Published online Jun 26, 2025. doi: 10.12998/wjcc.v13.i18.100610
Revised: October 10, 2024
Accepted: January 24, 2025
Published online: June 26, 2025
Processing time: 190 Days and 2.1 Hours
Birt-Hogg-Dubé (BHD) syndrome is a rare genetic disorder associated with mutations in the BHD gene, which can manifest symptoms at any age, including dermatological and pulmonary complications, as well as renal tumors. This study presents a case of a BHD patient who experienced spontaneous pneumothorax, aiming to enhance the understanding of this syndrome.
A 42-year-old female patient presented with left-sided chest pain and tightness lasting three days. Chest computed tomography scans revealed left-sided pneumothorax and multiple pulmonary bullae. Physical examination indicated decreased vocal fremitus and tympanic percussion on the left side. A thorough family history revealed a pattern of pulmonary disorders, including emphysema, spontaneous pneumothorax, and lung cancer among relatives. Genetic testing identified a heterozygous frameshift mutation in the FLCN gene at the 17p11.2 locus. Based on the clinical presentation, imaging findings, family history, and genetic results, the patient was suspected to have BHD syndrome.
We present a case of a heterozygous mutation in the FLCN gene in a patient with BHD syndrome, aiming to review the associated clinical characteristics and genetic mechanisms of this condition. This case serves as a reference point to offer insights into the diagnosis of multiple pulmonary cysts and spontaneous pneumothorax of unknown etiology in clinical practice.
Core Tip: Birt-Hogg-Dubé (BHD) syndrome is a rare autosomal dominant genetic disorder. The non-specific nature of its symptoms often results in frequent misdiagnoses and overlooked cases. In this report, we describe a patient with BHD syndrome who presented solely with multiple pulmonary bullae and pneumothorax. This case serves as a focal point for an in-depth discussion regarding the diagnosis, clinical presentation, prognosis, and management strategies for BHD syndrome.
- Citation: Li MZ, Deng J. Birt-Hogg-Dubé syndrome - a rare genetic disorder complicated by pneumothorax: A case report. World J Clin Cases 2025; 13(18): 100610
- URL: https://www.wjgnet.com/2307-8960/full/v13/i18/100610.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i18.100610
Birt-Hogg-Dubé (BHD) syndrome is an uncommon genetic disorder initially documented by Birt, Hogg, and Dubé in 1977[1,2]. It is primarily attributed to mutations in the BHD gene (folliculin gene), which is located on chromosome 17 and is responsible for encoding the folliculin protein[3,4]. Although the precise role of this protein within cells has not been fully elucidated, it is recognized for its pivotal involvement in cell proliferation, differentiation, and metabolic regulation. The inheritance pattern of BHD syndrome follows an autosomal dominant trait, indicating that the presence of a single mutated BHD gene is adequate for disease manifestation. Consequently, individuals with BHD syndrome are 50% likely to transmit this mutated gene to their offspring. Research indicates[1,4,5] that approximately 80% of patients have a familial history of the disorder, whereas 20% of cases arise from spontaneous mutations.
The prevalence of BHD syndrome varies between 1 in 200000 and 1 in 300000 individuals on the basis of geographical data[1,4,6,7]. Onset age displays considerable diversity, with symptoms potentially emerging from infancy to advanced age. Notable features of the syndrome include skin and lung abnormalities, which are frequently accompanied by the emergence of kidney tumours. Spontaneous pneumothorax represents a prevalent complication of BHD syndrome, but the precise underlying mechanisms remain unclear.
This manuscript presents a clinical case study of a confirmed BHD syndrome patient encountering spontaneous pneumothorax, with the intention of enriching comprehension of this condition.
The patient had left-sided chest pain and tightness persisting for 3 days.
The patient is a 42-year-old female who presented with left-sided chest pain and tightness that has lasted for three days. She reported no history of trauma, previous similar episodes, or recent strenuous physical activity. An emergency electrocardiogram (ECG) indicated sinus bradycardia and low voltage in the left precordial leads. Vital signs showed a heart rate of 56 beats per minute, a respiratory rate of 18 breaths per minute, blood pressure of 124/84 mmHg, and oxygen saturation of 98%. Acute myocardial infarction was ruled out based on these findings.
The patient has no significant past medical history, including no history of cardiovascular disease, cerebrovascular disease, or substance use (tobacco or alcohol).
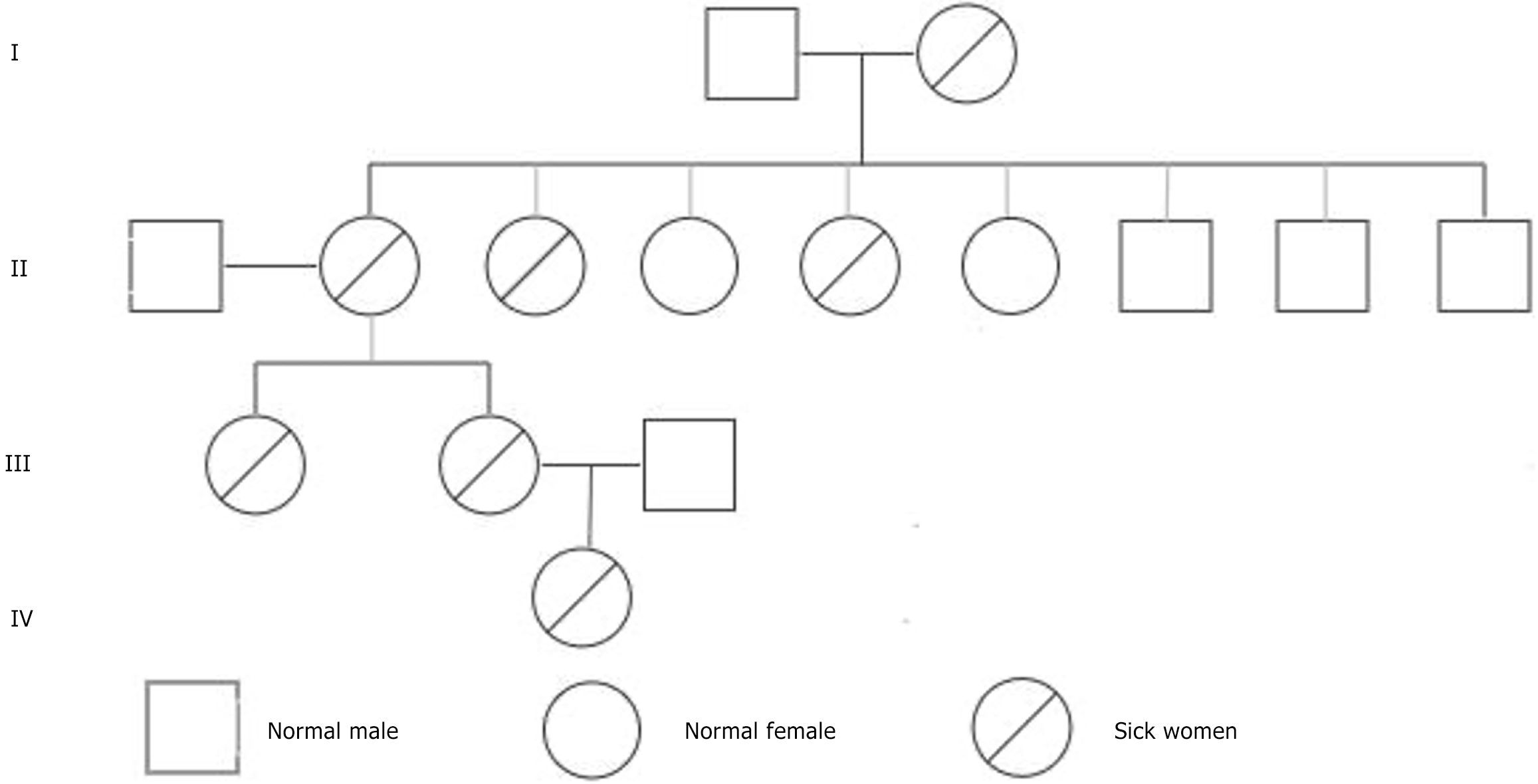
The patient has a significant family history of pulmonary disorders: (1) Grandmother: Emphysema, which led to her death; (2) Mother: Experienced unilateral spontaneous pneumothorax requiring surgical intervention; (3) One aunt: Developed pulmonary nodules that progressed to lung cancer; (4) Another aunt: Had numerous untreated pulmonary cysts; and (5) Sister: Experienced bilateral spontaneous pneumothorax on separate occasions, each managed surgically.
Physical examination revealed decreased vocal fremitus and tympanic percussion on the left side of the chest. No notable changes were observed in the skin of the face or anterior chest.
Genetic testing revealed a heterozygous FLCN gene variant, specifically a frameshift mutation at the 17p11.2 locus of the FLCN gene (Table 1).
| Gene | Chromosomal location | Reference transcript | Location | cDNA level | Protein level | Status | Mutation classification | |
| A | FLCN | 17p11.2 | NM-144997.6 | Exon11 | c.1285delC | p.(His429fs) | Hybrid | Pathogenic mutation |
| B | FLCN | 17p11.2 | NM-144997.6 | Exon11 | c.1285delC | p.(His429fs) | Hybrid | Pathogenic mutation |
| C | FLCN | 17p11.2 | NM-144997.6 | Exon11 | c.1285delC | p.(His429fs) | Hybrid | Pathogenic mutation |
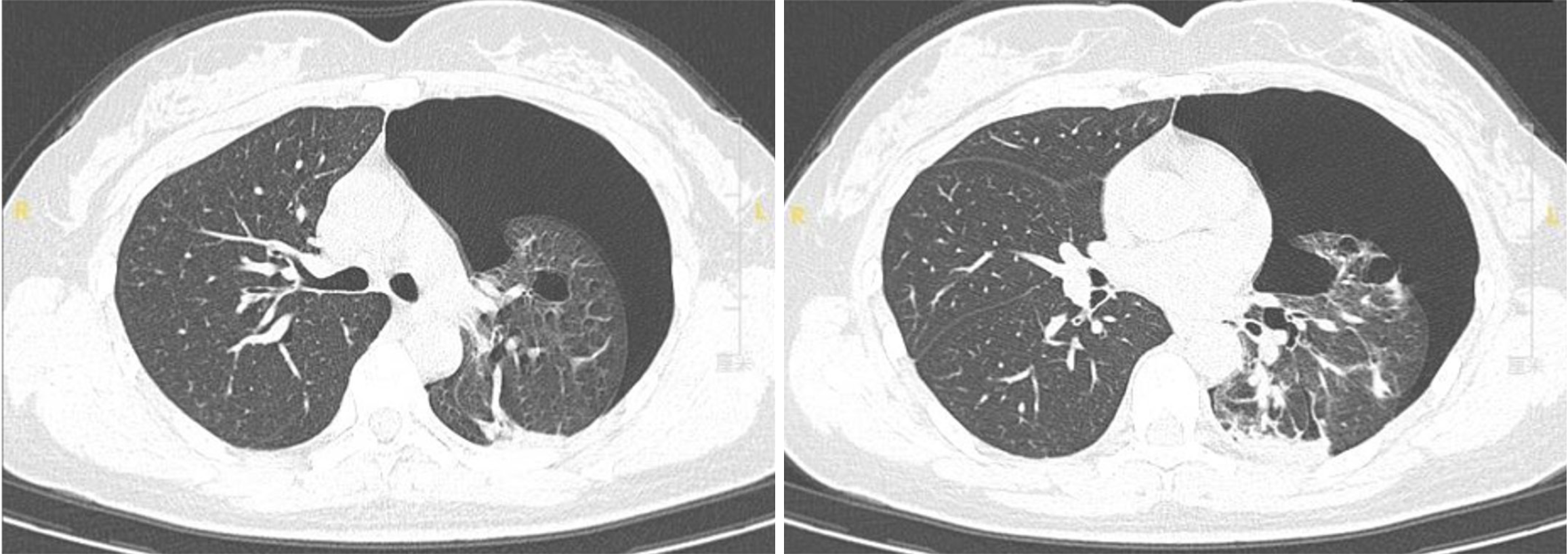
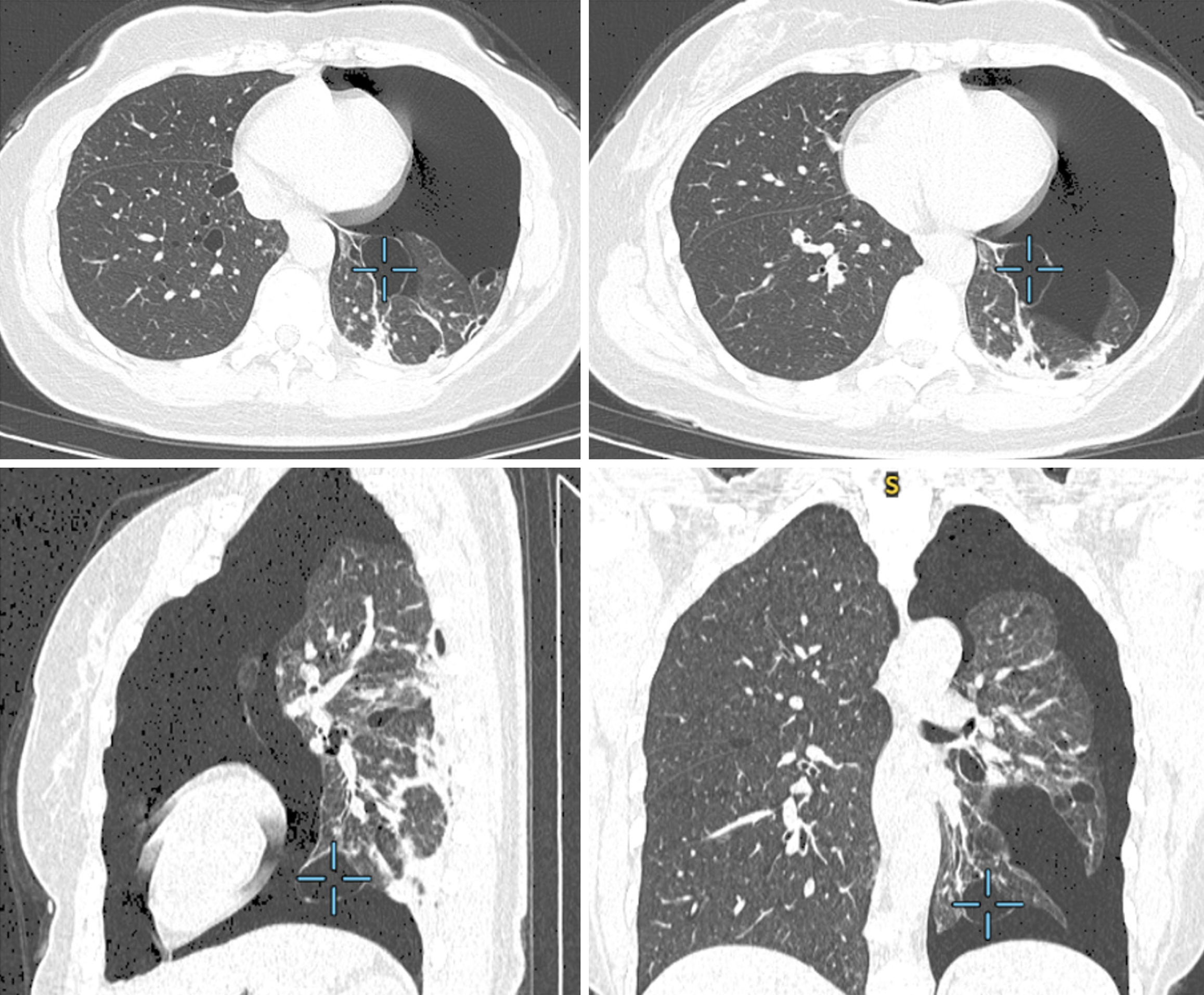
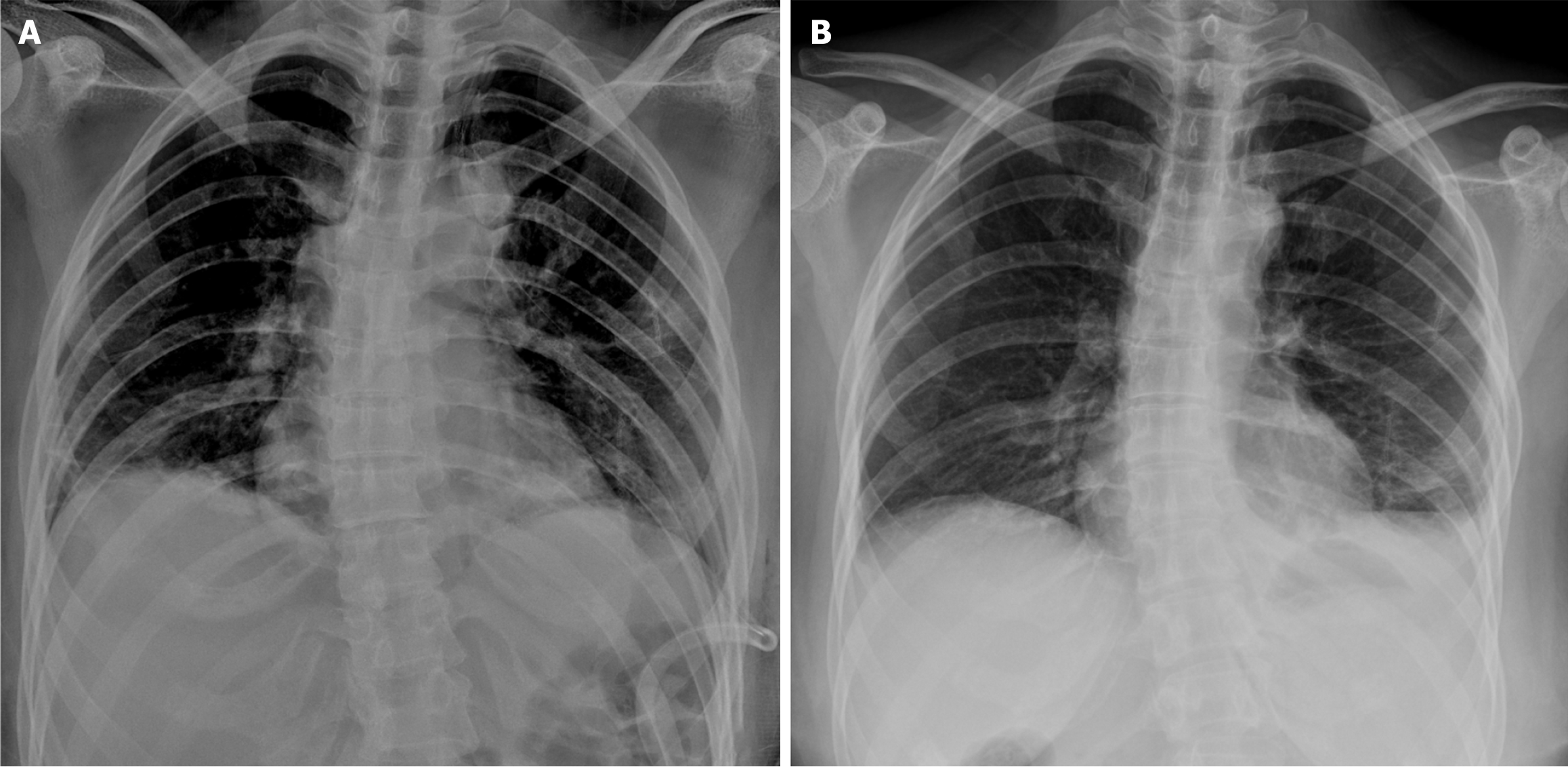
Emergency chest computed tomography (CT; Figures 1 and 2) scans showed left-sided pneumothorax and multiple pulmonary bullae in both lungs.
In light of the patient's complex clinical presentation, a multidisciplinary team was assembled, comprising specialists in pulmonology, genetics, and cardiology. The pulmonologist assessed the chest CT findings, confirming the presence of left-sided pneumothorax and multiple pulmonary bullae, and discussed potential management strategies for these pulmonary complications. The geneticist reviewed the patient's family history of pulmonary disorders and the results of genetic testing, highlighting the significance of the identified heterozygous FLCN gene variant. The cardiologist evaluated the ECG findings, which indicated sinus bradycardia and low voltage, ensuring that any cardiac issues were appropriately managed and did not contribute to the patient's respiratory symptoms. This collaborative approach aimed to formulate a comprehensive management plan that addressed both the immediate respiratory concerns and the underlying genetic condition.
Based on the patient's clinical presentation, imaging studies, family medical history, and genetic test results, the final diagnosis was confirmed as BHD syndrome, inherited through her maternal lineage (Figure 3). This diagnosis explains the patient's left-sided pneumothorax, the presence of multiple pulmonary bullae, and the notable family history of associated pulmonary disorders.
We recommended surgical intervention for the patient, thoroughly explaining the associated risks and prognosis, and obtained her informed consent for the procedure. Routine preoperative examinations were performed, revealing that the patient tested positive for Treponema pallidum antibodies. A complete blood count, along with liver and kidney function tests, C-reactive protein, and erythrocyte sedimentation rate, were all within normal ranges, and no respiratory pathogens were cultured from sputum over a two-day period.
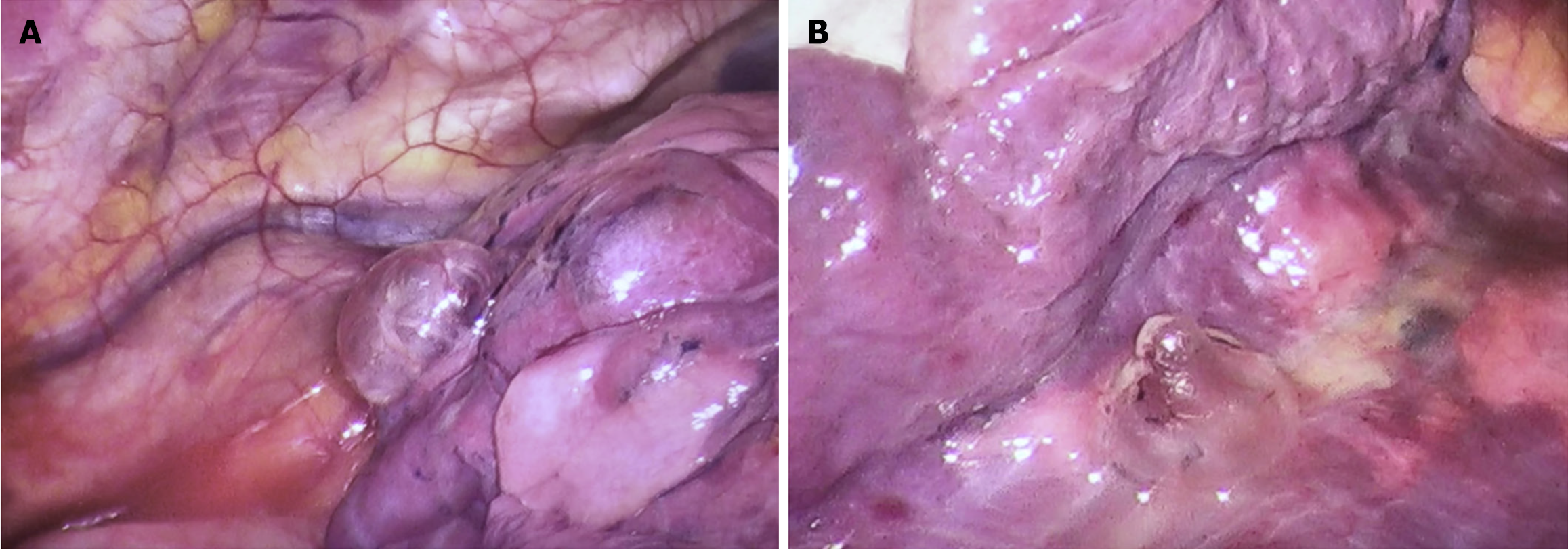
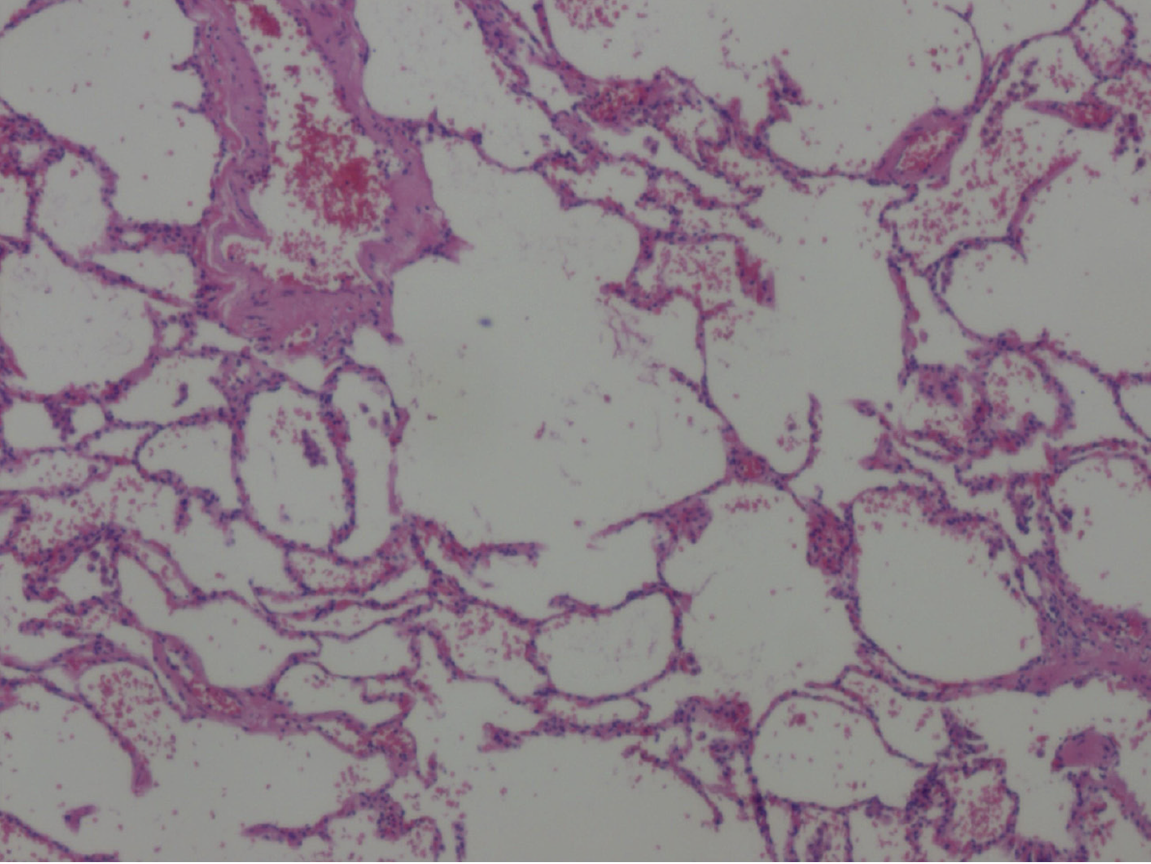
After comprehensive preoperative preparation, the patient underwent video-assisted thoracoscopic bullectomy and pleurodesis. Intraoperatively, a roughly 1.0 cm-long observation orifice was established at the 7th intercostal space along the left midaxillary line for the thoracoscope. An incision measuring 2 cm at the 4th intercostal space along the left anterior axillary line was designated as the operative port. Exploration unveiled approximately 80 mL of pale yellow turbid fluid within the pleural cavity devoid of adhesions. Various bullae were identified in distinct locations, including the left lung apex, the anterior segment of the left upper lobe, in proximity to the left upper lobe oblique fissure, and adjacent to the mediastinum beside the left lower lobe ligament, manifesting as clustered bubble-like structures (Figure 4). Among them, the largest bulla measured approximately 3.0 cm × 3.0 cm. The bullae were completely excised, and following suction and reinflation of the lung, no significant air leakage was noted. Closure of the pleural cavity was conducted along each intercostal space, and a 24 French chest tube was placed through the observation port. Histological evaluation of the excised tissue during the surgical intervention substantiated findings consistent with a pulmonary bulla (Figure 5).
Upon emergence from anesthesia, the patient expressed incisional pain; however, her vital signs remained stable, and she reported no other significant discomfort. Symptomatic management, including analgesia and fluid supplementation, was initiated. On the first postoperative day, the chest tube drained 150 mL of serosanguineous fluid, which increased to 180 mL on the second day, decreased to 100 mL on the third day, and further to 80 mL on the fourth day. During this timeframe, there was no significant air leakage noted in the chest drainage bottle, and the water column fluctuated appropriately. The patient did not report any specific discomfort aside from wound pain, leading to the removal of the chest tube on the fifth postoperative day.
The patient’s postoperative incision exhibited primary intention healing. A follow-up examination conducted one month after the surgery indicated a well-healed thoracic cage, as assessed by chest X-ray (Figure 6), with the patient reporting no symptoms of chest pain, tightness, or dyspnea. Additionally, no significant complications were observed.
BHD syndrome, a rare genetic autosomal dominant condition stemming from germline mutations in the tumor suppressor gene FLCN responsible for encoding folliculin. Notably, 90% of documented BHD cases originate from Europe and the United States, with limited recognition in Southeast Asia, particularly in China. In the Western regions, 82% to 92% of BHD instances prominently feature distinctive skin manifestations, whereas in Asian cohorts, these manifestations are observed in only 20% to 29% of cases, potentially contributing to underdiagnosis. Asian individuals with BHD frequently present pulmonary cysts and spontaneous pneumothorax, occurring at a rate of approximately 58%. Consequently, a family history of pneumothorax in Asian populations can serve as a pivotal diagnostic indicator for BHD syndrome[8,9]. Thus, a comprehensive exploration of the clinical presentations, genetic underpinnings, and therapeutic approaches of BHD syndrome is crucial to deepen comprehension of the disorder and enhance diagnostic accuracy.
BHD syndrome, while relatively uncommon in clinical settings, is frequently subject to misdiagnosis or delayed diagnosis owing to its varied clinical manifestations. The identification of BHD syndrome hinges predominantly on clinical symptoms and germline mutations within the FLCN gene. Thus, when suspicion of BHD arises, a thorough exploration of the individual's and family's medical history concerning skin lesions, pneumothorax, pulmonary cysts, and renal tumors is advised, as these serve as pivotal diagnostic indicators for the syndrome. The current diagnostic criteria proposed are delineated in the subsequent table (Table 1)[6,8-12], albeit necessitating formal validation.
For individuals fulfilling the clinical diagnostic criteria for BHD, the option of FLCN sequence analysis can be pursued. In instances where sequence analysis fails to reveal any pathogenic variants, alternative methodologies like multiplex ligation-dependent probe amplification should be contemplated to identify deletions or duplications. It is crucial to emphasize that a non-detectable result in FLCN gene testing does not definitively rule out the diagnosis[6,9].
Cutaneous manifestations: The predominant clinical presentation of BHD syndrome manifests as skin lesions, observed in roughly 80%-90% of cases in Europe and the United States, featuring distinctive skin alterations. Conversely, among Asian individuals, skin manifestations are less prevalent and less distinctive[6,9,13]. The characteristic skin manifestations in BHD patients encompass multiple, smooth, asymptomatic round or oval papules, commonly situated on the face, neck, and upper chest[2,7,14-16], appearing as fibrofolliculomas, trichodiscomas, and angiofibromas. While skin tumors in BHD syndrome are predominantly benign, instances of malignant melanoma have been documented[6,9]. Studies by Sattler et al[17] revealed an overall melanoma incidence of 6.0% in BHD patients, markedly surpassing the general population's 1.8%. Nonetheless, further investigations are imperative to ascertain whether FLCN gene mutations elevate the susceptibility to malignant skin neoplasms. Notably, the identification of skin fibromas during dermatological assessments should trigger suspicion of BHD syndrome, prompting comprehensive evaluations encompassing pulmonary and renal assessments.
Pulmonary manifestations: Another distinctive clinical feature observed in individuals with BHD syndrome is the occurrence of lung cysts[18] and recurrent pneumothorax. Imaging studies reveal that over 80% of BHD patients exhibit multiple lung cysts. Characteristic chest CT findings include bilateral multiple thin-walled cysts with well-defined borders[7], often presenting as oval, round, or irregular shapes ranging in size from a few millimeters to several cen
Data indicates that 90% of Asian individuals with BHD experience recurrent pneumothorax, a rate 60% higher than in Western populations[13], with recurrence rates reaching 40%-75%[2,7]. Although lung involvement typically represents the initial manifestation in patients, the triad phenotype of BHD (involving the skin, lungs, and kidneys) demonstrates clinical variability among affected individuals, with some presenting solely with pneumothorax[10,20]. In a study involving 12 families[21], at least two first-degree relatives in each family exhibited spontaneous pneumothorax, without concurrent renal or skin manifestations in genetically affected individuals.
The current BHD syndrome patient under our care presents solely with unexplained spontaneous pneumothorax, devoid of skin or renal symptoms. Several family members have a history of spontaneous pneumothorax or lung cysts. Therefore, when confronted with a patient displaying unexplained multiple lung cysts or recurrent pneumothorax, particularly in the context of familial occurrences, maintaining a high index of suspicion for BHD syndrome is crucial to avert misdiagnosis or oversight.
Renal manifestations: Renal tumors stand out as the most critical characteristic manifestation in individuals diagnosed with BHD syndrome[6,9,18], with an estimated incidence rate ranging from 12% to 34%[6,9,14,16]. Research indicates[12] that the average age at which BHD patients develop renal tumors is 50.4 years, with the earliest diagnosis of BHD-related renal tumors reported at 20 years of age[6,9,14]. In a risk evaluation involving BHD family members, those affected by the syndrome presented a sevenfold increased of developing renal tumors compared to genetically unaffected relatives, and a fiftyfold increased risk of experiencing spontaneous pneumothorax[6,9,14,19]. The predominant histological subtype of BHD renal tumors is the hybrid oncocytic tumor, which constitute approximately half of all cases. Furthermore, these tumors can manifest as chromophobe renal cell carcinoma, papillary tumors, and clear cell renal cell carcinoma[2,7,14,19]. Prognosis primarily hinges on the prevalence of renal cell carcinoma and the histological classification of the renal tumor. Hence, early detection through imaging studies or familial screening plays a pivotal role in determining the optimal timing of surgical intervention for patients, as well as in evaluating postoperative renal function and overall prognosis.
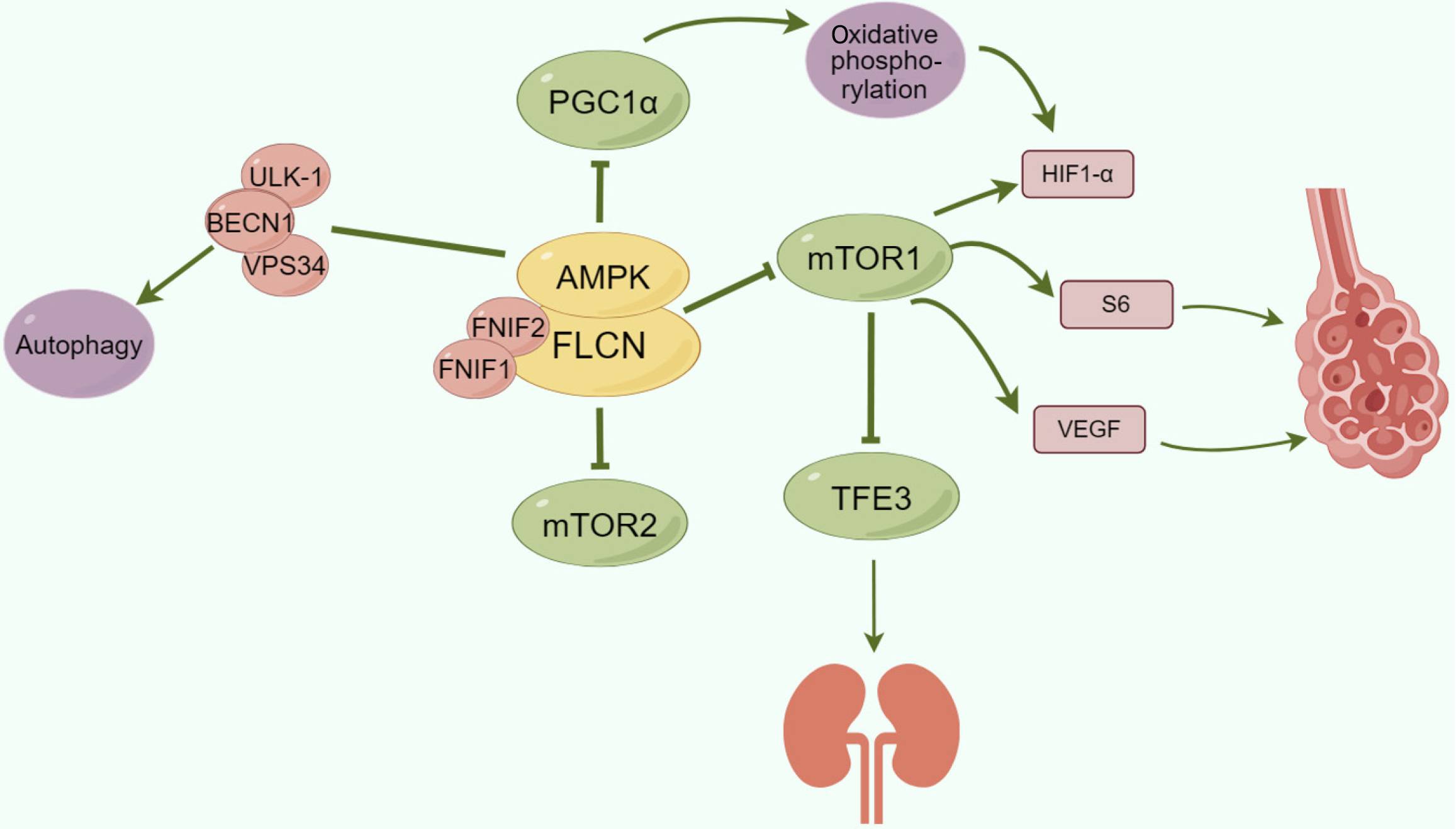
Folliculin serves as a tumour suppressor, with FLCN representing the gene encoding folliculin, which is recognized as a tumour suppressor gene[6,9]. Deletion or functional mutations in FLCN are linked to increased susceptibility to BHD-related tumours[6,8,9]. Recent investigations[3,4,14,22] have highlighted the involvement of FLCN in diverse metabolic pathways, encompassing the regulation of the mTOR pathway, the modulation of PGC1α and mitochondrial biogenesis, the control of TFE3/TFEB transcriptional activity, and the management of cellular autophagy[3,23]. It has been proposed that through these signalling and metabolic pathways, FLCN may impact the balance between cell proliferation and apoptosis, culminating in disease progression.
To date, two folliculin-interacting proteins, FNIP1 and FNIP2, have been identified[3,24]. FNIP1 interacts with the carboxyl terminus of FLCN and AMPK, while FNIP2 shares 49% homology with FNIP1[4,15,24]. Consequently, FNIP1, FNIP2, and FLCN collaborate synergistically or independently to form a FLCN complex that interacts with AMPK, thereby modulating mTOR signalling. Studies utilizing FLCN knockout mouse models have demonstrated that FLCN deficiency triggers mTORC1 activation[3,5]. Moreover, as a suppressor of AMPK, FLCN loss induces the phosphorylation of the autophagy proteins ULK-1, VPS34, and BECN1, promoting cellular autophagy. Investigations have revealed[3,4,23] that FLCN loss-induced AMPK activation leads to the upregulation of PGC1α. When the expression of PGC1α, a transcription factor in BHD tumours, is elevated, it enhances oxidative phosphorylation in cancer cells, resulting in the accumulation of reactive oxygen species, subsequently activating HIF1α and fostering aerobic glycolysis.
Furthermore, FLCN has been identified as a negative regulator of another MiTF family member, TFE3[4,5,24]. In the absence of FLCN in cells and tissues, mTORC1 activation enables TFE3 to evade mTORC1 phosphorylation, facilitating its nuclear translocation and transcriptional activity[22]. The transcriptional activity of TFE3 contributes to the development of aggressive renal cell carcinoma. In lung cyst cells, researchers[18] have detected the positive expression of mTOR and S6, alongside the identification of hypoxia-inducible factor 1α and VEGF. It is postulated that FLCN activates mTOR signalling, thereby governing downstream molecules such as the S6 protein and VEGF and promoting cyst formation (Figure 7).
Treatment options for BHD syndrome are currently limited and are primarily centred on symptom alleviation. For example, addressing skin fibrofolliculomas may involve surgical excision or laser therapy. The management of renal lesions necessitates the regular monitoring of kidney function and tumour screening. Pulmonary lesions can be conservatively managed or require surgical intervention, contingent upon the patient's clinical status. Given the notable recurrence rate of pneumothorax in BHD patients, surgical resection and pleurectomy are commonly recommended[6,10], with studies demonstrating a markedly reduced recurrence rate postsurgery compared with conservative approaches[6,11].
Conversely, pulmonary care in BHD individuals involves considerations regarding air travel or diving activities. Fluctuations in atmospheric pressure during these pursuits may heighten the risk of pneumothorax. Guidelines from the British Thoracic Society caution that individuals with lung cysts or bullae face a risk of barotrauma while diving, with lung cysts serving as a contraindication for diving[10,11]. With respect to air travel, existing evidence does not indicate significant risks associated with this mode of transportation. Generally, most BHD patients should be able to safely engage in air travel. Nonetheless, patients with persistent or recent pneumothorax episodes are advised to refrain from air travel. If they experience unexplained chest tightness, chest pain, or breathing difficulties before boarding, they should postpone their travel plans. Additionally, patients should be informed that BHD cystic lung disease typically has a minimal impact on lung function. Nevertheless, individuals with compromised lung function or extensive pulmonary cystic changes should undergo routine lung function assessments and adhere to timely physician follow-up.
For individuals diagnosed with BHD, diligent screening for renal involvement is imperative. It is recommended that all BHD patients commence renal tumour screening around the age of 20, with subsequent screening every 3-4 years to facilitate early detection and intervention. Screening modalities primarily include renal ultrasound, CT, and magnetic resonance imaging (MRI), although ultrasound may lack sensitivity in detecting small renal lesions. Research indicates[11] that for renal lesions measuring 1.5-2.0 cm, the detection rate with ultrasound is merely 58%. Given the lifelong need for renal surveillance in BHD patients and to mitigate excessive radiation exposure from CT scans, renal MRI is presently the preferred modality. In the event of a renal tumour diagnosis during screening, subsequent diagnostic and treatment strategies should be tailored on the basis of factors such as tumour size and quantity. Treatment modalities typically encompass surgical excision, radiofrequency ablation, and cryotherapy. Presently, the recommended surgical threshold is a renal lesion size of 3 cm, with lesions smaller than 3 cm warranting preservation techniques such as radiofrequency ablation or cryotherapy to safeguard kidney function and avert metastatic spread.
As the majority of skin lesions in BHD patients are benign and pose a low risk of malignant transformation, specific pharmacological treatments for skin lesions are generally unnecessary. Nevertheless, for cosmetic purposes, certain patients may opt for surgical interventions such as excision, volume reduction, ablative laser therapy (CO2 or Erb-Yag laser), or electrocautery. Notably, skin lesions also carry a risk of recurrence.
On the basis of our experiential insights and pertinent literature, upon establishing that a patient's pulmonary symptoms, such as multiple pulmonary bullae complicated by pneumothorax, stem from BHD syndrome, we advocate proactive consideration of additional surgical measures. The rationale is delineated as follows: (1) Surgical intervention, coupled with pleural space closure, notably reduces the probability of postoperative pneumothorax recurrence; (2) The prevailing trend towards minimally invasive surgical approaches has advantages such as reduced trauma and expedited recovery; and (3) After surgery, the patient's quality of life will considerably improve, with a reduction in psychological distress.
| 1. | Ghosh S, Farver CF. Birt-Hogg-Dubé Syndrome. Radiology. 2022;302:514. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Lu YR, Yuan Q, Liu J, Han X, Liu M, Liu QQ, Wang YG. A rare occurrence of a hereditary Birt-Hogg-Dubé syndrome: A case report. World J Clin Cases. 2021;9:7123-7132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Ramirez Reyes JMJ, Cuesta R, Pause A. Folliculin: A Regulator of Transcription Through AMPK and mTOR Signaling Pathways. Front Cell Dev Biol. 2021;9:667311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Schmidt LS, Linehan WM. FLCN: The causative gene for Birt-Hogg-Dubé syndrome. Gene. 2018;640:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Marziali V, Geropoulos G, Frasca L, Longo F, Patrini D, Panagiotopoulos N, Crucitti P. Focus on the pulmonary involvement and genetic patterns in Birt-Hogg-Dubè syndrome: Literature review. Respir Med. 2020;168:105995. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Daccord C, Good JM, Morren MA, Bonny O, Hohl D, Lazor R. Birt-Hogg-Dubé syndrome. Eur Respir Rev. 2020;29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Boone PM, Scott RM, Marciniak SJ, Henske EP, Raby BA. The Genetics of Pneumothorax. Am J Respir Crit Care Med. 2019;199:1344-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Yukawa T, Fukazawa T, Yoshida M, Morita I, Kato K, Monobe Y, Furuya M, Naomoto Y. A Case of Birt-Hogg-Dubé (BHD) Syndrome Harboring a Novel Folliculin (FLCN) Gene Mutation. Am J Case Rep. 2016;17:788-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 9. | Garg A, Herts BR. Birt-Hogg-Dube syndrome. J Urol. 2012;188:1343-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Dal Sasso AA, Belém LC, Zanetti G, Souza CA, Escuissato DL, Irion KL, Guimarães MD, Marchiori E. Birt-Hogg-Dubé syndrome. State-of-the-art review with emphasis on pulmonary involvement. Respir Med. 2015;109:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Gupta N, Seyama K, McCormack FX. Pulmonary manifestations of Birt-Hogg-Dubé syndrome. Fam Cancer. 2013;12:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, Nordenskjöld M, Hansen TV, Solly J, Maher ER; European BHD Consortium. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 13. | Hao S, Long F, Sun F, Liu T, Li D, Jiang S. Birt-Hogg-Dubé syndrome: a literature review and case study of a Chinese woman presenting a novel FLCN mutation. BMC Pulm Med. 2017;17:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat Rev Urol. 2015;12:558-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, Gunji Y, Kikkawa M, Iwakami S, Hino O, Takahashi K, Seyama K. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 2010;47:281-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, Wei MH, Schmidt LS, Davis L, Zbar B, Choyke P, Steinberg SM, Nguyen DM, Linehan WM. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Sattler EC, Ertl-Wagner B, Pellegrini C, Peris K, Reithmair M, Schädle N, Ruzicka T, Steinlein OK. Cutaneous melanoma in Birt-Hogg-Dubé syndrome: part of the clinical spectrum? Br J Dermatol. 2018;178:e132-e133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Furuya M, Nakatani Y. Birt-Hogg-Dube syndrome: clinicopathological features of the lung. J Clin Pathol. 2013;66:178-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Lee JE, Cha YK, Kim JS, Choi JH. Birt-Hogg-Dubé syndrome: characteristic CT findings differentiating it from other diffuse cystic lung diseases. Diagn Interv Radiol. 2017;23:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Gunji Y, Akiyoshi T, Sato T, Kurihara M, Tominaga S, Takahashi K, Seyama K. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet. 2007;44:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Kumar K, Ross C. Birt-Hogg-Dubé syndrome presenting with spontaneous pneumothorax and extensive pulmonary cysts in the absence of skin lesions or renal pathology. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kobayashi K. Incidental detection of asymptomatic pneumothorax resulting in a diagnosis of Birt-Hogg-Dubé syndrome. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Napolitano G, Di Malta C, Esposito A, de Araujo MEG, Pece S, Bertalot G, Matarese M, Benedetti V, Zampelli A, Stasyk T, Siciliano D, Venuta A, Cesana M, Vilardo C, Nusco E, Monfregola J, Calcagnì A, Di Fiore PP, Huber LA, Ballabio A. A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dubé syndrome. Nature. 2020;585:597-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 24. | Min H, Ma D, Zou W, Wu Y, Ding Y, Zhu C, Lin A, Song S, Liang Q, Chen B, Zhang B, Wan Y, Ye M, Pan Y, Wen Y, Yi L, Gao Q. FLCN-regulated miRNAs suppressed reparative response in cells and pulmonary lesions of Birt-Hogg-Dubé syndrome. Thorax. 2020;75:476-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |