consistency of our findings with prior experience and underscores the importance of surgical exploration for conclusive diagnosis. Various materials have been documented in the repair of CSF fistulas; although a single-layer closure achieves a closure rate of approximately 75.4%, a multilayer closure approach generally leads to a success rate of 100%[16]. The use of postoperative lumbar drainage is not standard practice[17,18]. In cases involving patients at high risk for recurrent CSF leaks and multiple repair failures, the placement of a lumboperitoneal or ventriculoperitoneal shunt may be warranted[19].
Endoscopic techniques for repairing CSF leaks offer numerous advantages, including safety, minimal tissue damage, elimination of facial scarring, and rapid postoperative recovery, establishing it as the primary method for managing CSF leaks[20]. Thorough preoperative imaging assessment is essential for determining the most appropriate surgical technique for addressing skull base defects. In our experience, ensuring adequate visualization of bone defects, careful removal of all surrounding mucosa, multilayer closures, and proficiency in various surgical methods are critical for achieving successful outcomes in CSF leaks repairs[21]. Furthermore, advancements in navigational technology have started to significantly enhance surgical outcomes through improved localization via imaging[10].
Conservative medical management is frequently employed as a preliminary treatment strategy; however, it often proves ineffective for the long-term management and resolution of a substantial number of patients suffering from spontaneous intracranial hypotension, with pooled efficacy estimates for conservative treatment ranging between 18% and 37%[22]. A case report by Tilak et al[23] suggests that, in the absence of other contraindications for delaying repair, a trial of acetazolamide therapy could be considered as an initial option in the management of isolated spontaneous CSF rhinorrhea and it can help some patients avoid surgical intervention. For patients with persistent CSF leaks who choose conservative treatment, the primary risk is meningitis. Although the incidence of meningitis remains uncertain, and many patients may never experience it, it is prudent to engage in careful monitoring and provide patient education regarding the associated risks[15].
In conclusion, local meningeal protrusion or meningoencephalocele resulting from benign intracranial hypertension can precipitate CSF leaks at various anatomical locations. Nevertheless, this type of CSF leaks generally demonstrates favorable responses to mucosal interventions. The challenges associated with treatment as well as diagnostic and therapeutic considerations for this patient have been clearly delineated. It is underscored that in instances where CSF leaks cannot be detected through imaging modalities or when diagnostic techniques are constrained, undertaking proactive exploratory surgery may be more effective in resolving the diagnostic and therapeutic conundrum. Despite the inherent risks associated with surgical intervention, the proficiency of experienced surgeons combined with the application of endoscopic methodologies positions this approach as a highly feasible option. Future studies should put emphasis on longer follow-up time and adequate long-term monitoring due to the underlying chronicity of increased intracranial pressure[24].
The authors are grateful to the patient in this study for his collaboration.
| 1. | Hiremath SB, Gautam AA, Sasindran V, Therakathu J, Benjamin G. Cerebrospinal fluid rhinorrhea and otorrhea: A multimodality imaging approach. Diagn Interv Imaging. 2019;100:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Vemuri NV, Karanam LSP, Manchikanti V, Dandamudi S, Puvvada SK, Vemuri VK. Imaging review of cerebrospinal fluid leaks. Indian J Radiol Imaging. 2017;27:441-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Bidot S, Levy JM, Saindane AM, Oyesiku NM, Newman NJ, Biousse V. Do Most Patients With a Spontaneous Cerebrospinal Fluid Leak Have Idiopathic Intracranial Hypertension? J Neuroophthalmol. 2019;39:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Cooper T, Choy MH, Gardner PA, Hirsch BE, McCall AA. Comparison of Spontaneous Temporal Bone Cerebrospinal Fluid Leaks From the Middle and Posterior Fossa. Otol Neurotol. 2020;41:e232-e237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Schraven SP, Bisdas S, Wagner W. Synchronous spontaneous cerebrospinal fluid leaks in the nose and ear. J Laryngol Otol. 2012;126:1186-1188. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Thomeer HG, Schreurs C, van Doormaal TP, Straatman LV. Management and Outcomes of Spontaneous Cerebrospinal Fluid Otorrhoea. Front Surg. 2020;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Dong D, Chen X, Cai F, Huang S, Li C, Zhao Y. Correlation between Pneumatization Variants of Paranasal Sinuses and Skull Base and Spontaneous Cerebrospinal Fluid Rhinorrhea: A Case-Control Study. ORL J Otorhinolaryngol Relat Spec. 2023;85:156-162. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Hendriks T, Thompson A, Boeddinghaus R, Tan HEI, Kuthubutheen J. Radiological findings in spontaneous cerebrospinal fluid leaks of the temporal bone. J Laryngol Otol. 2021;135:403-409. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Dogan SN, Salt V, Korkmazer B, Arslan S, Islak C, Kocer N, Kizilkilic O. Intrathecal use of gadobutrol for gadolinium-enhanced MR cisternography in the evaluation of patients with otorhinorrhea. Neuroradiology. 2020;62:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Hwang SH, Kim SW, Kim DH. Efficacy of Imaging Methods in the Detection and Diagnosis of Cerebrospinal Fluid Rhinorrhea. Laryngoscope. 2023;133:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 11. | Hernandez-Montero E, Caballero E, García-Ibanez L. Surgical management of middle cranial fossa bone defects: meningoencephalic herniation and cerebrospinal fluid leaks. Am J Otolaryngol. 2020;41:102560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kapitanov DN, Shelesko EV, Potapov AA, Kravchuk AD, Zinkevich DN, Nersesyan MV, Satanin LA, Sakharov AV, Danilov GV. [Endoscopic endonasal diagnosis and treatment of skull base meningoencephalocele]. Zh Vopr Neirokhir Im N N Burdenko. 2017;81:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zahedi FD, Subramaniam S, Kasemsiri P, Periasamy C, Abdullah B. Management of Traumatic and Non-Traumatic Cerebrospinal Fluid Rhinorrhea-Experience from Three Southeast Asian Countries. Int J Environ Res Public Health. 2022;19:13847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Li S, Lu T, Wang Y, Guo M, Ma R, Li S, Ruan B. Spontaneous Cerebrospinal Fluid Rhinorrhea and Otorrhea: A Case Report and Literature Review. Ear Nose Throat J. 2023;1455613231158797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Cheng E, Grande D, Leonetti J. Management of spontaneous temporal bone cerebrospinal fluid leak: A 30-year experience. Am J Otolaryngol. 2019;40:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Kutz JW Jr, Tolisano AM. Diagnosis and management of spontaneous cerebrospinal fluid fistula and encephaloceles. Curr Opin Otolaryngol Head Neck Surg. 2019;27:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Dallan I, Cambi C, Emanuelli E, Cazzador D, Canevari FR, Borsetto D, Tysome JR, Donnelly NP, Rigante M, Georgalas C, Alobid I, Molteni G, Marchioni D, Shahzada AK, Scarano M, Seccia V, Pasquini E. Multiple spontaneous skull base cerebrospinal fluid leaks: some insights from an international retrospective collaborative study. Eur Arch Otorhinolaryngol. 2020;277:3357-3363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Georgalas C, Oostra A, Ahmed S, Castelnuovo P, Dallan I, van Furth W, Harvey RJ, Herman P, Kombogiorgas D, Locatelli D, Meco C, Palmer JN, Piltcher O, Sama AM, Saleh H, Sindwani R, Van Zele T, Woodworth BA. International Consensus Statement: Spontaneous Cerebrospinal Fluid Rhinorrhea. Int Forum Allergy Rhinol. 2021;11:794-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Jolly K, Gupta KK, Bhamra N, Aslanidou A, Batra R, Ahmed SK. Endonasal endoscopic management of spontaneous cerebrospinal fluid rhinorrhoea: The Birmingham UK experience. Asian J Endosc Surg. 2023;16:68-76. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Peng A, Li Y, Xiao Z, Wu W. Exploration of endonasal endoscopic repair of pediatric cerebrospinal fluid rhinorrhea. Int J Pediatr Otorhinolaryngol. 2011;75:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Huang Z, Huang Q, Cui S, Qiu E, Xian J, Yang B, Huo M, Zhou B. Endoscopic Repair of Spontaneous Cerebrospinal Fluid Leaks in the Lateral Recess of the Sphenoid Sinus. Otolaryngol Head Neck Surg. 2022;167:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | D'Antona L, Jaime Merchan MA, Vassiliou A, Watkins LD, Davagnanam I, Toma AK, Matharu MS. Clinical Presentation, Investigation Findings, and Treatment Outcomes of Spontaneous Intracranial Hypotension Syndrome: A Systematic Review and Meta-analysis. JAMA Neurol. 2021;78:329-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 23. | Tilak AM, Koehn H, Mattos J, Payne SC. Preoperative management of spontaneous cerebrospinal fluid rhinorrhea with acetazolamide. Int Forum Allergy Rhinol. 2019;9:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Piemontesi JA, Samson LA, Alqunaee MD, Javer AR. Multiple spontaneous cerebrospinal fluid leaks: a rare case report and review of literature. AME Case Rep. 2022;6:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |