Published online Jun 26, 2025. doi: 10.12998/wjcc.v13.i18.101882
Revised: January 28, 2025
Accepted: February 12, 2025
Published online: June 26, 2025
Processing time: 150 Days and 17.7 Hours
Breast hamartomas are rare benign breast tumors, with an incidence rate of 0.8%-4.8%. Further, the coexistence of hamartomas and carcinoma is also uncommon. Our case report presents a unique instance where invasive ductal carcinoma (IDC) and ductal carcinoma in situ were found both inside and outside a breast hamartoma. This is the second case reported in the literature.
A 51-year-old woman presented with a 6.0 cm breast tumor on mammography and ultrasound, with suspicious areas indicative of malignant transformation. Biopsy of the suspicious area confirmed IDC with intraductal carcinoma. Breast magnetic resonance imaging showed typical hamartoma changes with irregular areas of abnormal enhancement both inside and outside. A breast-conserving surgery was performed, and postoperative pathology confirmed mammary hamartoma, concurrent with IDC and intraductal carcinoma occurring both inside and outside the hamartoma. Subsequently, appropriate adjuvant therapy was initiated. Currently, the patient is in good condition. Breast cancer may be located both inside and outside the ipsilateral mammary hamartoma, which is difficult to detect preoperatively, especially when there is a focus of intraductal carcinoma, requiring accurate assessment of the tumor extent by modern imaging techniques. Early detection of the coexistence of cancer is clinically important as it can alter patient management.
This case emphasizes the importance of modern imaging techniques in accurately evaluating mammary hamartomas associated with malignancies prior to surgery.
Core Tip: This case reports a rare instance of concurrent invasive ductal carcinoma and ductal carcinoma in situ inside and outside a breast hamartoma in a 51-year-old woman. Imaging suggested suspicious malignancy within the hamartoma, confirmed by postoperative pathology. It highlights the importance of modern imaging in preoperative evaluation of breast hamartomas associated with malignancies. Thorough assessment is crucial for determining safe margins in breast-conserving surgery and reducing postoperative recurrence risk.
- Citation: Wei L, Tian Z, Wang ZY, Liu WJ, Li HB, Zhang Y. Concurrent invasive ductal carcinoma and ductal carcinoma in situ arising inside and outside a breast hamartoma: A case report. World J Clin Cases 2025; 13(18): 101882
- URL: https://www.wjgnet.com/2307-8960/full/v13/i18/101882.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i18.101882
Breast hamartomas are rare, benign lesions resulting from the abnormal development of residual mammary ductal buds, fibrous tissue, and adipose tissue, typically encapsulated Hogeman and Osbterg[1] first described this lesion in 1968, with the term "hamartoma" introduced by Arrigoni et al[2]. in 1971. Breast hamartomas account for 0.8%-4.8% of benign breast tumors[3,4]. Clinically, they present as slow-growing, localized tumors with clear margins, often misdiagnosed as fibroadenomas or lipomas. The most common subtypes are adenolipomas and chondrolipomas. Although generally benign and not increasing breast cancer risk, there is a possibility of associated malignancies. Over ten cases of breast cancer associated with breast hamartomas have been reported, with most cancer lesions occurring within the hamartoma[5-9]. This report details a rare case of invasive ductal carcinoma (IDC) with ductal carcinoma in situ occurring both inside and outside a breast hamartoma.
A 51-year-old woman was admitted to our hospital due to the discovery of a mass in the right breast for 2 days.
Two days ago, during a physical examination at another hospital, the patient was found to have an abnormal hypoechoic nodule in the right breast through ultrasound examination. The nodule measured approximately 1.11 cm × 0.81 cm × 1.44 cm, with unclear borders and an irregular shape, classified as Breast Imaging Reporting and Data System 4B. She was referred to our breast clinic for further evaluation.
There was no history of past illness.
There was no personal and family history.
Physical examination revealed a palpable, oval mass in the outer right breast, approximately 6.0 cm × 5.0 cm × 5.0 cm, firm with smooth surfaces and good mobility. However, near the nipple, the mass was hard with unclear borders and poor mobility.
Not applicable.
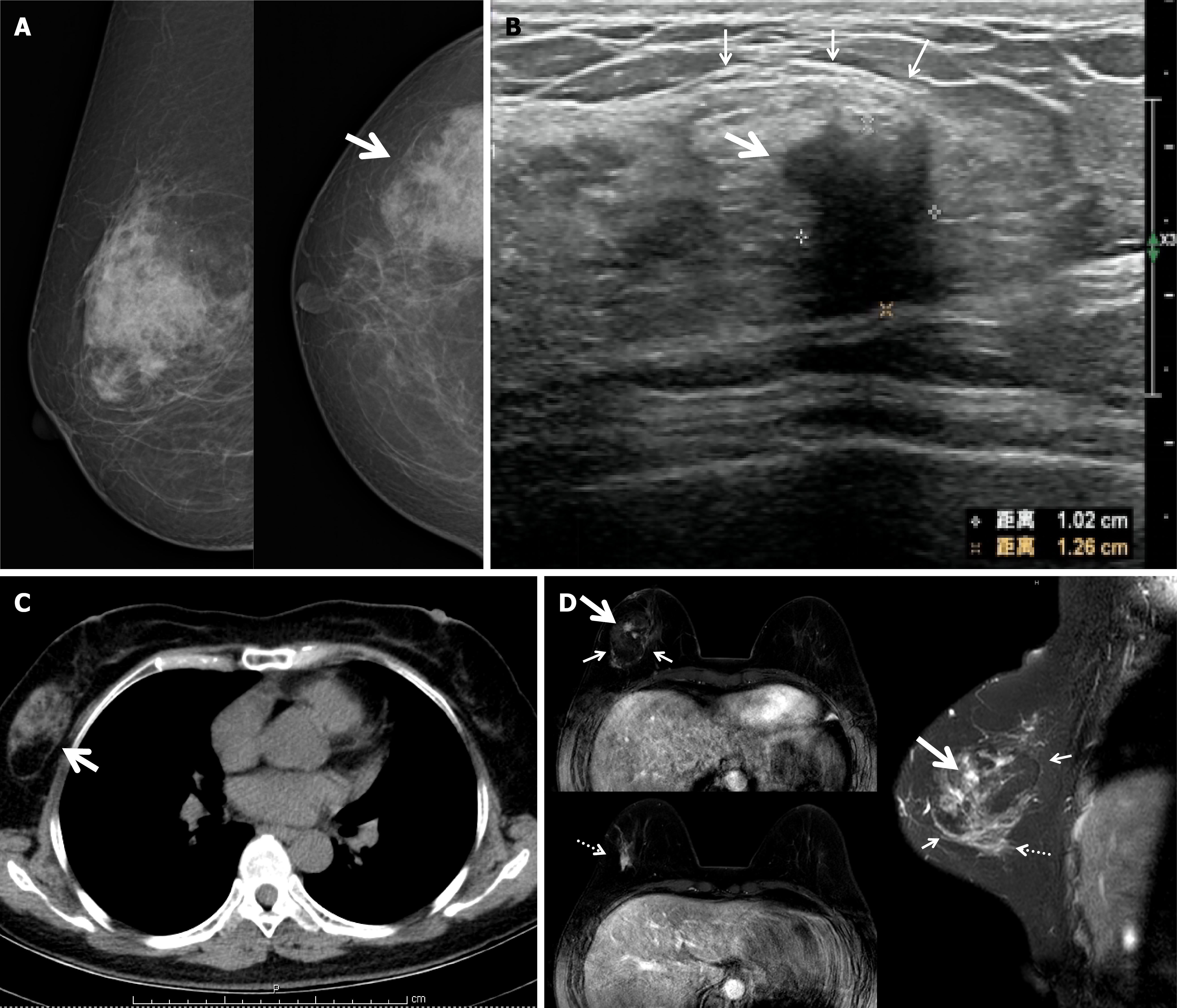
Mammography showed an irregular density increase in the outer right breast, measuring approximately 8.3 cm × 5.7 cm (Figure 1A). Due to discrepancies between the ultrasound findings and physical examination, a repeat ultrasound was performed. It revealed an irregular hypoechoic structure in the outer right breast, measuring approximately 1.02 cm × 1.26 cm, with unclear borders and blood flow signals, suggesting a suspicious malignancy. Additionally, an uneven hypoechoic structure with a suspected capsule, measuring approximately 5.5 cm × 3.5 cm × 3.0 cm, was observed in the outer right breast near the suspicious hypoechoic structure (Figure 1B). Chest computed tomography showed an oval mass in the outer right breast with uneven density (Figure 1C). Magnetic resonance imaging (MRI) clearly showed a 5.4 cm hamartoma with an IDC and ductal carcinoma in situ lesion within it. Additionally, a suspicious lesion outside the hamartoma exhibiting a type 2 enhancement curve suggestive of malignancy was detected (Figure 1D).
Ultrasound-guided biopsy of the irregular hypoechoic structure revealed IDC with low-grade ductal carcinoma in situ.
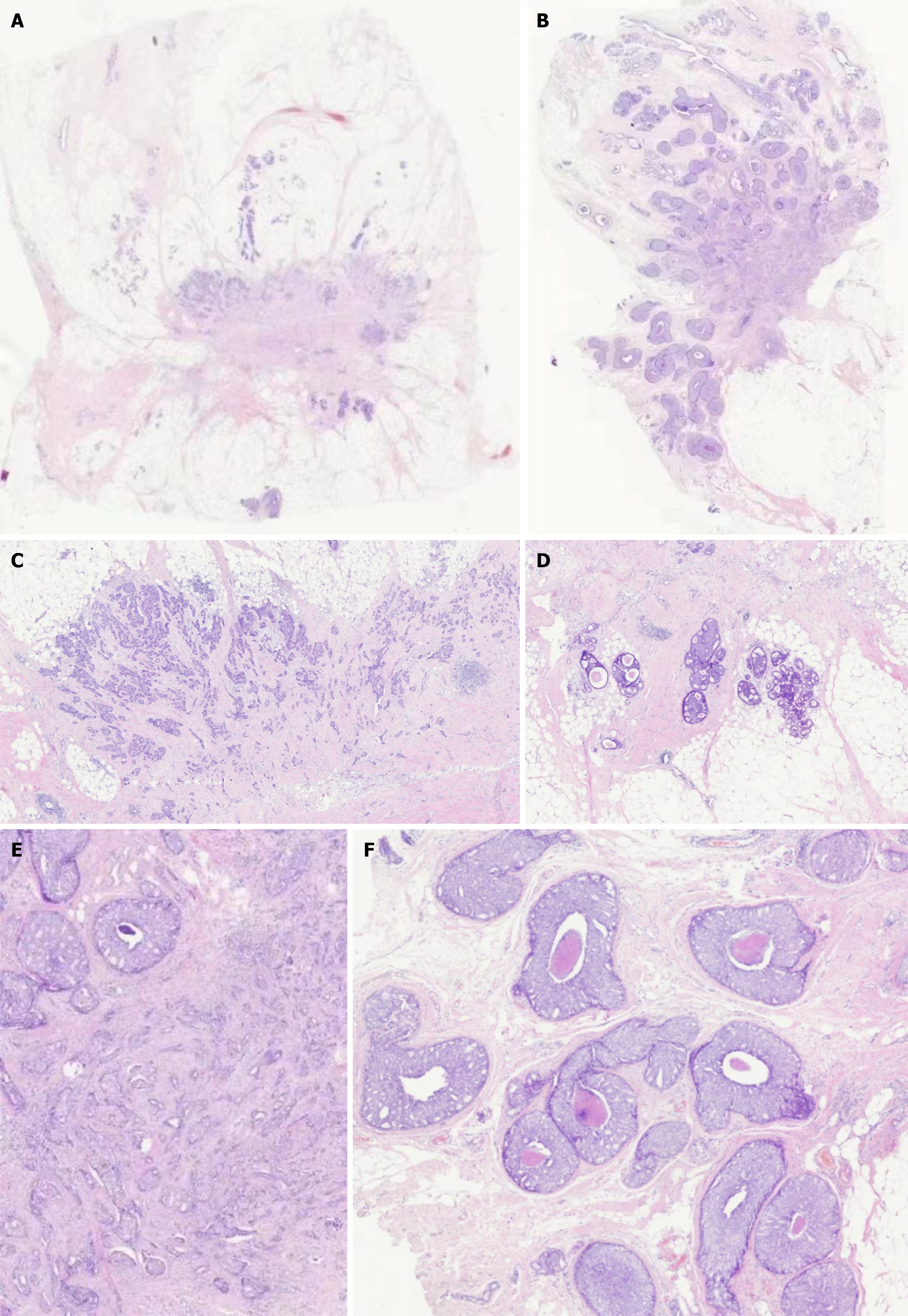
Postoperatively, pathology showed IDC (grades I-II) with two cancer foci, both being IDC. One focus was within the hamartoma, measuring approximately 1.0 cm × 0.5 cm, and the other was outside the hamartoma, with a maximum microscopic diameter of approximately 0.7 cm. Both foci contained abundant low-grade to intermediate-grade ductal carcinoma in situ with comedo-type necrosis (Figure 2). Immunohistochemistry revealed: (1) Estrogen receptor (100% 3+); (2) Progesterone receptor (90% 2+); (3) Human epidermal growth factor receptor 2 (1+); (4) Ki67 (10%+); (5) Calponin (-); (6) P63 (-); (7) Cytokeratin (CK) 5/6 (-); and (8) CK8 (+).
The patient expressed a desire for breast-conserving surgery and therefore underwent breast-conserving surgery and sentinel lymph node biopsy. Postoperatively, she received adjuvant radiotherapy and is currently undergoing letrozole treatment.
During the 19-month follow-up, no recurrence or metastasis has been detected.
The incidence of breast cancer associated with hamartomas is very low. The first case was reported by Mendiola et al[5] in 1982. To date, 19 cases have been reported in the literature[6,10-12]. In previous reports, the median age of patients was 59 years (range: 25-78 years)[6,13,14]. The patient in this case is 51 years old, younger than the median age.
In this case, a palpable mass was found in the lateral right breast, characterized by clear boundaries and good mobility, consistent with benign hamartoma features as reported in the literature. However, the periphery of the mass near the nipple was hardened, with unclear boundaries and poor mobility, more indicative of malignancy, distinguishing this case from others.
Mammography in this case revealed typical benign hamartoma features, such as uneven glandular density, with no signs of malignancy like calcification. In previous reports, mammography was performed in 10 cases, and in addition to the typical appearance of hamartomas, suspicious malignant features such as clustered microcalcifications, polymorphic microcalcifications, spiculated masses, and twisted amorphous calcifications were observed[6,15-17]. In contrast, no such features were found in this case.
Breast MRI performed in this case indicated not only the typical lobulated abnormal signal of a mammary hamartoma but also an irregular abnormal enhancement signal within the hamartoma, confirmed by biopsy as an IDC with ductal carcinoma in situ. Additionally, a local abnormal enhancement signal below the hamartoma was detected, with a dynamic enhancement curve (TICmax) showing a plateau (type II), suggesting malignancy. The pathological diagnosis from the surgically removed lesion confirmed the biopsy results. This lesion was not detected in the physical examination, ultrasound, or mammography, suggesting that breast MRI may be a valuable imaging tool for detecting malignant lesions associated with mammary hamartomas.
In previously reported cases, 14 were diagnosed as IDC or ductal carcinoma in situ, 4 as lobular carcinoma, and 1 as mixed ductal and lobular carcinoma[6,18-20]. Our case was diagnosed as IDC with a significant component of low-grade ductal carcinoma in situ, consistent with the majority of reported cases. Among the three cases with reported molecular subtypes, estrogen and progesterone receptors were either negative or weakly positive[7,9,12]. In our case, both the invasive lesion within the hamartoma and the one outside it were strongly positive for estrogen and progesterone receptors, with a molecular subtype of Luminal A, differing from previous reports.
In our case, the foci of invasive carcinoma and ductal carcinoma in situ were located both within and outside the hamartoma. Despite the distance between the two foci, their molecular subtypes were similar, suggesting that they may have originated as a single central lesion within the hamartoma, similar to the case reported by Anani and Hessler[7] in 2012. The mechanism by which the ductal carcinoma in situ component migrated outside the hamartoma remains unclear. We propose the following potential mechanisms: (1) There may be an anastomosis between the ductal systems of the hamartoma and the surrounding gland. Ohtake et al[21] found multiple anastomoses between different mammary ductal systems. There is no true fibrous capsule between normal breast tissue and hamartomas, only compressed adjacent breast tissue. It is possible that anastomoses between the lobules could allow ductal carcinoma in situ within the hamartoma to spread outside via ductal anastomoses. However, apart from Ohtake et al's study[21], other studies have not found such connections[22], suggesting that these anastomoses may not be common and further research is needed; and (2) Ductal carcinoma in situ may spread within the breast ducts toward the nipple and then to adjacent mammary ductal lobular systems, which we consider the most likely mechanism of dissemination. Studies have found that different ductal systems connect together behind the areola, forming 5-8 common collectors[23]. Ductal carcinoma in situ may spread to adjacent ductal lobular units via these common collectors and then retrogradely spread peripherally, forming extensive ductal spread. Epigenetic events such as telomere crisis[24] could then allow the tumor cells to break through the basement membrane and migrate into adjacent tissues[25], forming invasive lesions. The pathology and MRI findings of our reported case further support this possibility.
This case demonstrates that although most mammary hamartomas are benign, there is a potential for malignancy. Breast MRI serves as a useful tool in detecting malignant lesions associated with mammary hamartomas. The ductal carcinoma arising from a mammary hamartoma can also cause metastasis, whereby cancer cells spread to adjacent or distant ductal lobular units. Therefore, a thorough evaluation of the lesion's nature and defined margins is necessary before proceeding with surgical treatment.
| 1. | Hogeman KE, Ostberg G. Three cases of postlactational breast tumour of a peculiar type. Acta Pathol Microbiol Scand. 1968;73:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Arrigoni MG, Dockerty MB, Judd ES. The identification and treatment of mammary hamartoma. Surg Gynecol Obstet. 1971;133:577-582. [PubMed] |
| 3. | Fisher CJ, Hanby AM, Robinson L, Millis RR. Mammary hamartoma--a review of 35 cases. Histopathology. 1992;20:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Sevim Y, Kocaay AF, Eker T, Celasin H, Karabork A, Erden E, Genc V. Breast hamartoma: a clinicopathologic analysis of 27 cases and a literature review. Clinics (Sao Paulo). 2014;69:515-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Mendiola H, Henrik-Nielsen R, Dyreborg U, Blichert-Toft M, Al-Hariri JA. Lobular carcinoma in situ occurring in adenolipoma of the breast. Report of a case. Acta Radiol Diagn (Stockh). 1982;23:503-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Choi N, Ko ES. Invasive ductal carcinoma in a mammary hamartoma: case report and review of the literature. Korean J Radiol. 2010;11:687-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kai M, Tada K, Tamura M, Gomi N, Horii R, Akiyama F, Iwase T. Breast cancer associated with mammary hamartoma. Breast Cancer. 2012;19:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Lambert J, Jerjir N, Casselman J, Steyaert L. Invasive lobular carcinoma arising in a hamartoma of the breast: a case report. Clin Breast Cancer. 2015;15:e63-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Komaki K, Funagayama M, Saitoh T, Maeda Y, Hayashi T, Kanematsu M, Tangoku A. Ductal carcinoma in situ of the breast arising in encapsulated mammary hamartoma ; A case report. J Med Invest. 2020;67:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Pervatikar SK, Rao R, Dinesh US, Parameswaraiah S. Large mammary hamartoma with focal invasive ductal carcinoma. Indian J Pathol Microbiol. 2009;52:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kemp TL, Kilgore MR, Javid SH. Invasive ductal carcinoma arising within a large mammary hamartoma. Breast J. 2015;21:196-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Fukai S, Yoshida A, Akiyama F, Tsunoda H, Lefor AK, Kimura J, Sakamoto T, Suzuki K, Mizokami K. Ductal Carcinoma in situ of the breast in sclerosing adenosis encapsulated by a hamartoma: A case report. Int J Surg Case Rep. 2018;45:9-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Coyne J, Hobbs FM, Boggis C, Harland R. Lobular carcinoma in a mammary hamartoma. J Clin Pathol. 1992;45:936-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Anani PA, Hessler C. Breast hamartoma with invasive ductal carcinoma. Report of two cases and review of the literature. Pathol Res Pract. 1996;192:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Baron M, Ladonne JM, Gravier A, Picquenot JM, Berry M. Invasive lobular carcinoma in a breast hamartoma. Breast J. 2003;9:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lee EH, Wylie EJ, Bourke AG, Bastiaan De Boer W. Invasive ductal carcinoma arising in a breast hamartoma: two case reports and a review of the literature. Clin Radiol. 2003;58:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Ruiz-Tovar J, Reguero-Callejas ME, Aláez AB, Ramiro C, Rojo R, Collado MV, González-Palacios F, Muñoz J, García-Villanueva A. Infiltrating ductal carcinoma and ductal carcinoma in situ associated with mammary hamartoma. Breast J. 2006;12:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Mester J, Simmons RM, Vazquez MF, Rosenblatt R. In situ and infiltrating ductal carcinoma arising in a breast hamartoma. AJR Am J Roentgenol. 2000;175:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Tse GM, Law BK, Pang LM, Cheung HS. Ductal carcinoma in situ arising in mammary hamartoma. J Clin Pathol. 2002;55:541-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kuroda N, Sugimoto T, Numoto S, Enzan H. Microinvasive lobular carcinoma associated with intraductal spread arising in a mammary hamartoma. J Clin Pathol. 2002;55:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ohtake T, Kimijima I, Fukushima T, Yasuda M, Sekikawa K, Takenoshita S, Abe R. Computer-assisted complete three-dimensional reconstruction of the mammary ductal/lobular systems: implications of ductal anastomoses for breast-conserving surgery. Cancer. 2001;91:2263-2272. [PubMed] [DOI] [Full Text] |
| 22. | Going JJ, Moffat DF. Escaping from Flatland: clinical and biological aspects of human mammary duct anatomy in three dimensions. J Pathol. 2004;203:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Halliwell TM. Atlas of Ultrasound and Ductal Echography of the Breast (1995) Blackwell Science,Germany. Clin Radiol. 1996;. |
| 24. | Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, Kuo WL, Ljung BM, Chew K, Myambo K, Miranda M, Krig S, Garbe J, Stampfer M, Yaswen P, Gray JW, Lockett SJ. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Casasent AK, Schalck A, Gao R, Sei E, Long A, Pangburn W, Casasent T, Meric-Bernstam F, Edgerton ME, Navin NE. Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing. Cell. 2018;172:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |