Published online Jun 16, 2025. doi: 10.12998/wjcc.v13.i17.101593
Revised: December 10, 2024
Accepted: January 23, 2025
Published online: June 16, 2025
Processing time: 151 Days and 4.9 Hours
Epithelioid angiosarcoma (EA) is an aggressive, malignant endothelial-cell tumor of vascular or lymphatic origin. EA often arises from deep soft tissues such as pleura, breast, bone and gastrointestinal tract. It usually affects patients aged 60-70 years and is associated with high recurrence and metastasis rates with surgical resection as the primary treatment of choice. Overall survivals are generally poor, ranging from 6 to 16 months. More than 50% of patients died of disease within 2 to 3 years of diagnosis.
We present a rare case of EA of the cervical spine causing a C6 pathological fracture complicated by severe kyphosis. The patient received C4-7 posterior laminectomy and C2/3/4/7/T1 transpedicular screw fixation, followed by anterior C5-6 corpectomy with allograft bone fusion and cervical plate fixation. Postoperative radiotherapy was administered without delay. However, the patient died of rapidly progressive acute respiratory distress syndrome 3 weeks after the second surgery.
EA with spinal involvement is extremely rare. Early detection and cord decompression may prevent neurological deterioration and preserve better quality of life.
Core Tip: Epithelioid angiosarcoma (EA) is an aggressive and rare malignant tumor, particularly with spinal involvement. This case report presents a case of cervical spinal EA in a 63-year-old male with a pathological fracture causing spinal cord compression. The patient underwent staged surgeries with decompressive laminectomy, corpectomy, and bone fusion, followed by radiotherapy. Despite aggressive management, the patient succumbed to acute respiratory distress syndrome. This case emphasizes the importance of early diagnosis, timely tumor decompression to prevent neurological deterioration, and the challenges in managing EA due to its high recurrence and metastasis rates. Further research is needed to improve therapeutic outcomes.
- Citation: Nan YH, Chiu CD, Chen WL, Chen LC, Chen CC, Cho DY, Guo JH. Epithelioid angiosarcoma of the cervical spine: A case report. World J Clin Cases 2025; 13(17): 101593
- URL: https://www.wjgnet.com/2307-8960/full/v13/i17/101593.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i17.101593
Angiosarcoma is a subtype of soft-tissue sarcomas and is an aggressive, malignant endothelial-cell tumor originating from vascular or lymphatic tissues[1]. Epithelioid angiosarcoma (EA) is a rare variant of angiosarcoma that is characterized by poorly-differentiated neoplastic cells with an epithelioid morphology[2-4]. Although the majority of cases occur in deep soft tissues, cases have been described arising from skin, adrenal gland, thyroid gland, and bone[2,5,6]. EA has a male predilection and generally occurs in adult life, with the highest incidence diagnosed in the seventh decade. In addition, early nodal and solid organ metastases, especially to the lungs, bone, soft tissue, and skin, are common. Within 2–3 years of diagnosis, more than 50% of the patients die of this disease[6,7]. Here, we present a rare case of cervical spinal EA in a 63-year-old male, demonstrating a pathological fracture and spinal cord compression.
A 63-year-old male visited our neurosurgical outpatient department because of progressive neck weakness with a drop posture for 1 month.
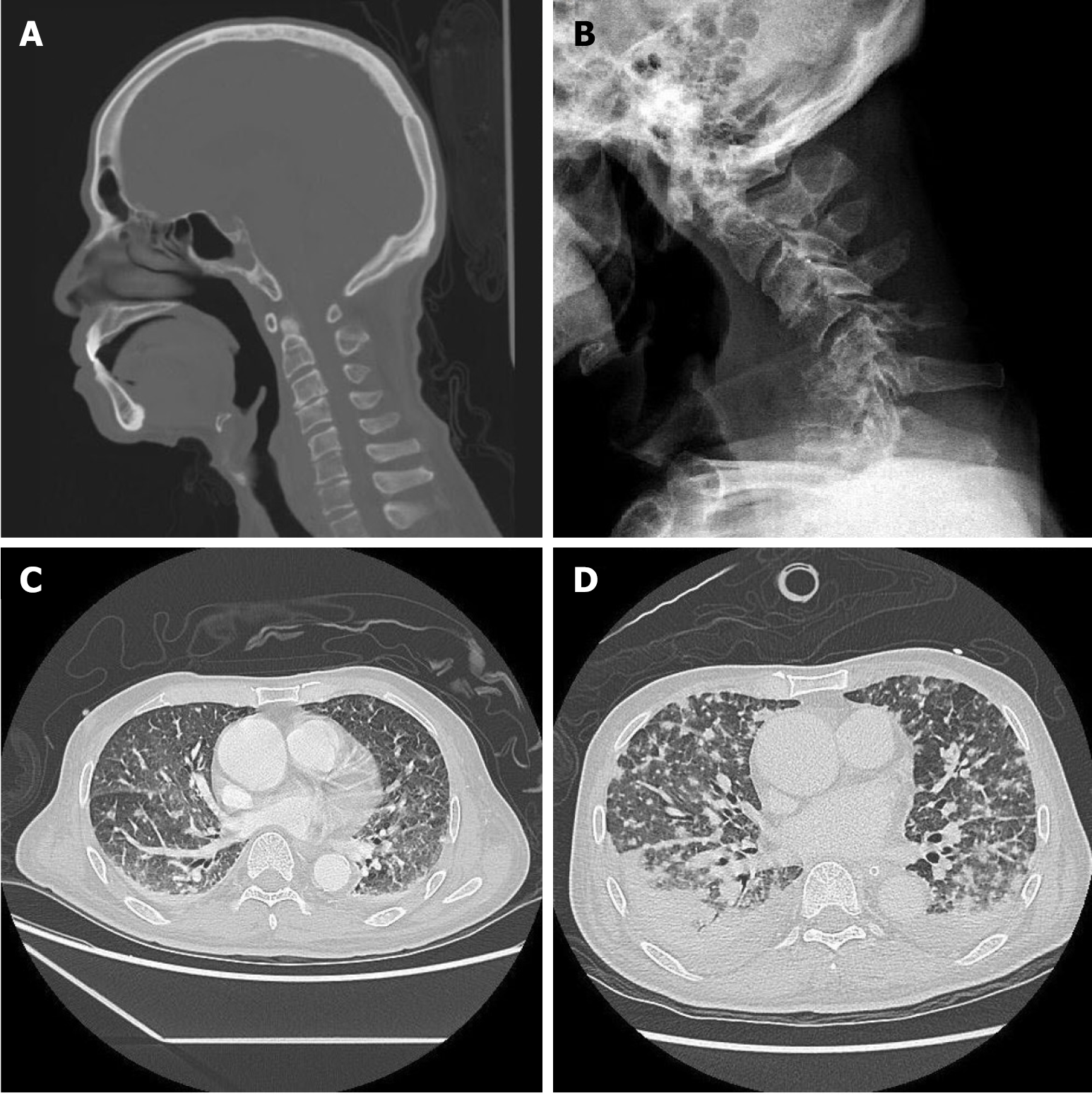
He was involved in a traffic accident with a minor traumatic head injury approximately 1 month prior to this visit. Computed tomography (CT) of the head and neck at that time revealed no traumatic intracranial hemorrhage, and no obvious cervical spinal fracture, facet lock, kyphotic deformity, or alignment deformity of the spine (Figure 1A). A progressive neck weakness with a drop to the right side was noted. The patient was then admitted for further evaluation and management.
He had the history of hypertension, nasopharyngeal carcinoma post concurrent chemoradiation therapy 10 years ago and left putamen lacunar infarction 4 months ago.
There is no obvious personal or family history related to this disease.
Physical examination showed kyphotic cervical posture with limitations in flexion-extension and lateral bending. Progressive weakness of the right upper limb was observed after a grade 1/5 muscle strength, but no muscle wasting was observed.
The tumor marker examination reported alpha-fetoprotein 1.69 ng/mL (< 9.0: Negative), carcinoembryonic antigen 1.34 ng/mL (< 5.0: Negative), prostate-specific antigen (PSA) 1.46 ng/mL (< 4.0: Negative), CA-125 75.6 U/mL (< 35: Negative), CA 15-3 4.0 U/mL (< 23.5: Negative) and CA 19-9 17.9 U/mL (< 35: Negative). The sputum tuberculosis culture results were negative. Sputum fungal cultures were also negative.
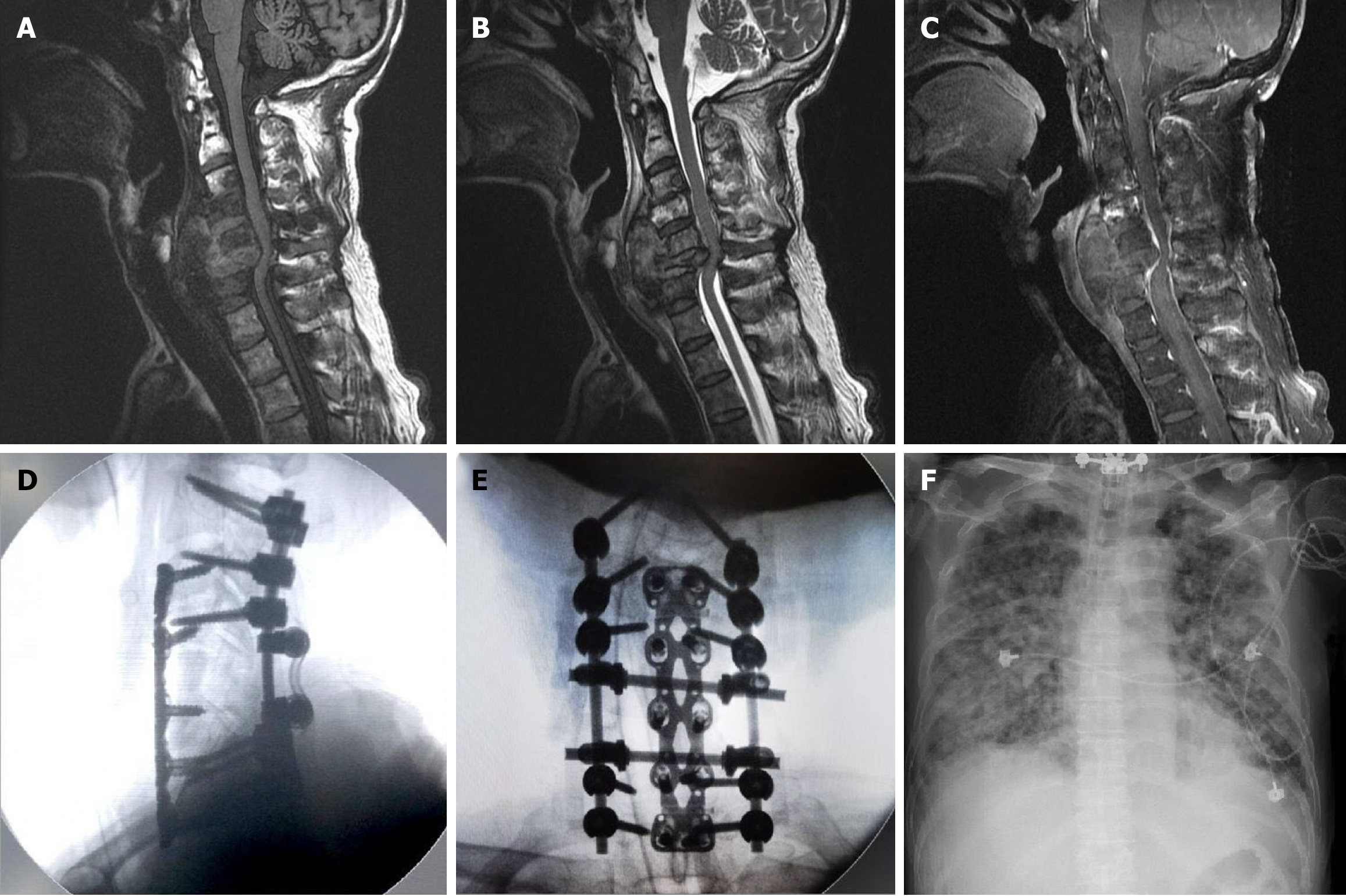
Cervical spine radiograph showed a C6 burst fracture with a severe kyphotic deformity (Figure 1B). Contrast-enhanced chest-abdomen-pelvis CT revealed no obvious solid organ tumors; however, diffuse nodules were noted in both lungs. Multiple bone metastases could not be ruled out (Figure 1C). Magnetic resonance imaging of the cervical spine revealed central necrotic, mixed with some fluid and hematoma lesion infiltration in prevertebral and paravertebral space of C4-5-6-7 vertebra, causing C6 pathological fracture with spinal cord compression (Figure 2A-C).
Under the impression of a cervical pathological fracture of unknown etiology causing spinal cord compression, surgical treatment was planned. The patient received the first stage of C4-7 posterior decompressive laminectomy and C2/3/4/7/T1 transpedicular screw fixation with posterolateral allograft bony fusion. All procedures were performed under somatosensory and motor-evoked potential intraoperative monitoring. As bony destruction caused instability at the C5/6 level, the patient underwent a second stage of C5-6 corpectomy with tibial bone graft fusion and plate fixation 5 days later (Figure 2D and E). A well-margin, capsulated tumor mass (measuring 7.8 cm × 2.0 cm × 2.4 cm in length × depth × width) with severe adhesion and erosive fragility to the C6 vertebral body was found during surgery.
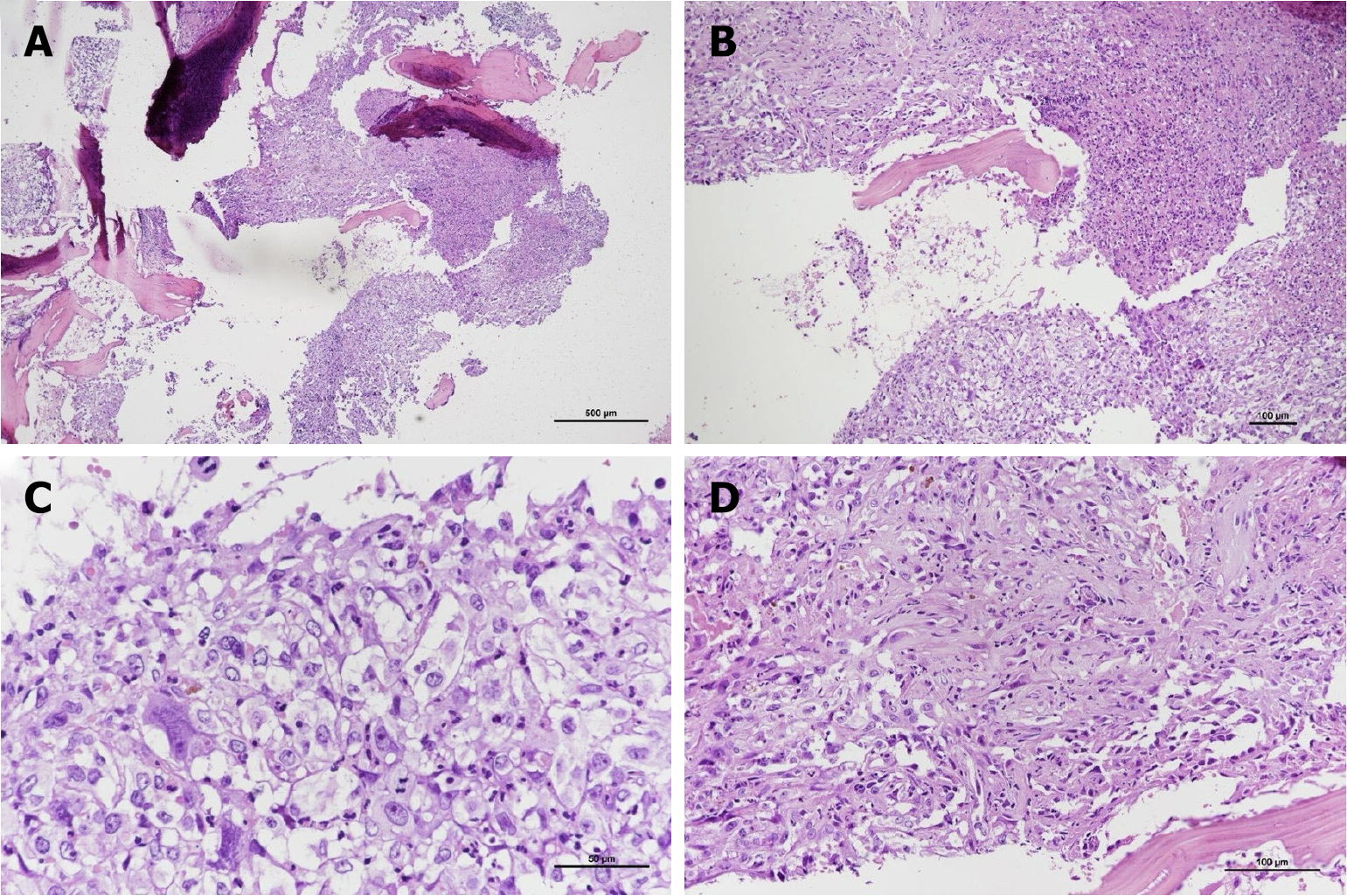
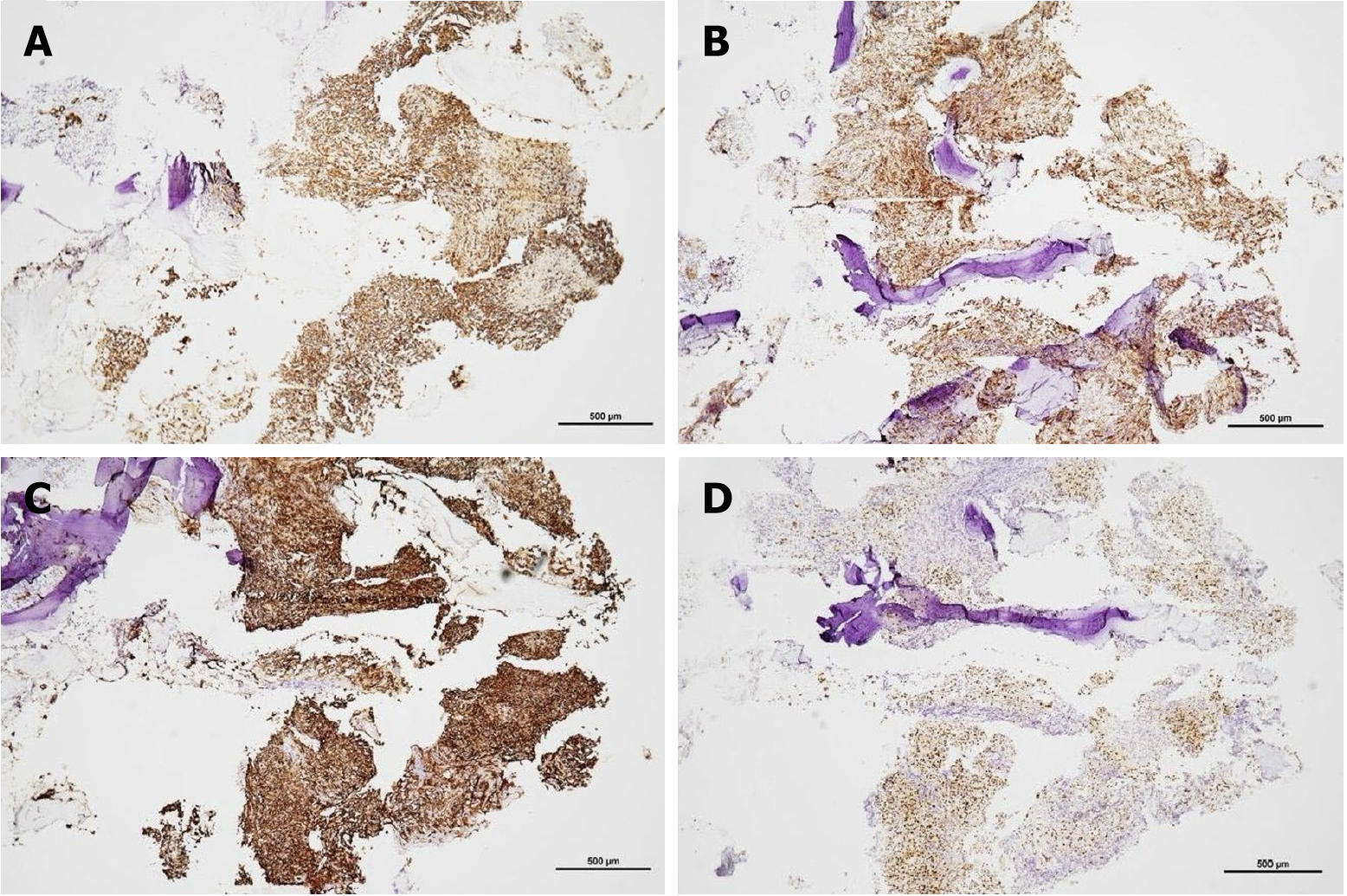
Histopathological examination revealed that the tumor was mostly composed of large, pleomorphic, round to polygonal epithelioid cells arranged in a solid sheet pattern. Focal hemorrhage with hemosiderin deposition and patchy tumor necrosis were also observed. The foci of neutrophilic infiltrates were also observed (Figure 3A and B). The cells exhibited an abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli. Mitotic cells were also observed (Figure 3C). Anastomotic vascular channels lined with epithelioid endothelial cells with intervening fibrovascular stroma were also observed (Figure 3D). Immunohistochemical stain revealed cytokeratin (CK) AE1/AE3 (+), CD31(+), CD34 (+), ERG (+), vimentin (+), EBER-ISH (-), CK7 (-), CK20 (-), CK5/6 (-), PSA (-), TTF-1 (-), CDX2 (-), PAX8 (-), P40 (-), S100 (-), CD68 (-), and INI-1 (focal expression). These findings supported the diagnosis of EA rather than metastatic nasopharyngeal carcinoma (Figure 4).
Surprisingly, our patient started to have bloody sputum 2 days after the second surgery. The chest CT revealed rapid progression of the tumor metastasis in both the lungs and pleura (Figure 1D). Lung biopsy for pathological confirmation was considered but was not performed due to rapid pulmonary deterioration with unstable oxygenation. The patient underwent only one adjuvant radiotherapy session.
Unfortunately, the patient died of the rapid progression of acute respiratory distress syndrome related to pulmonary metastasis 3 weeks after the second surgery (Figure 2F).
Angiosarcoma is a subtype of soft-tissue sarcoma and is an aggressive, malignant endothelial cell tumor originating from vascular or lymphatic tissue[1]. Angiosarcoma of bone is rare and accounts for less than 1% of primary skeletal malig
There are only a few publications on EA of bone origin[2,4,7], let alone on spinal cases. We searched the PubMed database for spinal EA, and enrolled and summarized all 11 spinal EA cases (Table 1)[2-5,7-11]. The highest incidence of EA is observed in the seventh decade of life. However, the average age of onset of spinal EA was 56.3 years, including two very young adults (i.e., cases 3.25 years and 5.27 years). The age at onset of spinal EA appears to be lower than that of non-spinal EA. This implies that spinal EA is more aggressive than non-spinal EA. EA has a male predilection; however, spinal EA does not appear to have a sex predominance. The initial presentation of spinal EA varies, without any specific symptoms. In all 11 Limited cases, a high incidence of spinal involvement was observed in the T-L spine. Lung metastases from spinal EA are uncommon.
| Ref. | Age | Sex | Underlying disease | Initial presentation | Primary location | Spine location | Metastasis | Operation | Chemotherapy | Radiotherapy | Outcome |
| Ghanem et al[8] | 54 | Male | DM; HTN | Acute back pain and intermittent claudication | Abdominal Aorta | T-L VB | Lung | Resection of Abdominal aorta lesion with aorta repair and angioplasty of right renal artery | Ifosamide, vincristin, etoposid, carboplatin and epirubicin | Local radiation therapy | DOD (17 months) |
| Deshpande et al[2] | 76 | Female | Unknown | Unknown | Right Iliac bone and L4 VB | L4 VB | No | Biopsy | No | Palliative radiation | DOD (7 weeks) |
| Sanchez-Mejia et al[9] | 25 | Female | No | Weakness, fatigue, and low back pain | S1 VB | S1 VB | Retroperitoneal lymphadenopathy | Sacral laminectomy with resection of tumor | Doxorubicin and ifosfamide | 600 cGy of external-beam radiotherapy to sacrum and retroperitoneum | AWD (3 months) |
| Marthya et al[3] | 65 | Female | No | Progressive pain over mid-thoracic spine for 3 months | T4 VB and posterior 1/3 of left 4th rib | T4 VB | No | En bloc resection of left 4th rib and decompression of T4 VB | No | 3000 cGy direct on field in 10 fractions | NED (2 years) |
| Ponce et al[10] | 27 | Male | Unknown | Back pain | T9 VB | T9 VB | Unknown | Thoracoscopic biopsy of T9 VB | No | Postoperative palliative radiotherapy | AWD (43 months) |
| Chen et al[7] | 62 | Female | Unknown | Unknown | Femur, Thoracic VB | Thoracic VB | No | Amputation of Femur | No | Yes | DOD (10 months) |
| Chen et al[7] | 53 | Male | Unknown | Unknown | Sacrum | Sacrum | No | Radical resection of tumor | No | No | NED (1.5 months) |
| Lang et al[4] | 76 | Male | No | Progressive pain over mid-lumbar spine for 5 months | L4 VB | L4 VB | No | L4 laminectomy and L4 VB biopsy | No | No | AWD (6 months) |
| Swaika et al[11] | 49 | Female | No | Weight loss, early satiety, nausea, and vomiting for 2 months | Liver, Spleen, Lumbar VB, and right Iliac bone | Lumbar VB | Unknown | Liver biopsy; Iliac bone marrow biopsy | Unknown | Unknown | Unknown |
| Lemus et al[5] | 69 | Male | Unknown | Bilateral upper and lower limbs weakness for 2 months | Extramedullary tumor at C7 level | C7 | Unknown | C6-7 laminectomy with near-total resection of tumor | No | No | AWD (Unknown) |
| Our case | 63 | Male | NPC | Progressive neck drop for 1 month | C6 VB | C6 VB | Lung | C4-7 laminectomy with TPS, C5-6 corpectomy | No | Local radiation therapy for one time | DOD (5 weeks) |
The radiographic appearance of spinal EA is nonspecific, and the diagnosis depends on histopathology and immunohistochemistry[3,4,7,12]. Radiologically, it presents as an osteolytic lesion without periosteal reaction[3]. In addition, osseous destruction, central necrosis, and marginal hyperperfusion of the soft tissue mass indicate the presence of an aggressive high-grade bone tumor; however, these findings are non-pathognomonic[4].
Histologically, the lesions are sheets of large polygonal epithelioid cells with marked nuclear pleomorphism and abundant eosinophilic cytoplasm[2,6,7,13]. Mitosis, necrosis, and hemorrhage are common findings in EA. Blood vessel formation can also be a feature[13]. Immunohistochemically, EA is immunopositive for epithelial markers (i.e., keratin and cytokeratin), vascular endothelial markers (i.e., CD31 and CD34), and factor VIII-associated antigen[2,6,12,13]. CD 31 is expressed in approximately 90% of angiosarcomas, but in less than 1% of carcinomas[2,4,12]. CD34 is expressed in more than 90% of vascular tumors, but is less specific because of its expression in several other soft tissue tumors. In addition, the immunoreactivity of the factor VIII-associated antigen can be informative for the diagnosis of bony EA[8,12]. In contrast, intermediate filament vimentin is strongly expressed in epithelioid vascular tumors[13].
EA presents diagnostic challenges as it can closely resemble metastatic lesions. Most patients do not survive beyond one year after being diagnosed with EA, highlighting its nature as an extremely aggressive and high-grade tumor. One of the primary differential diagnoses for EA is epithelioid hemangioendothelioma (EHE), which is classified as a low-grade malignancy. Key distinguishing features of EHE include prominent cellular atypia, the absence of a chondromyxoid matrix, and a limited presence of cord-like arrangements of endothelioid cells. Accurately differentiating EA from metastases and EHE is crucial since it influences clinical management strategies and can significantly impact patient prognosis[2-4,6,7,9].
The tumor markers in this case were within normal limits. At the time of presentation, the chest radiograph revealed no obvious tumor masses or nodules. However, the cervical spine radiograph identified a burst fracture at the C6 vertebra. A contrast-enhanced whole-body CT scan did not detect any solid organ tumors but revealed several diffuse nodules in both lungs and a pathological fracture at C6, accompanied by kyphotic deformity. Magnetic resonance imaging of the cervical spine demonstrated a large soft tissue tumor (measuring 7.8 cm × 2.0 cm × 2.4 cm) in the prevertebral and paravertebral regions involving the C4, C5, C6, and C7 vertebrae. This tumor caused a pathological fracture at C6 and spinal cord compression. Based on these findings, we concluded that the cervical spine is the primary site of origin.
Given the indeterminate cause of spinal cord compression and the rapid deterioration of muscle strength in the right upper limb, we prioritized addressing the most critical issue first, postponing the lung biopsy for the diffuse lung nodules to a later stage. The surgical plan involved a wide resection of the cervical spinal tumor. This procedure was comprised of a posterior decompressive laminectomy and transpedicular screw fixation, followed by an anterior corpectomy, which included bone graft fusion and plate fixation to ensure structural stability.
Complete resection of the EA is difficult because of its infiltrative nature and propensity for metastasis. In cases of neurological compromise, resection may also improve neurological function[8]. Previous studies all suggest that EA requires wide resection combined with adjuvant chemotherapy and/or radiotherapy, though the adjuvant therapies have not been shown conclusively to improve outcomes[2,3,5,12]. Postoperative radiotherapy seems to decrease the local recurrence and increase survival time. This phenomenon was observed in 11 patients with limited spinal EA (Table 1).
The effectiveness of the utility of preoperative embolization for angiosarcoma is still debatable, yet there are some reports advocating the preoperative embolization of angiosarcoma. Intuitively, embolization may facilitate resection by the way of reducing intraoperative blood loss[9]. Our patient only underwent radiation therapy once because of unstable oxygenation, and unfortunately died of acute respiratory distress syndrome due to the rapid progression of EA in the lung.
Although EA with spinal involvement is extremely rare, early diagnosis and the ability to distinguish it from other diseases with similar presentations are required, especially when associated with neurological deficits. Early decompression of the tumor may prevent neurological deterioration and preserve the patient’s quality of life. Preoperative embolization may facilitate wide resection by reducing intraoperative blood loss. Postoperative chemotherapy and/or radiotherapy remains controversial, although postoperative radiotherapy appears to decrease local recurrence.
| 1. | Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 670] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 2. | Deshpande V, Rosenberg AE, O'Connell JX, Nielsen GP. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol. 2003;27:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Marthya A, Patinharayil G, Puthezeth K, Sreedharan S, Kumar A, Kumaran CM. Multicentric epithelioid angiosarcoma of the spine: a case report of a rare bone tumor. Spine J. 2007;7:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lang J, Chen L, Chen B, Chen K, Liu A, Li J, Wang J. Epithelioid angiosarcoma of the spine: A case report of a rare bone tumor. Oncol Lett. 2014;7:2170-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Lemus LF, Minervini MH. Epithelioid Angiosarcoma Causing Spinal Cord Compression. Cureus. 2021;13:e14325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Chen Y, Shen D, Sun K, Bao D, Song Q, Wang G, Chen D, Yan T, Guo W. Epithelioid angiosarcoma of bone and soft tissue: a report of seven cases with emphasis on morphologic diversity, immunohistochemical features and clinical outcome. Tumori. 2011;97:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ghanem N, Riede U, Uhrmeister P, Weigang E, Altehoefer C. Epithelioid angiosarcoma of the aorta. Vasa. 2002;31:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Sanchez-Mejia RO, Ojemann SG, Simko J, Chaudhary UB, Levy J, Lawton MT. Sacral epithelioid angiosarcoma associated with a bleeding diathesis and spinal epidural hematoma: case report. J Neurosurg Spine. 2006;4:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ponce FA, Killory BD, Wait SD, Theodore N, Dickman CA. Endoscopic resection of intrathoracic tumors: experience with and long-term results for 26 patients. J Neurosurg Spine. 2011;14:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Swaika A, Jiang L. Metastatic epithelioid angiosarcoma with bone marrow involvement. Blood. 2016;127:1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Yang Z, Tao H, Ye Z, Yang D. Multicentric epithelioid angiosarcoma of bone. Orthopedics. 2012;35:e1293-e1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Balicki D, Buhrmann R, Maclean J, Cooper B, Minassian H, Wang NS, Hüttner I. Multicentric epithelioid angiosarcoma of the bone. Pitfalls in clinical and morphological diagnosis. Blood Cells Mol Dis. 1996;22:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |