Published online Jun 26, 2025. doi: 10.12998/wjcc.v13.i18.101471
Revised: January 25, 2025
Accepted: February 18, 2025
Published online: June 26, 2025
Processing time: 164 Days and 21.7 Hours
Luscan-Lumish syndrome (LLS) is a rare genetic congenital anomaly syndrome characterised by neurodevelopmental disorders, including delayed psychomotor development, behavioral difficulties, relative or true macrocephaly and, in individual cases, ocular abnormalities. This paper aims to present the case of a child with ocular abnormalities associated with LLS.
A 10-year-old girl born at 40 weeks gestation with features of dysmorphia, neurodevelopmental disorders, genetically confirmed LLS, convergent strabismus and suspected congenital glaucoma. Eye examination, ultrasound, optical coherence tomography (OCT), perimetry and electrophysiological study [pattern visually evoked potentials (VEP)] were performed. Best-corrected distance visual acuity was 0.5 in the right eye (correction -1.0 Dsph, -1.0 Dcyl, axis 180°) and 0.62 in the left eye (correction -2.0 Dsph). Near visual acuity (Snellen Chart) with the above correction was -0.5 D. A cycloplegic refraction test yielded -1.25 Dsph, -1.25 Dcyl, axis 165° in the right eye, and -2.0 Dsph, -0.25 Dcyl, axis 154° in the left eye. Intraocular pressure was 15 mmHg in both eyes. OCT of the maculae showed no abnormalities. In both eyes, the average ganglion cell layer and inner plexiform layer thickness was 73 μm. OCT of the optic nerve disc showed an average retinal nerve fibre layer thickness of 89 μm in the right eye and 81 μm in the left eye, with symmetry of 90%. The rim area was 1.59 mm2 and 1.74 mm2 in the right and left eye, respectively. The disc area was 2.77 mm² in the right eye and 2.89 mm2 in the left. The average cup-to-disc ratio was 0.64 in the right eye and 0.62 in the left eye. Ocular ultrasound depicted single extra echoes inside the vitreous chamber; otherwise, there were no abnormalities. Right and left eyeball lengths were 24.59 mm and 24.51 mm, respectively. Kinetic perimetry revealed no visual field defects, while static testing showed single relative scotomas. The mean defect was 4.7 dB in the right and 2.6 dB in the left eye. The loss variance values were 4.8 and 3.8 dB for the right and left eye, respectively. Pattern VEP test revealed normal values of P100 Latency. Wave amplitude in the right eye was 50% at a visual angle of 1.0° and 30% at 15’. Due to the rarity of LLS, it seems interesting to present the child ophthalmological examination with changes in the electrophysiological examination.
Although eye abnormalities are infrequently described in children with LLS, the patients should undergo eye examinations, especially as they may have central nervous system anomalies that may give rise to visual impairments. Generally, children with genetically determined congenital syndromes should receive regular ophthalmic check-ups for a thorough evaluation of the eyes and prognosis of the development of visual function.
Core Tip: Children with Luscan-Lumish syndrome (LLS) have ocular pathologies, which include strabismus, astigmatism, visual impairment associated with optic nerve hypoplasia or abnormalities in the visual cortex. Retinal vascular telangiectasias, glaucoma or cataracts are also described. This paper aims to present the case of a child with ocular abnormalities associated with LLS.
- Citation: Wójcik-Niklewska B, Filipek E. Luscan-Lumish syndrome: A case report. World J Clin Cases 2025; 13(18): 101471
- URL: https://www.wjgnet.com/2307-8960/full/v13/i18/101471.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i18.101471
Luscan-Lumish syndrome (LLS) is a rare, genetically determined congenital syndrome caused by various pathogenic variants in the SETD2 gene[1-3]. It is characterised by neurodevelopmental disorders featuring delayed psychomotor development, behavioural disorders, primarily autism spectrum disorders, and relative or true macrocephaly. Some children develop tonic-clonic epileptic seizures with onset between 3 and 10 years of age. Brain imaging studies may reveal type I Chiari and/or Dandy-Walker malformations, dilatation of the brain ventricles, and absence or hypoplasia of the corpus callosum. Speech delay is a characteristic feature. Other less common disorders include excessive height and weight[1-3]. Endocrine disorders leading to precocious puberty, hypothyroidism and growth hormone deficiency may also occur. Hearing loss is often found on audiological examination[4]. Children with LLS also suffer from frequent respiratory tract infections, ear infections and sinus infections, but no specific immune disorders have been described. In addition, sleep apnoea, gastrooesophageal reflux, constipation, heart defects, scoliosis and joint hypermobility have been observed[5]. In individual cases, ocular pathologies, which include strabismus, astigmatism, visual impairment associated with optic nerve hypoplasia or abnormalities in the visual cortex are observed. Retinal vascular telangiectasias, glaucoma or cataracts are also described.
This paper aims to present the case of a girl with ocular abnormalities associated with LLS.
A ten-year-old girl diagnosed with LLS was admitted for an eye examination to the Department of Paediatric Ophthalmology, Professor K. Gibiński University Hospital Center, Medical University of Silesia, Katowice, Poland.
The girl remains under the care of a speech therapist due to slurred speech, a psychologist due to autism spectrum disorder and intellectual disability and an ENT specialist due to a slight hearing loss.
Sucking abnormalities were observed in infancy, resulting in poor weight gain up to 4 years of age. Incidents of sleep apnoea and morning hypoglycaemia occurred. Psychomotor retardation was noted in the first year of life. Due to hypotonia, the child required rehabilitation from 4 months of age. She spoke her first words at three years of age and simple sentences at six years of age; autism was diagnosed at that time. Social and intellectual development was delayed. Head magnetic resonance imaging (MRI) performed at six months of age revealed hypoplasia of the corpus callosum. Encephalography revealed abnormalities resembling epileptic seizures despite the absence of seizure episodes. At four years of age, the child presented for genetic consult for the first time. Physical examination revealed hypertelorism, a prominent forehead with a high frontal hairline, a broad nasal bridge, a short philtrum, a pointed chin, a narrow mouth, low-set ears, broad distal phalanges of the fingers of both hands and hypertrophy of the elbow joints. A wide-based gait and slurred speech were also observed. At four years of age, the child measured 101 cm (10th percentile) and weighed 13.3 kg (below the 3rd percentile). Consultation with specialists did not reveal any heart defects, digestive system or endocrine disorders.
The patient is the first child of young, healthy, and unrelated parents. The family history was noncontributory. The girl was born at 40 weeks gestation, with a weight of 2820 g, a length of 52 cm and an occipital frontal circumference of 34 cm.
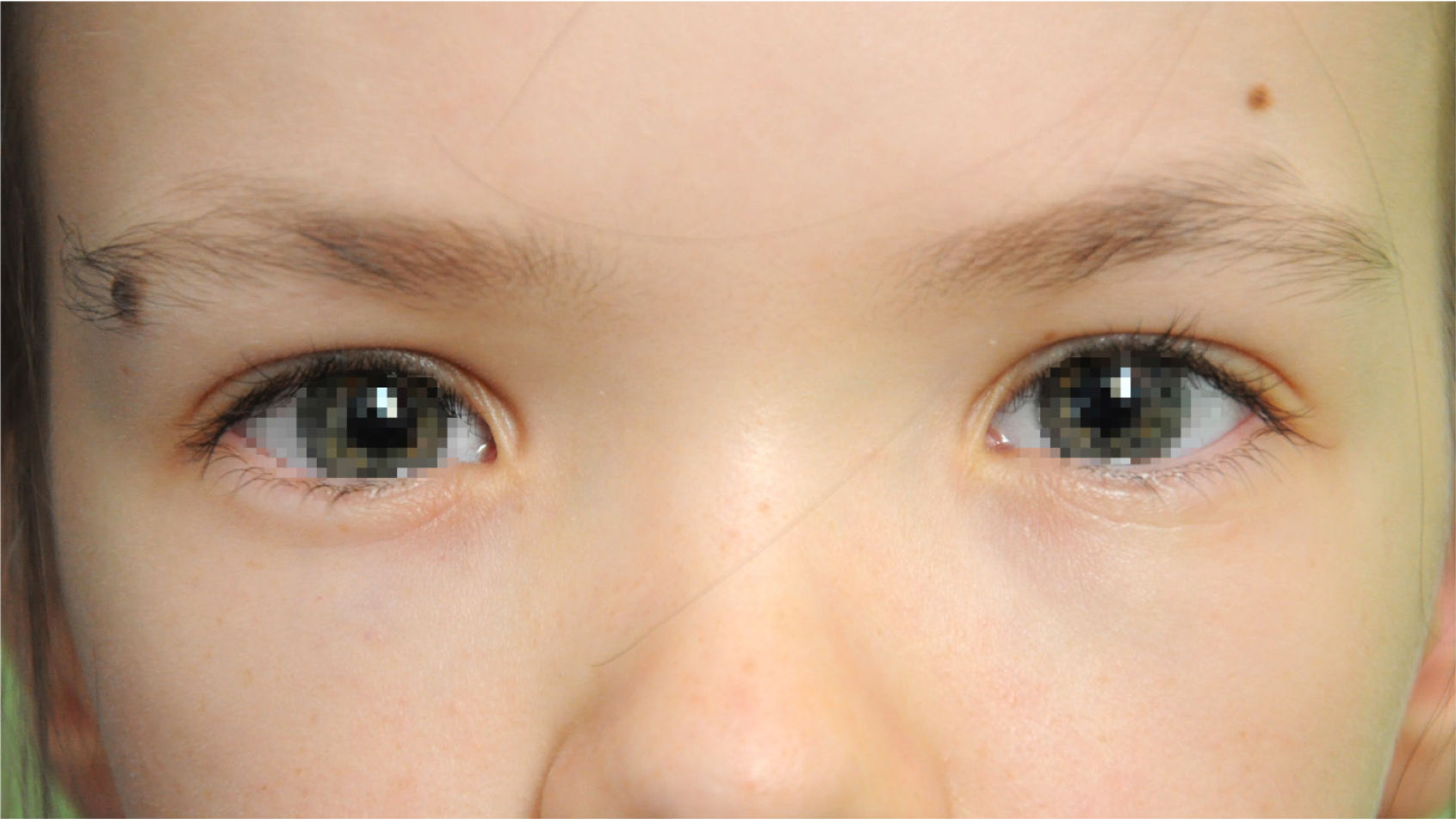
On the day of admission to the Department of Paediatric Ophthalmology, the child was in good general conditio. Physical examination revealed features of facial dysmorphia with hypertelorism. The girl spoke indistinctly and was reluctant to answer questions (Figure 1).
Eye examination revealed convergent strabismus of 11°, simultaneous perception without fusion and stereopsis, and normal pupillary response to light. The best-corrected distance visual acuity was 0.5 in the right eye (correction -1.0 Dsph, -1.0 Dcyl, axis 180°) and 0.62 in the left eye (correction -2.0 Dsph). Near visual acuity (Snellen Chart) with the above correction was -0.5 D. A cycloplegic refraction test yielded -1.25 Dsph, -1.25 Dcyl, axis 165° in the right eye, and -2.0 Dsph, -0.25 Dcyl, axis 154° in the left eye. Slit-lamp examination revealed no abnormalities in the anterior segments. The fundus showed pink optic nerve discs with clear borders and a cup-to-disc ratio of 0.6. Pink maculae with reflexes were observed; no vascular abnormalities were found.
Before hospitalisation in the Paediatric Ophthalmology Unit, the child underwent genetic testing of DNA isolated from peripheral blood. Whole exome sequencing using the SureSelectXT Human All Exon V5 Kit was applied, yielding the following results: Transcript at the cDNA level: C.4860dupA; transcript at the protein level: P.Gly1621ArgfsTer12. SETD2 gene; reference sequence: LRG_775t1; NM_014159.6; chromosomal position: Hg38 3: 047103402-C > CT.
Frequency in the genome aggregation database (gnomAD): 0. Heterozygous system. Genotype notation according to HGVS: LRG_775t1: c.[4860dupA]; [4860 =]. The selected variant was analysed using ADS in the patient, her parents, and two brothers. The presence of a pathogenic variant in SETD2 was ruled out in both parents and brothers.
The patient’s intraocular pressure was 15 mmHg in both eyes. Optical coherence tomography (OCT) of the anterior segment revealed the following findings: A corneal thickness of 506 μm in the right eye and 510 μm in the left eye; an anterior chamber depth of 3.05 mm in the right eye and 3.09 mm in the left eye; and a lens thickness of 3.73 mm in both eyes. No macula abnormalities were found. The average GCL-IPL thickness was 73 μm in both eyes. OCT of the optic nerve disc revealed an average retinal nerve fibre layer thickness of 89 μm in the right eye and 81 μm in the left eye, with symmetry of 90%. The rim area was 1.59 mm2 and 1.74 mm2 in the right and left eyes, respectively. The disc area was 2.77 mm2 in the right eye and 2.89 mm2 in the left eye. The average cup-to-disc ratio was 0.64 in the right eye and 0.62 in the left eye.
Ocular ultrasound revealed single extra echoes inside the vitreous chamber; otherwise, there were no abnormalities. The right and left eyeball lengths were 24.59 mm and 24.51 mm, respectively. Kinetic perimetry revealed no visual field defects, whereas static testing revealed single relative scotomas. The mean defect was 4.7 dB in the right and 2.6 dB in the left eye. The loss variance values were 4.8 and 3.8 dB for the right and left eyes, respectively. The pattern VEP test revealed normal P100 Latency values. The wave amplitude for the right eye was 50% at a visual angle of 1.0 deg and 30% at 15 min. An abnormal shape of the VEP response, i.e., an extended P100 wave peak, may suggest optic nerve atrophy/underdevelopment or central nervous system abnormalities.
The final diagnosis was LLS with an abnormal shape of VEP response.
No treatment was performed.
Follow-up visits have been scheduled.
LLS is a rare, genetically determined congenital syndrome with a variable phenotype. Eye disorders have also been described in patients with LLS. Our patient was found to have speech disorders characteristic of the syndrome, autism, intellectual disability, dysmorphia, hearing loss, ossification disorders and hypoplasia of the corpus callosum on magnetic resonance imaging. Due to problems with distance vision, the child was referred to the Paediatric Ophthalmology Department for a complete ophthalmic examination, which revealed convergent strabismus, myopia in both eyes, astigmatism in the right eye, lack of fusion and stereopsis, reduced levels of the retinal ganglion cell complex, changes in OCT of the optic disc, and an abnormal pattern VEP recording suggesting optic nerve underdevelopment or central nervous system anomalies, possibly related to hypoplasia of the corpus callosum. Fundus images did not show any vascular pathology. The child was prescribed corrective glasses and vision rehabilitation, and a follow-up appointment was scheduled.
Since LLS is a rare condition, ocular abnormalities are sporadically described, especially in paediatric patients.
Van Rij et al[2] described two patients with LLS: A 4.5-year-old boy had mild ptosis, whereas a 23-year-old female patient had slightly downslanted palpebral fissures and clear blue eyes. The ophthalmic evaluation was unremarkable in both patients. Wu et al[6] identified a novel pathogenic SETD2 variant in a 3-year-old Chinese boy but reported no eye disorders. Rabin et al[7] described 15 children with de novo variants in codon 1740 of SETD2. One child had normal eye images at two weeks of life; a repeat examination at 16 months of age revealed leukocoria, exudative detachment and telangiectasia around the periphery of the retina in the right eye, and blood vessels stop and star exudate in the macula of the left eye. Despite the use of a prophylactic laser, retinal detachment was found involving the upper quadrants.
Lumish et al[8] reported case of a 17-year-old girl with a de novo c.2028delT (P677 LfsX19) mutation in the SET domain-containing protein 2 (SET Domain Containing 2, Histone Lysine Methyltransferase) SETD2 gene. She presented with intellectual disabilities and autism spectrum disorder, did not walk until 18 months of age and began speaking at two years of age. Head MRI revealed Arnold-Chiari type I malformation and mild to moderate hydrocephalus of the third and lateral ventricles. However, the authors did not report any eye changes[8]. Suda et al[3] also did not mention ocular abnormalities, describing the case of a 20-year-old male with access to the results of an eye examination performed when he was 11 years old.
Although eye abnormalities are infrequently described in children with LLS, patients should undergo eye examinations, especially as they may have central nervous system anomalies that may give rise to visual impairments. Generally, children with genetically determined congenital syndromes should receive regular ophthalmic check-ups for a thorough evaluation of the eyes and prognosis of the development of visual function.
| 1. | Luscan A, Laurendeau I, Malan V, Francannet C, Odent S, Giuliano F, Lacombe D, Touraine R, Vidaud M, Pasmant E, Cormier-Daire V. Mutations in SETD2 cause a novel overgrowth condition. J Med Genet. 2014;51:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | van Rij MC, Hollink IHIM, Terhal PA, Kant SG, Ruivenkamp C, van Haeringen A, Kievit JA, van Belzen MJ. Two novel cases expanding the phenotype of SETD2-related overgrowth syndrome. Am J Med Genet A. 2018;176:1212-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Suda K, Fukuoka H, Iguchi G, Kanie K, Fujita Y, Odake Y, Matsumoto R, Bando H, Ito H, Takahashi M, Chihara K, Nagai H, Narumi S, Hasegawa T, Ogawa W, Takahashi Y. A Case of Luscan-Lumish Syndrome: Possible Involvement of Enhanced GH Signaling. J Clin Endocrinol Metab. 2021;106:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Zhang H, Wu W, Wang D, Lv Y, Zhao D, Wang L, Liu Y, Zhang K. Clinical and genetic features of luscan-lumish syndrome associated with a novel de novo variant of SETD2 gene: Case report and literature review. Front Genet. 2023;14:1081391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Pappas J, Rabin R. SETD2 Neurodevelopmental Disorders. 2021 Dec 30. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–. [PubMed] |
| 6. | Wu Y, Liu F, Wan R, Jiao B. A novel SETD2 variant causing global development delay without overgrowth in a Chinese 3-year-old boy. Front Genet. 2023;14:1153284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Rabin R, Radmanesh A, Glass IA, Dobyns WB, Aldinger KA, Shieh JT, Romoser S, Bombei H, Dowsett L, Trapane P, Bernat JA, Baker J, Mendelsohn NJ, Popp B, Siekmeyer M, Sorge I, Sansbury FH, Watts P, Foulds NC, Burton J, Hoganson G, Hurst JA, Menzies L, Osio D, Kerecuk L, Cobben JM, Jizi K, Jacquemont S, Bélanger SA, Löhner K, Veenstra-Knol HE, Lemmink HH, Keller-Ramey J, Wentzensen IM, Punj S, McWalter K, Lenberg J, Ellsworth KA, Radtke K, Akbarian S, Pappas J. Genotype-phenotype correlation at codon 1740 of SETD2. Am J Med Genet A. 2020;182:2037-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Lumish HS, Wynn J, Devinsky O, Chung WK. Brief Report: SETD2 Mutation in a Child with Autism, Intellectual Disabilities and Epilepsy. J Autism Dev Disord. 2015;45:3764-3770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |