Published online Jun 16, 2025. doi: 10.12998/wjcc.v13.i17.103350
Revised: December 25, 2024
Accepted: January 23, 2025
Published online: June 16, 2025
Processing time: 94 Days and 4 Hours
Traumatic subdural effusion is a common complication of traumatic brain injury, especially after decompressive craniectomy (DC). For neurosurgeons, early diag
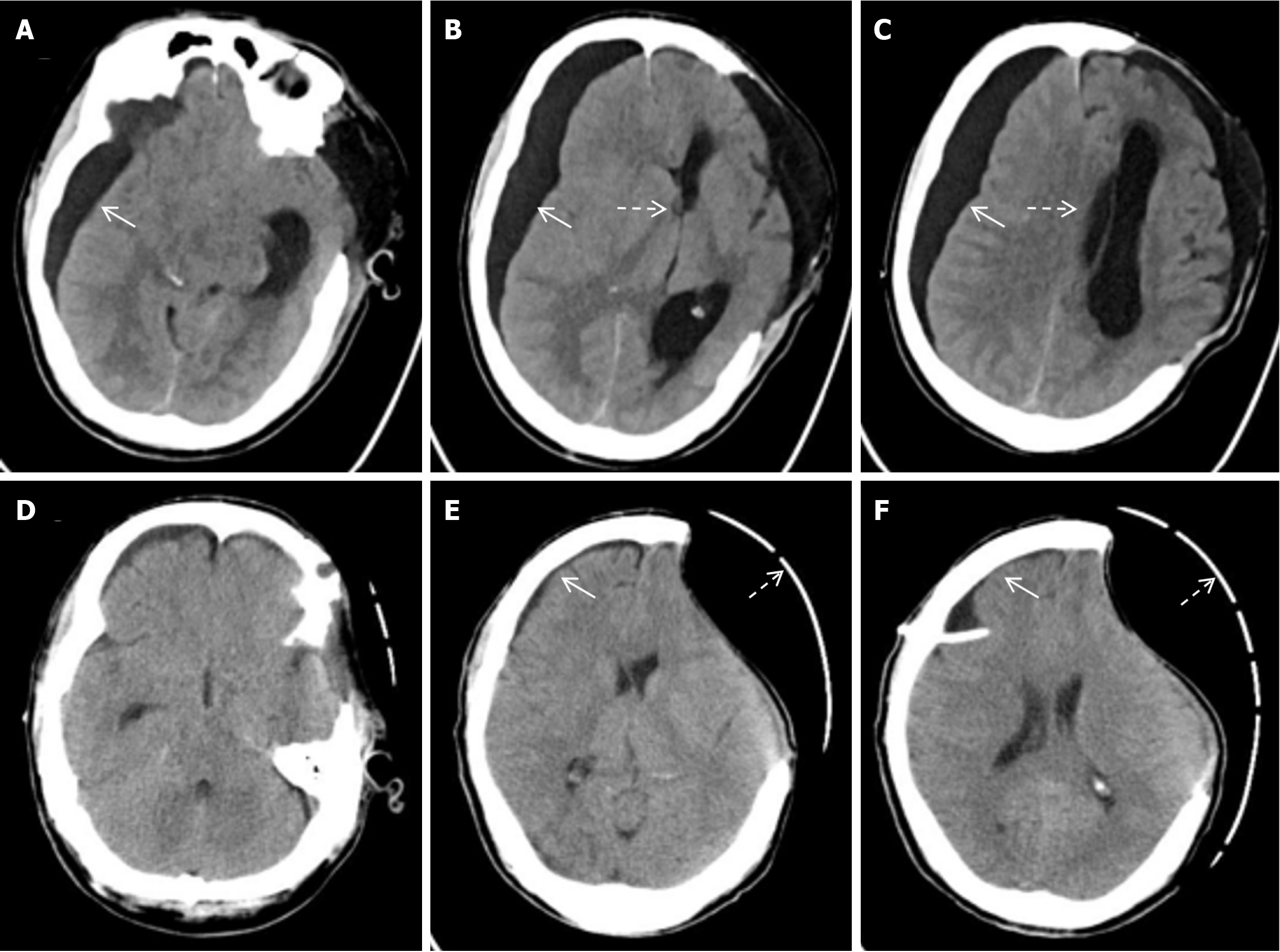
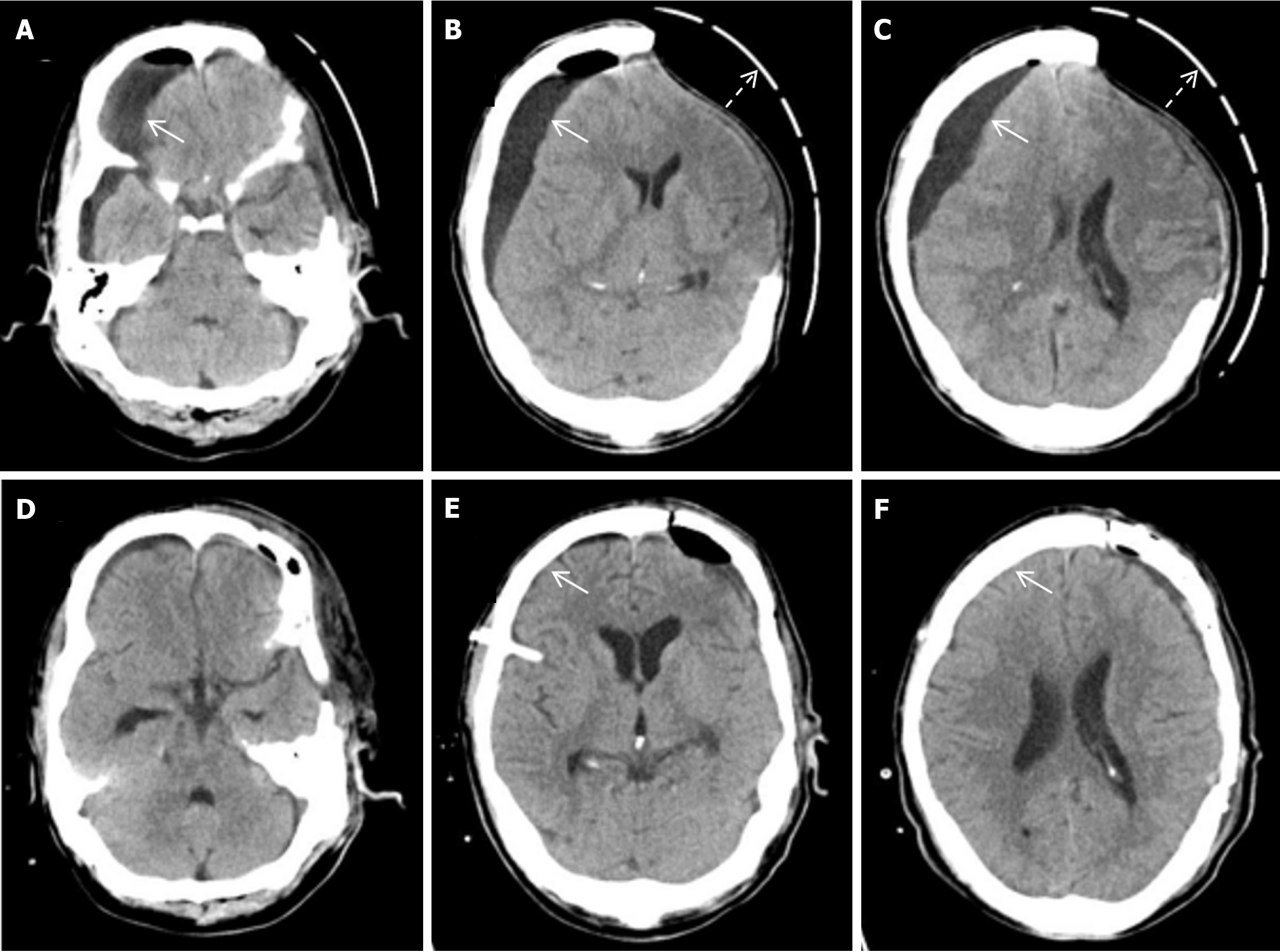
A 47 year old male underwent DC for traumatic brain herniation. After surgery, he developed stubborn subdural effusion (SDE) on the contralateral side and underwent multiple subdural drilling and drainage surgeries, but only tempora
For neurosurgeons, accurate assessment of the condition is necessary when treating patients with stubborn SDE after DC surgery, and timely cranioplasty can be performed to avoid multiple surgeries. This is a safe and effective surgical method for treating traumatic subdural effusion.
Core Tip: Traumatic subdural effusion is a common complication of traumatic brain injury, especially after decompressive craniectomy (DC). For neurosurgeons, early diagnosis and timely treatment are particularly important, which can help improve patient prognosis and enhance quality of life. This article reports a case of refractory subdural fluid accumulation after DC, providing reference for clinical work. Contralateral subdural effusion is one of the complications after DC surgery, especially in cases of stubborn subdural effusion, and cranioplasty is an effective treatment method. Our research can provide reference value for the clinical treatment of refractory subdural fluid accumulation after DC.
- Citation: Lin MJ. Intractable subdural effusion after decompressive craniectomy for traumatic brain injury: A case report. World J Clin Cases 2025; 13(17): 103350
- URL: https://www.wjgnet.com/2307-8960/full/v13/i17/103350.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i17.103350
Traumatic subdural effusion (TSE) is a common complication in patients with traumatic brain injury, particularly in those who have undergone decompressive craniectomy (DC). While most asymptomatic cases can be managed conservatively, some may progress to significant intracranial hypertension and associated neurological deficits, necessitating surgical intervention. Surgical options for TSE include external drainage, lumbar cistern drainage, hydrocephalus abdominal diversion, craniotomy for the resection of the hydrocephalic capsule, and subarachnoid communication surgery[1]. In cases complicated by skull defects, early cranioplasty is crucial to prevent the development of TSE[2]. Here, we present the case of a patient who, following DC and subsequent traumatic brain herniation, developed refractory TSE. The patient showed significant improvement after multiple surgical interventions and was discharged in stable condition.
4 hours of unconsciousness caused by car accident trauma.
Four hours ago, the patient was hit by a car and lost consciousness. The traffic police sent him to our emergency department for treatment. After completing the cranial computed tomography scan, it was found that there was bleeding in the left frontal and temporal regions and swelling of the brain.
Past physical health.
The patient is a non-smoker and has no history of alcohol abuse. The patient’s mother and father are healthy. No history of hypertension, diabetes, stroke, or myocardial infarction is present in the family.
On presentation, he was unconscious and intubated, with a Glasgow Coma Scale score of 5 (E1VTM4). The pupils were anisocoric, with the left pupil measuring approximately 4.0 mm and non-reactive to light, while the right pupil measured 3.0 mm with a sluggish light reflex. Neurological examination revealed absent coordination and overextension to painful stimuli, with normal muscle tone in the limbs. Bilateral Babinski signs were positive.
No obvious abnormalities were found in the laboratory examination.
Brain computed tomography shows left frontal and temporal hemorrhage with significant brain swelling.
Moderate traumatic brain injury.
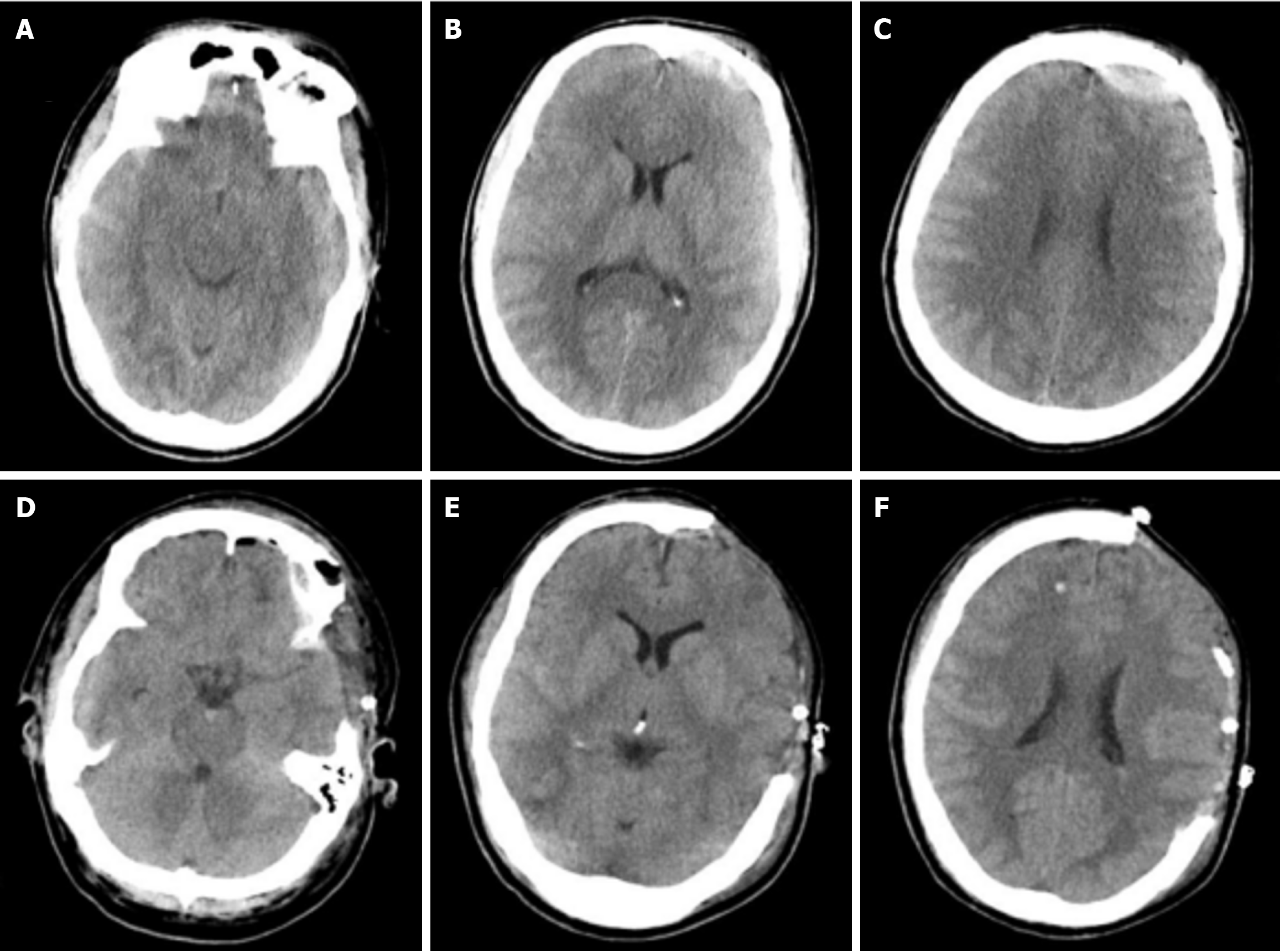
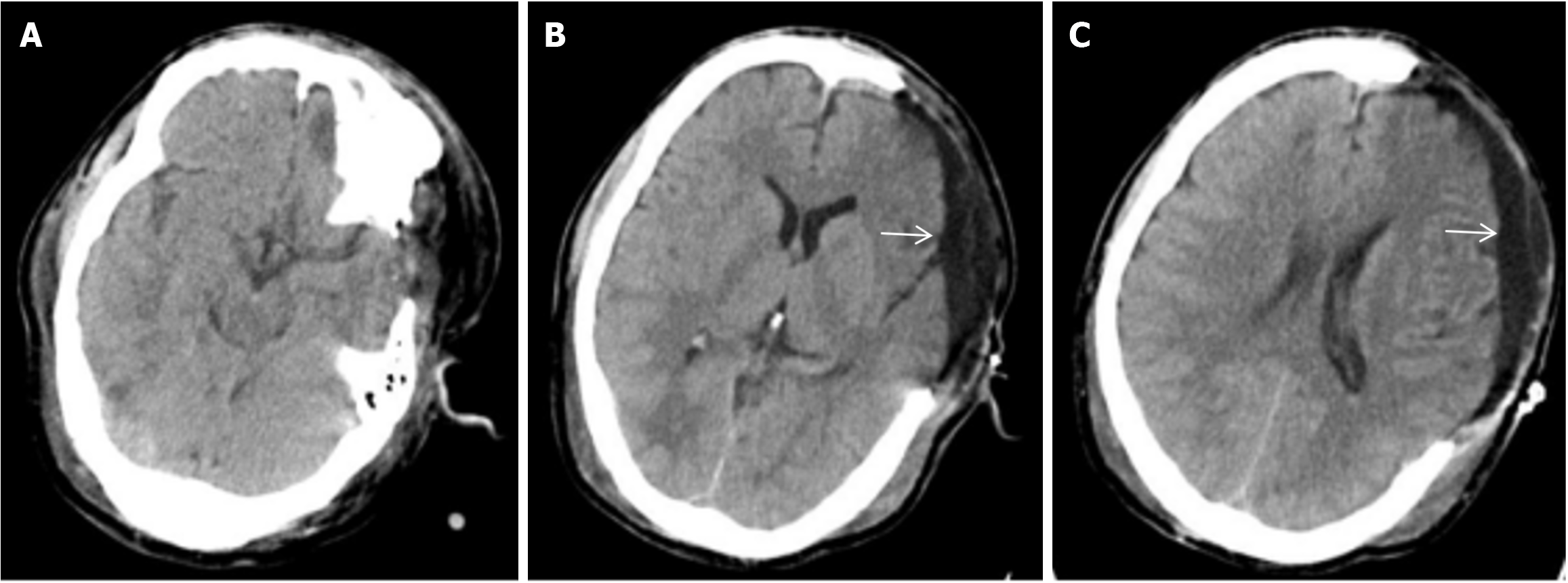
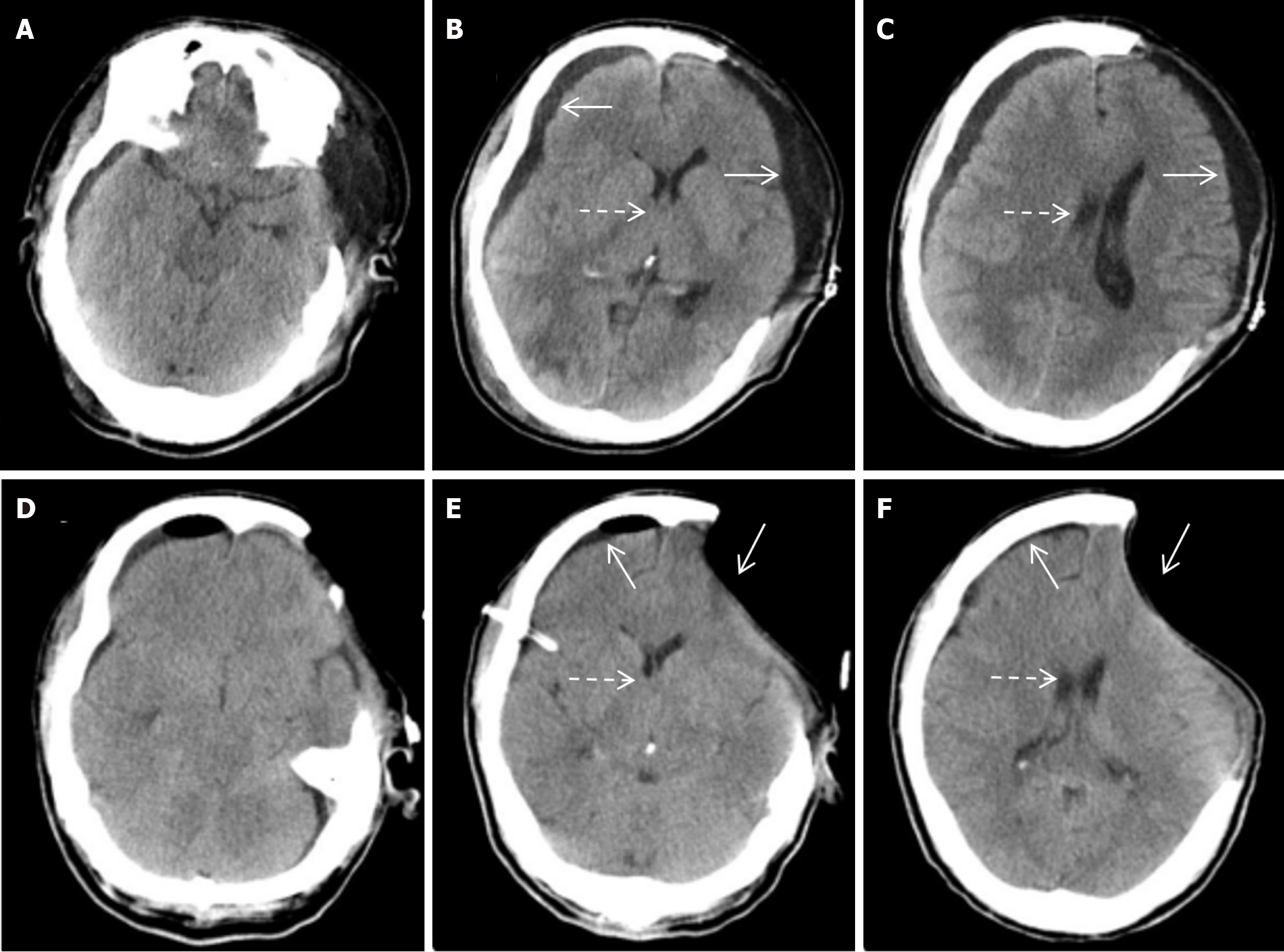
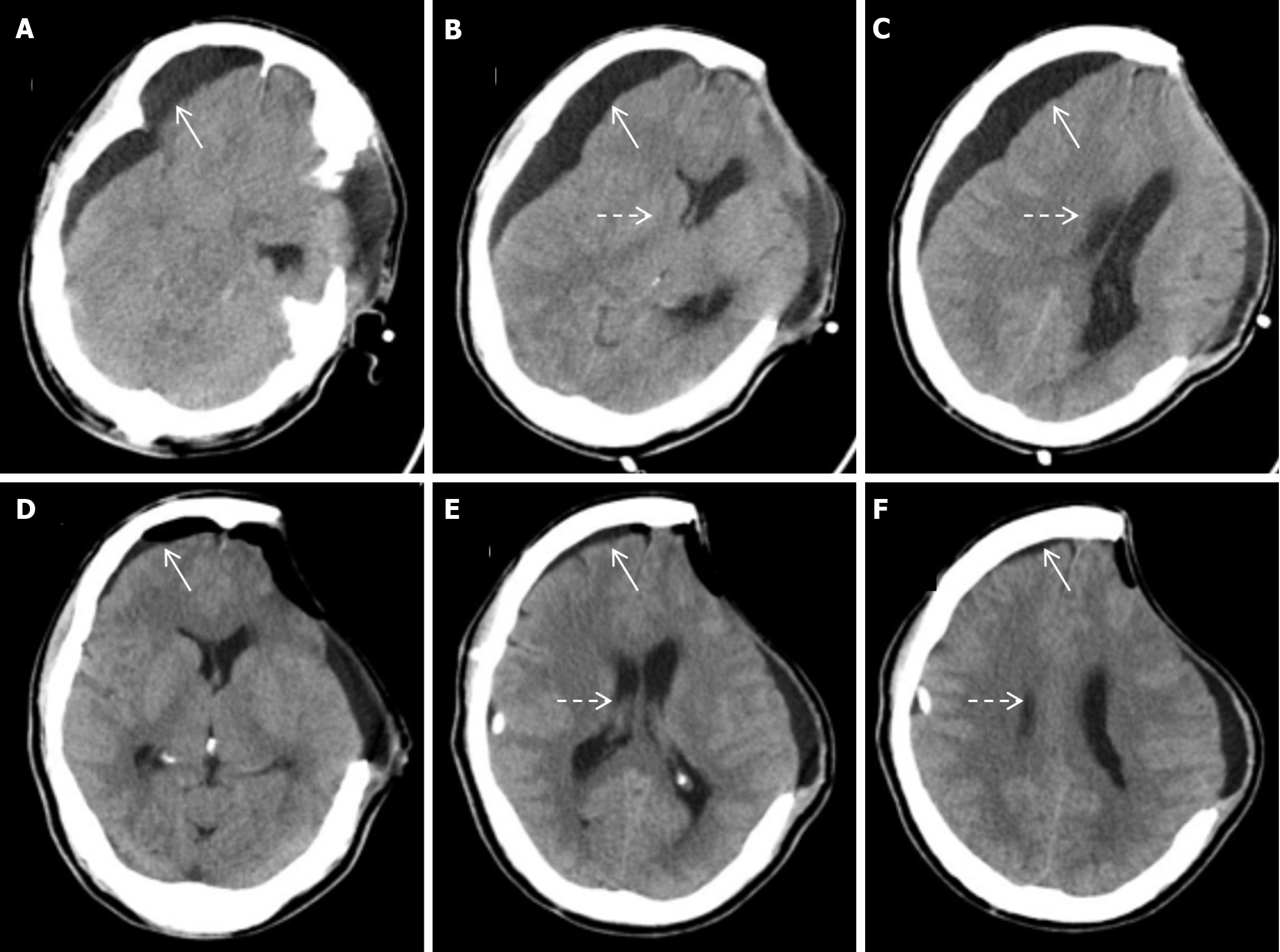
Emergency left intracerebral hematoma evacuation and left DC after admission (Figure 1). After surgery, he developed stubborn subdural effusion (SDE) on the contralateral side and underwent multiple subdural drilling and drainage surgeries (Figures 2,3,4 and 5), but only temporarily improved the patient’s symptoms. After the final cranioplasty, the contra
The patient did not experience any new contralateral neurological dysfunction, and the Glasgow prognostic score was 11 points (E4V1M6).
TSE is often caused by arachnoid tear and cerebrospinal fluid (CSF) circulation disorder caused by trauma. Its incidence rate is about 5%-21%, and the incidence rate in patients receiving DC is significantly higher than that in patients without DC[3-5]. At present, there is no consensus on the pathogenesis of TSE, and there are significant differences in its treatment methods. We will discuss and review the literature on its pathogenesis and treatment methods. At present, the main theories explaining the mechanism of SDE after DC surgery include one-way valve theory, blood-brain barrier disruption theory, and CSF absorption disorder theory[2,6]. The unidirectional valve theory suggests that external violence causes relative displacement between the brain tissue and the cranial cavity, resulting in tearing of the arachnoid membrane on the surface of the brain and forming a structural change similar to a unidirectional valve. And the pulsation of brain tissue caused by traumatic brain injury and the change in intracranial pressure gradient caused by increased intracranial pressure cause CSF to flow into the subdural space along the unidirectional valve, and CSF cannot flow back, thus accumulating in the subdural space. The theory of blood-brain barrier disruption suggests that traumatic brain injury disrupts the integrity of the blood-brain barrier, leading to increased vascular permeability, a large amount of high-density components such as plasma and proteins exuding, and a change in intracranial pressure gradient, further promoting fluid infiltration into the subdural space[7-9]. The theory of CSF absorption disorders suggests that subarachnoid hemorrhage caused by traumatic brain injury can cause aseptic intracranial inflammation, which leads to adhesion of the arachnoid membrane and blockage of the chorionic villi, resulting in obstruction of CSF reflux. At the same time, in severe traumatic brain injury, extensive cerebral vascular spasm can also hinder CSF reflux, causing absorption disorders of CSF[9-11].
We propose that a single theory may not sufficiently explain the development of SDE following DC. Traumatic brain injury disrupts the intrinsic structure and stability of the brain, involving intracranial tissue damage, blood-brain barrier disruption, and skull defects resulting from DC. These factors, coupled with changes in intracranial pressure (ICP) and exacerbation of CSF circulation disturbances due to ICP gradients, contribute to the pathophysiology of SDE. Additiona
Stubborn SDE can lead to brain swelling and worsening neurological function. While most cases of TSE following DC do not require specific treatment, a subset of patients with large effusions and symptoms of intracranial hypertension necessitate intervention. Treatment options include drilling and drainage, craniotomy for resection of the subdural fluid cyst wall and/or partial arachnoid resection, and peritoneal shunting. However, drilling and drainage are considered temporary measures. A review of the literature indicates that cranioplasty is the standard and most effective treatment for SDE after DC surgery[13-15]. In cases of refractory SDE, alterations in intracranial pressure gradients and the one-way valve function of the arachnoid membrane are thought to be key mechanisms contributing to the effusion. Early cranioplasty, or a combined approach with drilling and drainage, has proven to be more effective in these cases[16]. Looking back at the entire treatment process of this patient, we repeatedly performed puncture and drainage treatment on subdural fluid, but there is a certain lack of understanding. We believe that there are two basic strategies for treating the mechanism of subdural fluid accumulation. The first step is to ensure that CSF is drained from other areas, such as early drainage of the lumbar cistern or frontal angle drainage; The second is to maintain the integrity of the skull and perform skull repair surgery as early as possible. Therefore, in the treatment of this patient, while wearing a skull defect protective cap, lumbar drainage or frontal angle drainage can be used to reduce the trauma caused by repeated punctures, which may be a better treatment plan. Moreover, neurosurgeons must remain vigilant for the potential development of hydrocephalus in patients with SDE. Contralateral DC can disrupt CSF circulation, increasing the risk of hydrocephalus[17]. In such cases, timely ventriculoperitoneal shunting remains a safe and effective treatment[18]. In summary, contralateral SDE is a known complication following DC, particularly in cases of refractory SDE. Cranioplasty has proven to be an effective treatment for such cases. However, as the existing literature reports only a single case of stubborn SDE following DC surgery, further clinical case-control studies are needed to assess the efficacy of cranioplasty in managing refractory SDE after DC surgery.
For neurosurgeons, accurate assessment of the condition is necessary when treating patients with stubborn SDE after DC surgery, and timely cranioplasty can be performed to avoid multiple surgeries. This is a safe and effective surgical method for treating TSE.
| 1. | Yang XF, Wen L, Li G, Zhan RY, Ma L, Liu WG. Contralateral subdural effusion secondary to decompressive craniectomy performed in patients with severe traumatic brain injury: incidence, clinical presentations, treatment and outcome. Med Princ Pract. 2009;18:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Wan Y, Shi L, Wang Z, Sun G, Pan T, Zhang S, Zeng Y. Effective treatment via early cranioplasty for intractable contralateral subdural effusion after standard decompressive craniectomy in patients with severe traumatic brain injury. Clin Neurol Neurosurg. 2016;149:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Honeybul S, Ho KM. Decompressive craniectomy for severe traumatic brain injury: the relationship between surgical complications and the prediction of an unfavourable outcome. Injury. 2014;45:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Gong J, Li F, Wang H, Zhu S, Wu C. Traumatic subdural hydroma: clinical characteristics and classification. Injury. 2009;40:968-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Aarabi B, Chesler D, Maulucci C, Blacklock T, Alexander M. Dynamics of subdural hygroma following decompressive craniectomy: a comparative study. Neurosurg Focus. 2009;26:E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017;26:1118-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 7. | Yu J, Tang J, Chen M, Ren Q, He J, Tang M, Zhang X, Liu Z, Ding H. Traumatic subdural hygroma and chronic subdural hematoma: A systematic review and meta-analysis. J Clin Neurosci. 2023;107:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Park SH, Lee SH, Park J, Hwang JH, Hwang SK, Hamm IS. Chronic subdural hematoma preceded by traumatic subdural hygroma. J Clin Neurosci. 2008;15:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Wei L, Chang B, Geng Z, Chen M, Cao Y, Yao L, Ma C. Nomogram for predicting traumatic subdural effusion after mild traumatic brain injury. Front Neurol. 2022;13:947976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med Clin North Am. 2020;104:213-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 533] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 11. | Rufus P, Moorthy RK, Joseph M, Rajshekhar V. Post Traumatic Hydrocephalus: Incidence, Pathophysiology and Outcomes. Neurol India. 2021;69:S420-S428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg. 1985;63:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Wang H, Chen F, Wen L, Zhu Y, Chen Z, Yang X. Cranioplasty as the treatment for contralateral subdural effusion secondary to decompressive craniectomy: a case report and review of the relevant literature. J Int Med Res. 2020;48:300060520966890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Ling H, Yang L, Huang Z, Zhang B, Dou Z, Wu J, Jin T, Sun C, Zheng J. Contralateral subdural effusion after decompressive craniectomy: What is the optimal treatment? Clin Neurol Neurosurg. 2021;210:106950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Zhou W, Wang Z, Zhu H, Xie Z, Zhao Y, Li C, Xie S, Luo J, Li M, Yao J. Effects of Cranioplasty on Contralateral Subdural Effusion After Decompressive Craniectomy: A Literature Review. World Neurosurg. 2022;165:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Ouyang Q, Yang Y, Cheng J, Sun B, Ma Y. Is cranioplasty the optimal treatment for contralateral subdural effusion after decompressive craniectomy?: a case report. Ann Med Surg (Lond). 2024;86:1794-1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Shen X, Han Y, Li H, Dong Y, Yang D, Xu W, Zhang S. The risk factors associated with traumatic subdural effusion for patients with traumatic brain injury who did not undergo decompressive craniectomy. Acta Neurol Belg. 2023;123:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Wu R, Ye Y, Ma T, Jia G, Qin H. Management of subdural effusion and hydrocephalus following decompressive craniectomy for posttraumatic cerebral infarction in a patient with traumatic brain injury: a case report. BMC Surg. 2019;19:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |