Published online Jun 26, 2025. doi: 10.12998/wjcc.v13.i18.103571
Revised: January 15, 2025
Accepted: January 24, 2025
Published online: June 26, 2025
Processing time: 82 Days and 18.9 Hours
Breast cancer is a leading cause of cancer-related mortality among women worldwide, with invasive ductal carcinoma (IDC) being the most prevalent subtype. Lymph node metastasis is the primary prognostic indicator, typically evaluated via biopsy of the ipsilateral sentinel or axillary lymph nodes. Contralateral axillary metastasis (CAM) without ipsilateral involvement is exceedingly rare, particularly in early-stage breast cancer. This report presents a case of CAM in a patient with triple-negative breast cancer (TNBC), underscoring diagnostic and therapeutic complexities.
A 73-year-old female presented with left-sided early-stage IDC in February 2023. Despite a modified radical mastectomy and pathologically negative ipsilateral lymph nodes, a postoperative positron emission tomography (PET) scan detected fluorodeoxyglucose-avid nodes in the contralateral axilla. Biopsy confirmed metastatic ductal carcinoma with triple-negative status, resulting in an upstaged diagnosis of metastatic breast cancer, stage IV, M1. The patient underwent six cycles of adjuvant chemotherapy, with follow-up PET imaging showing regression of the contralateral lesion. This case highlights the importance of advanced imaging in TNBC for precise staging and treatment optimization.
This case highlights the aggressive nature of TNBC and the need for advanced imaging to ensure accurate staging and effective management.
Core Tip: We present a 73-year-old female with triple-negative breast cancer (TNBC) exhibiting contralateral axillary lymph node metastasis without ipsilateral involvement. Despite early-stage diagnosis and modified radical mastectomy, positron emission tomography (PET) imaging and biopsy later upstaged the disease to metastatic stage IV. This case highlights the aggressive nature of TNBC, its complex lymphatic spread, the potential utility of PET scans for accurate staging in high-risk early breast cancers and treatment for contralateral axillary metastasis. These findings suggest reconsidering mandatory PET imaging for early TNBC or other high-risk cases to ensure accurate staging and optimal systemic therapy for improved outcomes.
- Citation: Lin YT, Hong ZJ, Liao GS, Dai MS, Chao TK, Tsai WC, Sung YK, Chiu CH, Chang CK, Yu JC. Unexpected contralateral axillary lymph node metastasis without ipsilateral involvement in triple-negative breast cancer: A case report and review of literature. World J Clin Cases 2025; 13(18): 103571
- URL: https://www.wjgnet.com/2307-8960/full/v13/i18/103571.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i18.103571
Breast cancer is one of the most common malignancies affecting women worldwide and remains a primary cause of cancer-related deaths[1]. The management of breast cancer is complex and requires a multimodal approach. Treatment strategies are guided by factors such as lymph node status, which is typically evaluated via biopsy of the ipsilateral sentinel or axillary lymph nodes, tumor size, biology, disease stage, patient age, and underlying conditions, particularly in older individuals[2,3].
Among the various subtypes of breast cancer, invasive ductal carcinoma (IDC) is the most prevalent. IDC originates in the milk ducts and subsequently invades the surrounding breast tissues[4]. A particularly challenging subtype of IDC is triple-negative breast cancer (TNBC), which lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2/Neu)[5]. TNBC is associated with poorer prognosis, higher recurrence rates, and limited treatment options because it does not respond to hormonal or HER2-targeted therapies[6]. Nevertheless, recent studies have demonstrated that adding pembrolizumab to standard neoadjuvant chemotherapy improved prognosis in patients with early-stage TNBC[7,8]. These data emphasize the evolving landscape of TNBC treatment and highlight the importance of a precise stage to improve outcomes.
Considering the aggressive behavior and limited treatment options for TNBC, comprehensive diagnostic imaging and careful monitoring are critical for guiding treatment decisions and improving outcomes[9]. According to the National Comprehensive Cancer Network Guidelines, version 6, 2024, positron emission tomography (PET) or computed tomography (CT) is most beneficial and accurate for advanced disease, such as stage III or higher with invasive ductal histology. Nevertheless, these modalities may also be beneficial in selected circumstances for earlier stage disease, such as equivocal CT or bone scan results, suspicion of undetected nodal or distant disease, and treatment response assessment. Therefore, preoperative PET is currently not routinely performed in patients with early-stage breast cancer[10].
In addition to whether PET should be routinely performed for early-stage breast cancer, contralateral axillary metastasis (CAM) is another novel topic for breast cancer. CAM refers to the spread of cancer to the axillary lymph nodes on the side opposite to the primary breast tumor without the involvement of ipsilateral axillary lymph nodes[11]. CAM is a rare clinical presentation in breast cancer, with an incidence rate reported approximately 1.9%-6% in various studies[12,13]. Classifying CAM as synchronous or metachronous is essential for understanding its clinical implications and guiding management strategies. Synchronous CAM exhibits better outcomes in terms of overall survival than other metastatic diseases[13]. Nonetheless, patients with metachronous CAM exhibit survival similar to that of patients with distant metastasis[14].
Here, we describe a rare case of a 73-year-old woman diagnosed with TNBC who exhibited an unusual metastasis pattern, involving the contralateral axillary lymph node without the involvement of the ipsilateral or internal mammary lymph node. This case underscores the diagnostic challenges and complexities in managing advanced TNBC. It also emphasizes the potential role of advanced imaging techniques, such as whole-body PET, in achieving more accurate staging before adjuvant chemotherapy, whether for early detection or staging of metastatic disease.
A 73-year-old female presented with a palpable left breast mass for 4 months.
The patient reported a palpable mass in her left breast for 4 months. Mammography and ultrasonography categorized the mass as highly suspicious for neoplasm with breast imaging-reporting and data system (BI-RADS) category 4c in February 2023. A subsequent core needle biopsy revealed IDC, grade II, with triple-negative status (ER-, PR-, HER2-, Ki-67: 10%, Nottingham histological score 7). After additional evaluations, including abdominal sonography and chest X-ray, the clinical stage was determined as cT2N0M0, with the largest tumor measuring 22.6 mm × 12.6 mm. The patient was a candidate for neoadjuvant chemotherapy, and based on guidelines, we strongly recommended that she undergo neoadjuvant chemotherapy. Nevertheless, considering the relatively small tumor size in T2, her age, and frailty, she ultimately refused neoadjuvant chemotherapy and opted to proceed directly with surgery.
In 2018, the patient experienced a microvascular rupture and underwent brain surgery.
She had no relevant family history of breast cancer or other malignancies. However, she had some relevant personal histories related to breast cancer, including first childbirth after the age of 35 years and a breastfeeding duration of < 2 years.
Physical examination revealed a fixed mass in the left breast without palpable lymph nodes in the bilateral axillary or supraclavicular regions.
Preoperative CEA and CA153 Levels were 0.77 ng/mL and 7.8 U/mL, respectively, whereas postoperative levels were 1.52 ng/mL and 9.57 U/mL, respectively.
Core needle biopsy of the left tumor revealed IDC, grade II, with triple-negative status (ER-, PR-, HER2-, Ki-67: 10%, Nottingham histological score 7).
Pathology of modified radical mastectomy, left IDCs, grade III with triple-negative status (ER-, PR-, HER2-, Ki-67: 40%, Nottingham histological score 9) and free of tumor invasion in the surgical margin, lymph nodes (0/11), including sentinel node (0/3), level I (0/7), level II (0/1). No lymphovascular space or perineural invasion was identified.
Core needle biopsy of the right axillary lymph node revealed metastatic ductal carcinoma with triple-negative status (ER-, PR-, HER2-, Ki-67: 30%, GATA3+).
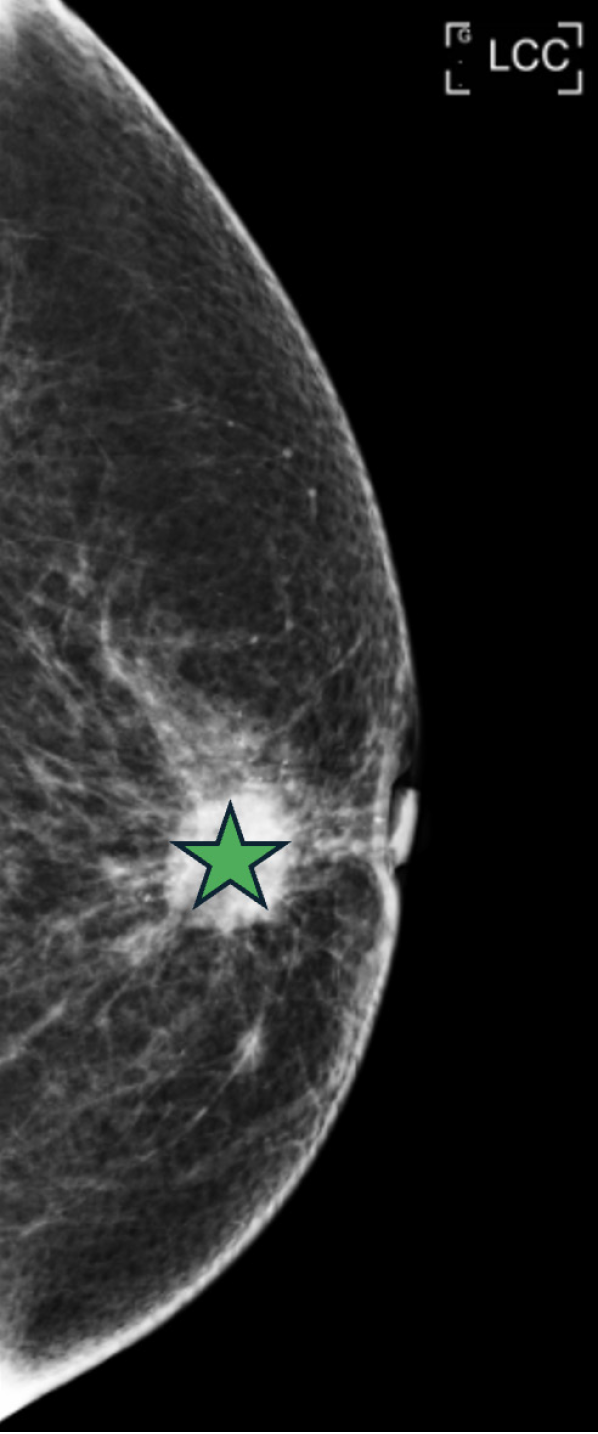
Mammography (Figure 1) revealed well-circumscribed lobular masses with pleomorphic calcifications in the left breast, classified as BI-RADS category 4c.
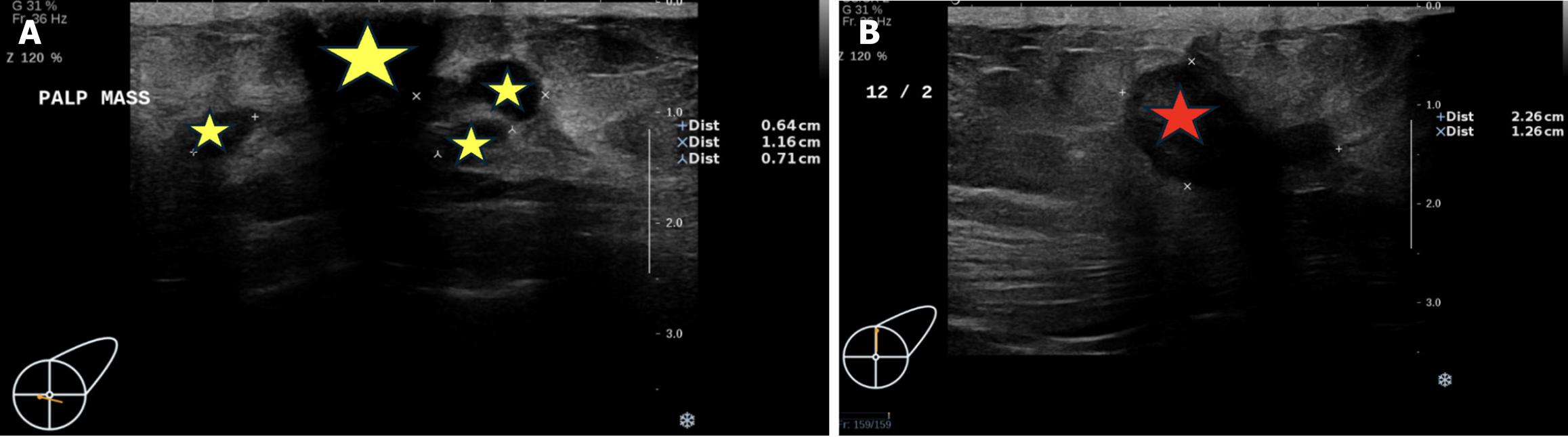
Breast sonography performed on February 6, 2023 (Figure 2), revealed multiple masses with poorly defined margins and a dilated duct. The largest mass, located at the 12 o’clock position of the left breast, measured 22.6 mm × 12.6 mm and was classified as BI-RADS category 4c. Enlarged lymph nodes were also detected in the left axillary fossa, which might be adenopathy. No obvious enlarged lymph nodes were detected in the right axillary fossa.
Abdominal sonography revealed a 0.8-cm hepatic cyst in segment 4 of the liver.
Modified radical mastectomy was performed on the left breast on February 17, 2023, which revealed two major tumors, with the larger tumor measuring 2.1 mm × 1.3 cm and the smaller one measuring 1.3 mm × 0.6 cm.
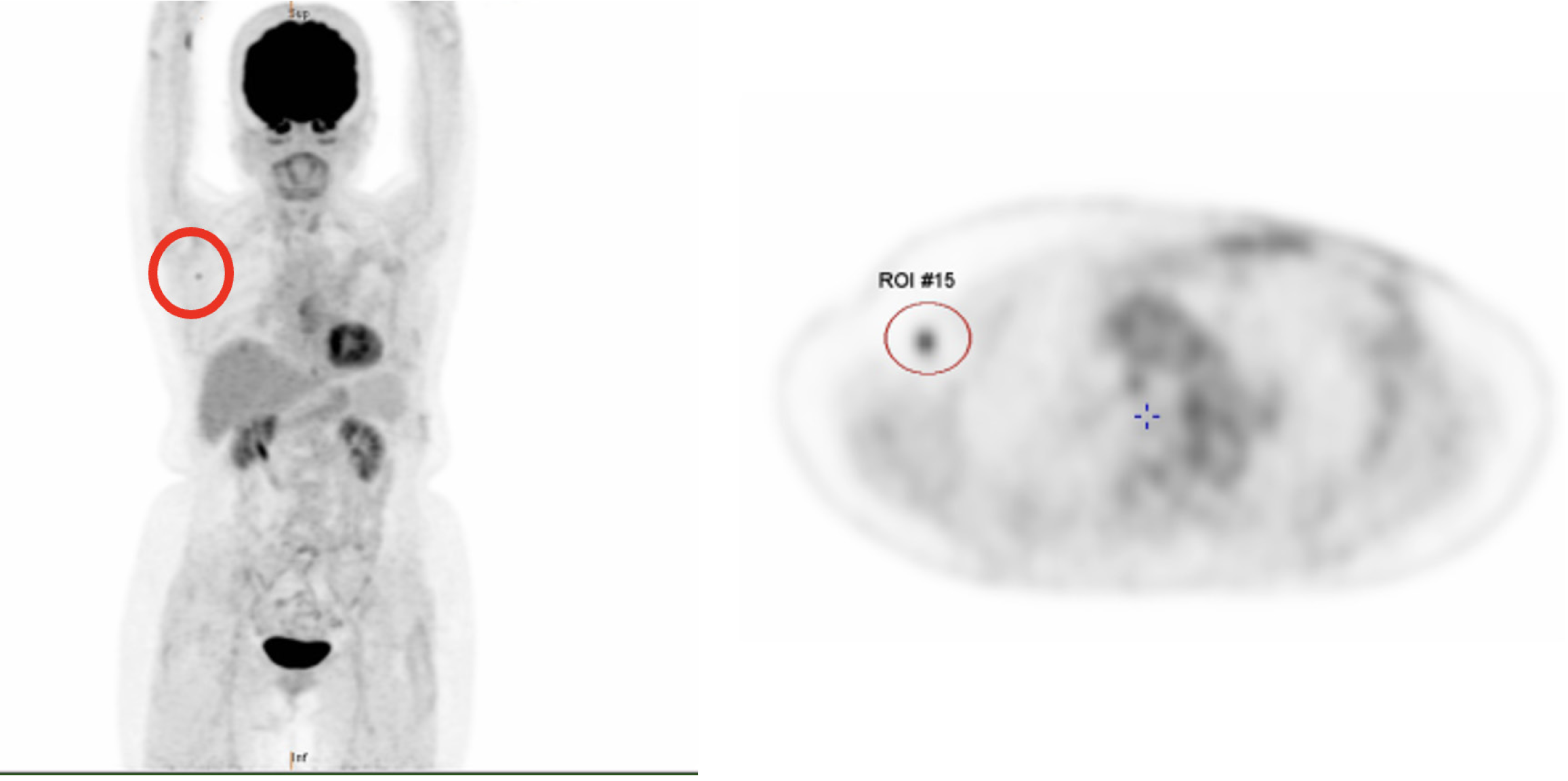
Whole-body PET performed in March 2023 (Figure 3) revealed mild fluorodeoxyglucose (FDG) avidity in the right axilla and right lobe of the liver. The FDG-avid nodule in the right axilla was confirmed as metastatic ductal carcinoma in the right axillary lymph node by needle biopsy. The FDG-avid lesion in the right lobe of the liver was suspicious for a cyst upon further evaluation by abdominal CT with contrast.
Abdominal CT with contrast performed in April 2023 identified that the liver lesion exhibited no significant enhancement after contrast administration, supporting the diagnosis of a cyst, consistent with the previous mild FDG avidity observed on PET.
Sono-guided core needle biopsy for mild FDG avidity over the right axillary region was performed on April 19, 2023, which revealed an enlarged lymph node in the right axillary fossa.
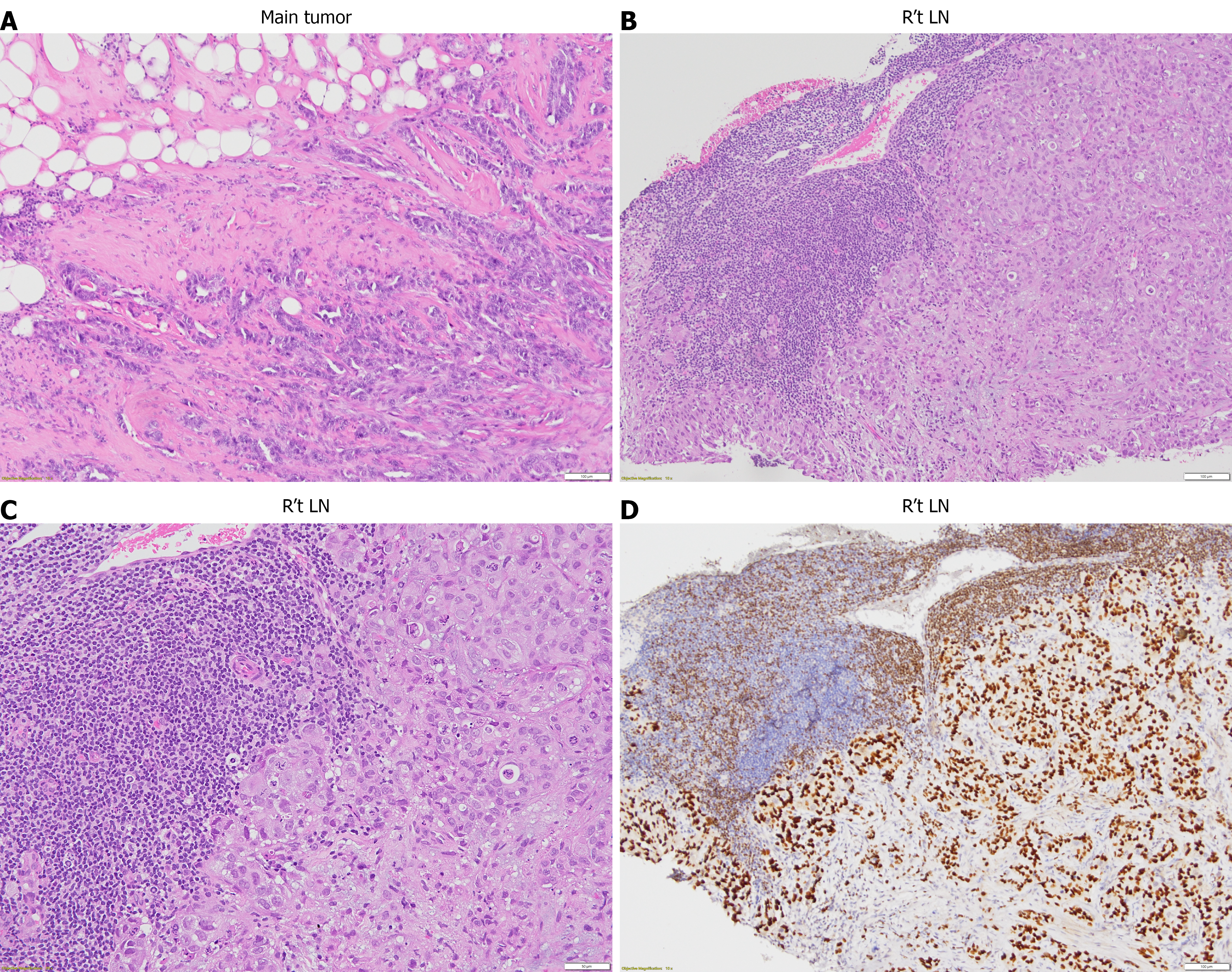
IDC, grade III, triple-negative (ER-, PR-, HER2-, Ki-67: 40%, Nottingham histological score 9), with metastatic involvement of the right axillary lymph node. Final staging: PT2N0M1, stage IV (Figure 4).
The patient underwent a modified radical mastectomy, followed by adjuvant chemotherapy with six cycles of docetaxel and cyclophosphamide, with dose reductions due to age and intolerable side effects, such as skin rash, pitting edema, hand-foot syndrome, myalgia, and nausea.
A PET scan performed in August 2023 demonstrated regression of the FDG-avid nodule in the right axilla (Figure 3) and no evidence of distant organ involvement. Radiotherapy to the right axillary region was planned; however, the patient and her family declined the treatment due to her age and underlying conditions. She experienced no significant complications following the course of treatment and surveillance.
TNBC is a distinct and challenging subtype of breast cancer characterized by the absence of targeted therapies[6]. It is associated with aggressive behavior, higher recurrence rates, and poorer prognosis compared with other subtypes of breast cancer[15]. In the present case, the patient exhibited a high Ki-67 index, which increased from 10% preoperatively to 40% in the final pathological analysis, signifying a highly proliferative tumor and further complicating the prognosis[16]. Hence, although this patient did not meet the criteria for preoperative PET, due to her strong refusal to undergo neoadjuvant chemotherapy and even her initial refusal to accept adjuvant chemotherapy and radiotherapy, err on the side of caution, we still arranged a postoperative PET after achieving pathologically free margins. Unexpectedly, the PET scan revealed CAM, resulting in the disease being reclassified as stage IV. Consequently, the patient agreed to proceed with adjuvant chemotherapy. Therefore, PET should be considered routinely in high-risk breast cancer, including TNBC.
The rare occurrence of CAM presents significant diagnostic and therapeutic challenges. This atypical presentation may necessitate modifications to postoperative adjuvant systemic therapy and radiotherapy. The observed pattern suggests that the lymphatic spread pathways in TNBC differ from those of other subtypes of breast cancer, potentially bypassing the conventional routes of local dissemination and resulting in unexpected metastatic sites. The absence of internal mammary and mediastinal lymph node involvement raises intriguing questions concerning the selective nature of metastatic spread in TNBC. In our case, we used an individualized strategy rather than a standard-of-care protocol because of the general conditions, including the patient’s comorbidities, preference, and the rare condition of CAM with TNBC.
The prognosis associated with CAM warrants further discussion. Although distant metastasis in breast cancer is typically associated with poorer prognosis, CAM may represent a distinct entity with unique clinical implications[17]. Emerging evidence suggests that synchronous CAM differs prognostically from metachronous CAM; therefore, an individualized strategy rather than a standard-of-care protocol should be used for each patient.
The concept of oligometastasis is relevant to isolated CAM as well as this case. Oligometastatic disease, characterized by limited metastatic burden, has been associated with better survival rates than polymetastatic disease[18]. A critical debate in the management of metastatic breast cancer lies in the choice between systemic therapy alone and a combination of systemic and localized therapies. Systemic therapy remains the standard of care for metastatic disease, due to its ability to address disseminated cancer cells[19,20]. Nevertheless, in cases of limited metastatic burden, combining systemic therapy with localized interventions, such as surgery and stereotactic body radiotherapy, has shown promise in improving outcomes[21,22].
Another consideration was whether contralateral axillary surgery was suitable for this patient. Although the role of contralateral axillary surgery in CAM is not well-established, it could provide several potential benefits, including local disease control and improved staging accuracy. Nonetheless, it is essential to carefully weigh its limitations, including the risk of surgical morbidity and uncertain survival benefits[23,24]. Further studies are required to elucidate the indications for contralateral axillary surgery in CAM, particularly in the context of TNBC.
Especially considering the complexities of TNBC, comprehensive imaging plays a vital role in its management. A previous study emphasized that preoperative PET scans significantly affect early-stage breast cancer cases with tumors ≥ 2 cm[25], demonstrating superior performance in detecting axillary lymph node metastases than other diagnostic imaging methods, although SLNB remains the gold standard technique[26,27]. The use of whole-body PET scans, although not routinely performed for early-stage breast cancer, may facilitate the early detection of distant metastases not evident on other imaging modalities such as abdominal sonography, chest X-ray, and even CT scans[10]. In our case, the identification of FDG-avid lesions in the right axilla emphasizes the importance of PET scans in achieving early and accurate staging of TNBC, which is essential for accurately guiding treatment decisions and improving prognostication[25,28].
The findings from this case prompt a discussion on whether whole-body PET scans should be routinely incorporated into the diagnostic workup for early-stage breast cancer. Although whole-body PET scans are not currently standard for early-stage breast cancer, concerns regarding cost, radiation exposure, and risk of false positives leading to overtreatment remain inadequately addressed[29]. Nevertheless, in aggressive subtypes such as TNBC and HER2-positive breast cancer, PET scans may provide significantly improved staging accuracy[28]. The detection of asymptomatic metastatic disease can lead to changes in staging and enable more precise adjuvant therapies, including chemotherapy and radiotherapy. This case presents such a scenario, where the patient’s disease was upgraded from stage IIA to stage IV based on PET findings and subsequent tissue diagnosis.
Therefore, further investigations are necessary to establish clear guidelines and identify which patients with early-stage breast cancer might benefit from routine whole-body PET scans and further treatment of CAM. This is particularly applicable for those with high-risk features such as aggressive pathology, a high Ki-67 index, large tumors, and immunocompromised status[30].
This case underscores the aggressive nature of TNBC and the importance of detailed diagnostic evaluation and follow-up, particularly considering the unusual patterns of metastasis[31,32]. The isolated CAM emphasizes the need for tailored treatment strategies and vigilant monitoring. The management of CAM must consider the patient’s clinical context, input from a multidisciplinary team, and the potential role of advanced imaging techniques, such as whole-body PET scans. Therapeutic decision-making should also consider factors such as the extent of the metastatic disease, the potential for localized treatments, and the patient’s overall prognosis and preferences. Although whole-body PET scans are not yet the standard for early-stage breast cancer, they may provide significant benefits in accurately staging aggressive subtypes such as TNBC[33]. Further studies are required to develop guidelines for the selective use of whole-body PET scans in early-stage TNBC, particularly for patients with high-risk features[34]. Moreover, studies focusing on optimizing the management of CAM through a combination of systemic and localized therapies, guided by multidisciplinary input and tailored to the individual patient, are necessary to optimize treatment strategies and improve outcomes.
The authors wish to acknowledge the assistance of the people in the Department of Surgery, Hematology and Oncology, Pathology, Nuclear Medicine and Radiology, Tri Service General Hospital, National Defense Medical Center. This report would not have been possible without their efforts in data collection and interprofessional collaboration in treating this patient.
| 1. | Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 836] [Article Influence: 209.0] [Reference Citation Analysis (0)] |
| 2. | DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 2028] [Article Influence: 338.0] [Reference Citation Analysis (0)] |
| 3. | Mamounas EP, Kuehn T, Rutgers EJT, von Minckwitz G. Current approach of the axilla in patients with early-stage breast cancer. Lancet. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Makki J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin Med Insights Pathol. 2015;8:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 5. | Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1973] [Article Influence: 219.2] [Reference Citation Analysis (0)] |
| 6. | Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3591] [Cited by in RCA: 3997] [Article Influence: 285.5] [Reference Citation Analysis (0)] |
| 7. | Schmid P, Cortes J, Dent R, McArthur H, Pusztai L, Kümmel S, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Im SA, Untch M, Fasching PA, Mouret-Reynier MA, Foukakis T, Ferreira M, Cardoso F, Zhou X, Karantza V, Tryfonidis K, Aktan G, O'Shaughnessy J; KEYNOTE-522 Investigators. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N Engl J Med. 2024;391:1981-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 101] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 8. | Ali MA, Aiman W, Shah SS, Hussain M, Kashyap R. Efficacy and safety of pembrolizumab based therapies in triple-negative breast cancer: A systematic review of clinical trials. Crit Rev Oncol Hematol. 2021;157:103197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429-4434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2803] [Cited by in RCA: 3582] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 10. | Groheux D, Hindie E. Breast cancer: initial workup and staging with FDG PET/CT. Clin Transl Imaging. 2021;9:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Zhao Q, Yang F, Wu HL, Mo M, Ling YX, Liu GY. Contralateral axillary lymph node metastasis in breast cancer: An oligometastatic-like disease. Breast. 2023;72:103589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Strazzanti A, Gangi S, Trovato C, Pacini N, Basile F. Contralateral lymph node metastasis in a woman with new primary breast cancer: Systemic desease or locoregional diffusion? Int J Surg Case Rep. 2018;53:400-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Díaz-Roldán J, Eguía-Larrea M, Rubio-Sánchez T, Muñoz-Bellvís L. Systematic review of synchronous contralateral axillary metastases in breast cancer: really M1 disease? Breast Cancer. 2022;29:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Jung JJ, Cheun JH, Kang E, Shin I, Byeon J, Lee H, Kim HK, Lee HB, Han W, Moon HG. Survival After Contralateral Axillary Metastasis in Breast Cancer. Ann Surg Oncol. 2024;31:5189-5196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2585] [Cited by in RCA: 3043] [Article Influence: 202.9] [Reference Citation Analysis (0)] |
| 16. | Lee J, Lee YJ, Bae SJ, Baek SH, Kook Y, Cha YJ, Lee JW, Son BH, Ahn SH, Lee HJ, Gong G, Jeong J, Lee SB, Ahn SG. Ki-67, 21-Gene Recurrence Score, Endocrine Resistance, and Survival in Patients With Breast Cancer. JAMA Netw Open. 2023;6:e2330961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 17. | Huston TL, Pressman PI, Moore A, Vahdat L, Hoda SA, Kato M, Weinstein D, Tousimis E. The presentation of contralateral axillary lymph node metastases from breast carcinoma: a clinical management dilemma. Breast J. 2007;13:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Terao M, Niikura N. Diagnosis of oligometastasis. Transl Cancer Res. 2020;9:5032-5037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 569] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 20. | Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397:1750-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 931] [Article Influence: 232.8] [Reference Citation Analysis (0)] |
| 21. | Yoon SM, Bazan JG. Navigating Breast Cancer Oligometastasis and Oligoprogression: Current Landscape and Future Directions. Curr Oncol Rep. 2024;26:647-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Burgaleta AM, Burguete AB, Gutiérrez LR, Nuín EB, Felipe GA, de la Vega FA. Local treatment in oligometastasis from breast cancer: an overview. Clin Transl Oncol. 2023;25:2861-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Zwimpfer TA, Schwab FD, Steffens D, Kaul F, Schmidt N, Geiger J, Geissler F, Heinzelmann-Schwarz V, Weber WP, Kurzeder C. Contralateral lymph node metastasis in recurrent ipsilateral breast cancer with Lynch syndrome: a locoregional event. World J Surg Oncol. 2023;21:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Zhang Z, Zhang Y, Sun X. Therapeutic options for contralateral axillary lymph node metastasis in breast cancer. Curr Probl Cancer. 2021;45:100706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Bernsdorf M, Berthelsen AK, Wielenga VT, Kroman N, Teilum D, Binderup T, Tange UB, Andersson M, Kjær A, Loft A, Graff J. Preoperative PET/CT in early-stage breast cancer. Ann Oncol. 2012;23:2277-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Ferahman S, Velidedeoglu M, Tosun Y, Albeniz G, Ulusan K, Celik V, Ferahman M, Gazioglu E. The effect of preoperative (18)F-FDG PET on the surgical decision in early breast cancer: 5-Year follow-up. Hell J Nucl Med. 2020;23:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Bove S, Fragomeni SM, Romito A, DI Giorgio D, Rinaldi P, Pagliara D, Verri D, Romito I, Paris I, Tagliaferri L, Marazzi F, Visconti G, Franceschini G, Masetti R, Garganese G. Techniques for sentinel node biopsy in breast cancer. Minerva Surg. 2021;76:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Hadebe B, Harry L, Ebrahim T, Pillay V, Vorster M. The Role of PET/CT in Breast Cancer. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 29. | Katal S, Eibschutz LS, Saboury B, Gholamrezanezhad A, Alavi A. Advantages and Applications of Total-Body PET Scanning. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Martei YM, Matro JM. Identifying patients at high risk of breast cancer recurrence: strategies to improve patient outcomes. Breast Cancer (Dove Med Press). 2015;7:337-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Cadoo KA, Fornier MN, Morris PG. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging. 2013;57:312-321. [PubMed] |
| 32. | Gündoğdu R, Gemici K, Kaya M, Turgut S. The effect of preoperative 18f Fdg-Pet on staging and treatment protocols in breast cancer patients. Ann Ital Chir. 2021;92:346-352. [PubMed] |
| 33. | Ko H, Baghdadi Y, Love C, Sparano JA. Clinical Utility of 18F-FDG PET/CT in Staging Localized Breast Cancer Before Initiating Preoperative Systemic Therapy. J Natl Compr Canc Netw. 2020;18:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Won KA, Spruck C. Triple‑negative breast cancer therapy: Current and future perspectives (Review). Int J Oncol. 2020;57:1245-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 35. | Amin MB, Gress DM, Meyer Vega LR, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Compton CC. AJCC Cancer Staging Manual, Eighth Edition. Cham: Springer, 2018. |