Published online Jun 16, 2025. doi: 10.12998/wjcc.v13.i17.101008
Revised: October 28, 2024
Accepted: January 21, 2025
Published online: June 16, 2025
Processing time: 169 Days and 8.1 Hours
Painless acute pancreatitis (PAP) is a slowly progressive disease that involves inflammation, scarring, and thickening of pancreatic cells, which can happen due to either alcohol, idiopathic, or genetic. Clinicians usually miss PAP due to lack of pain and additional symptoms of hypotension and fever can lead to an infectious work-up instead. In this case report, we discuss the importance of the rapid dis
A 47-years old male with past medical history of hypotension and alcohol abuse presented for loss of consciousness. Patient was found with pinpoint pupils, hypoglycemia, and hypotensive. He received Narcan, dextrose, and IV fluids and became responsive. In the emergency department, the patient was hypotensive and the physical exam was only significant for diaphoresis. Patient denied abdo
This case report featured PAP without chronic inflammation which is an even rarer disease than PAP which progressed to SAP.
Core Tip: This article highlights an atypical case of painless acute pancreatitis (PAP). This condition is typically a sequela of chronic pancreatitis. However, in this case report, the patient developed PAP in the absence of chronic pancreatitis features or abdominal pain. In addition, this disease course developed into severe acute pancreatitis associated with multiple end-organ damage.
- Citation: Sargon K, Al-Sabea N, Elango A, Scarbrough B, Wong J, Ebrahim S. Atypical presentation of painless acute pancreatitis: A case report. World J Clin Cases 2025; 13(17): 101008
- URL: https://www.wjgnet.com/2307-8960/full/v13/i17/101008.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i17.101008
Painless acute pancreatitis (PAP) is a chronic disease that involves episodes of inflammation resulting scaring and thickening of the pancreas, which can happen due to either alcohol, idiopathic, or genetic at rates of 32.4%, 56.9%, and 8.9%[1]. This condition can usually be undiagnosed due to the lack of pain and hidden, unexpected course. The diagnostic workup of PAP is similar to AP. However, the presentation of pain associated with AP leads clinicians to obtain labs such as amylase and lipase whereas PAP may lead clinicians to investigate other avenues of diagnosis and management. Most literature on PAP showed cases where PAP stems from multiple episodes of inflammation of the pancreas which is a stark contrast to the patient presented in this case report. Although rare, PAP can rapidly progress to severe AP (SAP) and due to the lack of classical pain symptoms can lead to an alternative diagnostic workup such as sepsis given its similar presentation. In this case report, we discuss the unlikely presentation of painless AP and its progression to SAP.
A 47-year-old male presented for loss of consciousness (LOC).
The patient was found with pinpoint pupils, hypoglycemia, and hypotensive. Patient received 2 milligrams (mg) of Narcan, dextrose, and a 1 L bolus of normal saline (NS) from Emergency medical services (EMS) and became responsive. Prior to syncope, the patient was watching TV and eating pizza when he suddenly lost consciousness for an unknown period without prior symptoms of headache, dizziness, shortness of breath, chest or abdominal pain, extremity numbness or weakness. He denies consumption of alcohol or illicit drugs within the past 24 hours. No similar episodes in the past.
The patient was hospitalized 3 years prior for acute alcoholic intoxication and underwent an abdominal computed tomography (CT) that was negative for acute findings or pancreatic changes.
The patient had a personal history of hypotension and no family history.
The patient was diaphoretic and not in acute distress. Review of systems were otherwise unremarkable.
Laboratory workup was significant for thrombocytopenia, hyponatremia, anion gap lactic acidosis, and elevated lipase. Urinalysis was positive for ketones and proteinuria. Urine drug screen and blood alcohol were unremarkable. Laboratory findings represented in Table 1.
| Labs | Value | Reference |
| Hemoglobin | 15.1 | 13.0-17.0 g/dL |
| Hematocrit | 44.1 | 38.6-49.2% |
| MCV | 110.9 | 80.0-100.0 fL |
| Platelets | 107 × 103 | 150-450 × 103/mcL |
| Sodium | 125 | 133-144 mEq/L |
| Potassium | 4.0 | 3.5-5.2 mEq/L |
| Chloride | 82 | 98-107 mEq/L |
| HCO3 | < 10 | 21-31 mEq/L |
| Creatinine | 1.35 | 0.70-1.30 mg/dL |
| BUN | 13 | 7-25 mg/dL |
| Calcium | 9.1 | 8.6-10.3 mg/dL |
| AST | 93 | 13-39 U/L |
| Total bilirubin | 3.5 | 0.3-1.0 mg/dL |
| Glucose | 210 | 70-99 mg/dL |
| Lactic acid | 7.7 | 0.5-2.0 mmol/L |
| Troponin | 377 | 0-20 pg/mL |
| Lipase | 557 | 11-82 U/L |
| CPK | 350 | 30-223 U/L |
| Cholesterol | 218 | < 200 mg/dL |
| Triglycerides | 313 | < 150 mg/dL |
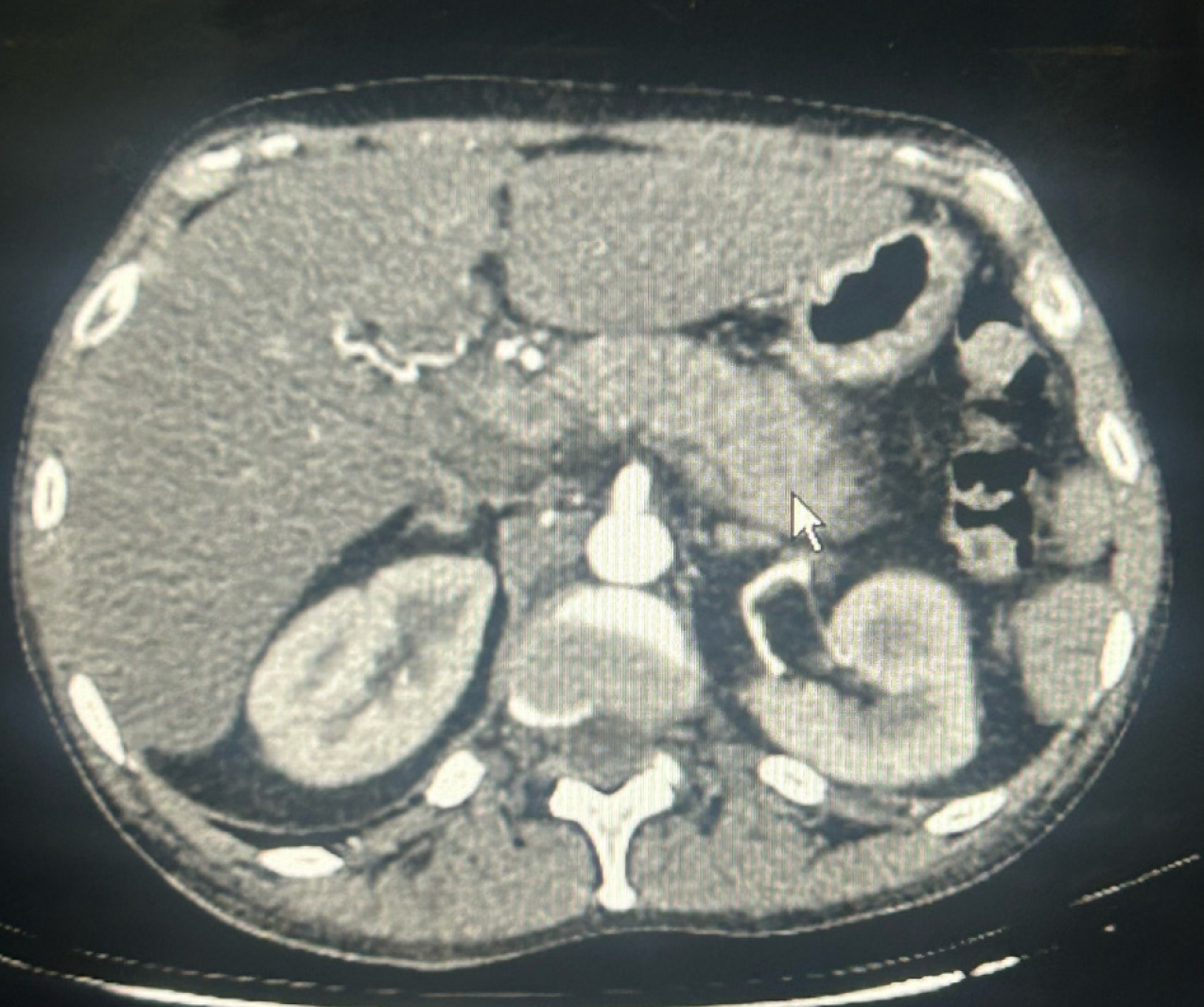
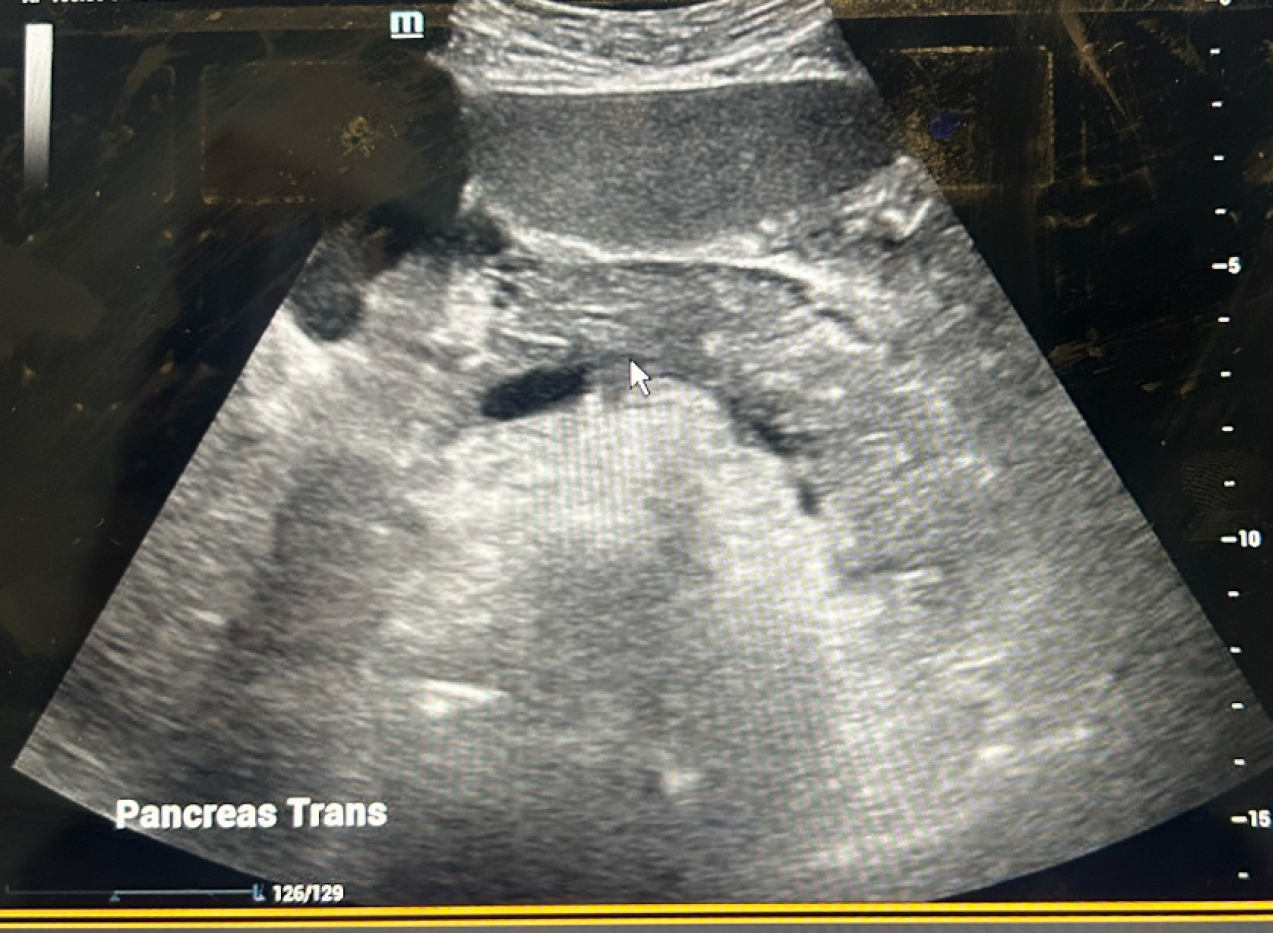
CT angiography chest/abdomen/pelvis with/without contrast showed hepatomegaly and AP without evidence of chronic changes, pancreatic duct dilation, necrosis, or pulmonary embolism (Figure 1). Abdominal ultrasound confirmed pancreatitis, hepatomegaly, and normal biliary tree (Figure 2). Transthoracic echocardiogram showed an ejection fraction (EF) of 35%-40%, stage 1 diastolic dysfunction, and moderate diffuse hypokinesis with regional variations; worsened from echocardiogram 3 years prior with EF 55%-60%.
PAP.
The patient received 1 Liter (L) NS bolus, 2 mg of Narcan for suspected acute opioid intoxication, and dextrose bolus for hypoglycemia per EMS.
In the emergency department, patient given dextrose boluses for multiple episodes of hypoglycemia and started on levophed for hypotension that was unresponsive to IV fluids. Patient became hemodynamically unstable with altered mental status and was intubated and sedated for acute hypoxic respiratory failure and airway protection.
Patient went into pulseless electrical activity (PEA) and underwent 2 rounds of cardiopulmonary resuscitation with epinephrine, bicarbonate, and calcium given.
Status-post PEA arrest and intubation, patient was started on empiric antibiotics for suspected aspiration pneumonia and amiodarone drip for sustained tachycardia. The patient was maxed out on 5 pressors and started on dexamethasone for increasing pressor requirements.
Patient ultimately went into distributive shock likely secondary to SAP in the setting of new-onset heart failure, kidney and liver dysfunction. Despite aggressive fluid resuscitation, ventilation and pressor support, and medical management, the patient’s condition resulted in death.
PAP was diagnosed based on positive imaging and elevated lipase three times the upper limit of normal with absence of epigastric and/or back pain radiation. PAP most commonly results from repeated episodes of AP in men (mean age of 44.7 years) who are heavy drinkers[2]. Approximately 96% of PAP cases were related to pancreatic calcium deposition, genetic predisposition, diabetes mellitus, and pancreatic exocrine insufficiency[2]. Characteristics of PAP include early satiety or GERD-like symptoms, obstructive jaundice, weight loss, steatorrhea, calcium deposits within the pancreas, and dilation of the pancreatic duct. Fever was reported in 75% of cases while jaundice was reported in 65.2% and fatigue in 40.9% of cases[3]. The patient did not experience the common characteristics associated with PAP except for lack of abdominal pain.
The incidence of PAP was first reported in 1948 with an incidence rate of 10%-15%, but more recent studies show this number could be as high as 51% which could be attributed to increasing availability of CT[4]. The incidence of PAP can be estimated up to 72% in patients newly diagnosed with diabetes mellitus[4].
PAP has a predilection for males (2.8 male: 1 female ratio) with ages ranging from 30-40 years old[2]. Incidence of PAP in patients with acute or chronic pancreatitis has yet to be determined, however it is found that an estimated 10% of all SAP cases to be painless often synchronously with hypotension[2]. The mortality rate of SAP is 45.63%[2]. Fortunately, due to improved diagnostic methods for earlier detection and newer procedural interventions the mortality of AP and SAP has significantly reduced[5].
There is a growing concern for increasing mortality rates associated with AP progressing to SAP, thus making it essential for clinicians to recognize the clinical and laboratory evidence with utmost haste. Scoring systems such as the Harmless Acute Pancreatitis score, modified Glasgow-Imrie Criteria (GIC), Bedside Index of Severity in Acute Pancreatitis (BISAP), Bedside Index for Acute Pancreatitis, Ranson criteria, Acute physiology and chronic health evaluation II (APACHEII), or modified Computed Tomography Severity Index (CTSI) score have been implemented to guide in the early prediction of disease severity. HAPS is an initial assessment that should be done within the first 30 minutes of admission and once non-SAP has been ruled out, the abovementioned scoring systems can be utilized.
The gold standard of staging and detecting complications of AP is Dynamic contrast-enhanced CT (DCT). DCT has been shown to have diagnostic sensitivity of 87% for pancreatic necrosis and detection rate of 90% of AP. CTSI is used to determine the morphological severity of AP as well as to predict the likelihood of pancreatic necrosis progression. However, it is important to note that each scoring system has its own limitations[6].
The Ranson and Glagow-Imrie scores require lab values obtained at a minimum of 48 hours as it was designed to predict morbidity and/or mortality outcomes at the time. Since the modified CTSI considers peripancreatic necrosis and pseudocyst formation (generally present from day 6-10 after initial onset of pancreatitis) as part of its criteria; which are present in the first 48 hours of AP, a CTSI score must be performed 48-72 hours after established diagnosis of AP[7]. During emergent circumstances, the Ranson, GIC and CTSI all require time-dependent criteria to be met which may obscure the diagnosis and potentially delay early treatment. BISAP includes mental status as part of its severity assessment, thus this scoring system is often subjective via requirement of baseline mental status[8]. This proves to be complicated for patients with a long standing history of the disease.
It is difficult to determine which scoring system is the most accurate and appropriate given the dynamic clinical signs and symptoms of AP. Of the scoring systems mentioned have a sensitivity and specificity of 55%-90% of predicting AP. Ong and Shelat showed that the Ranson score was more accurate in stratifying the severity and predicting mortality of AP when compared to scoring systems such as APACHE II, BISAP, and CTSI[9]. However, in a more recent study, Chauhan et al[10] compared the modified GIC, Ranson and APACHE II scores. It was noted that the APACHE II score had the highest sensitivity (79.17%) in predicting the severity of AP and the modified GIC was the most specific (97.83%).
In a retrospective study of 918 patients carried out by Li et al[11], the prediction of SAP prognosis and mortality were similar by using the BISAP, Ranson, GIC and APACHE II scoring system. It was concluded that Ranson and APACHE II were more suitable for younger patients (< 60 years old), BISAP performed well for elderly patients (> 60 years old) and both Ranson and Glasgow can be appropriately used for the general population[11]. Table 2 discusses each scoring system with interpretation in detail.
| Scoring System | Number of criterion | Criterion on Admission | Within 48 hours | Interpretation |
| Harmless Acute Pancreatitis Score (should not be used in isolation) | 3 | Peritonitis (rebound tenderness/guarding); Creatinine ≥ 2 mg/dL (177 µmol/L); Hematocrit ≥ 43% (male) or 39.6% (female) | Lower score suggests a more “harmless/ low-risk” course of pancreatitis | |
| Ranson (Initially derived from Alcoholic Pancreatitis therefore prognosis is better for other types of pancreatitis) | 11 | WBC > 16k; Age > 55 years; Glucose > 200 mg/dL (> 11.1 mmol/L); AST > 250; LDH > 350 | Hct drop > 10%; BUN increase > 5 mg/dL (> 1.79 mmol/L); Ca < 8 mg/dL (< 2 mmol/L); Arterial pO2 < 60 mmHg; Base deficit (24 - HCO3) > 4 mg/dL; Fluid needs > 6 L | Risk factors < 3: 1% mortality; Risk factors 3-4: 15% mortality; Risk factors 5-6: 40% mortality; Risk factors > 7: 100% mortality |
| Glasgow-Imerie | 8 | PaO2 < 59.3 mmHg (7.9 kPa); Age > 55 years; WBC > 15 × 10³/µL (109/L); Calcium < 8 mg/dL (2 mmol/L). BUN > 44.8 mg/dL (serum urea > 16 mmol/L); LDH > 600 IU/L. Albumin < 3.2 g/dL (32 g/L); Glucose > 180 mg/dL (10 mmol/L) | Serum Calcium < 2.0 mmol/L); Serum albumin < 32 g/L; LDH > 600 IU/L; AST/ALT > 200 IU/L | > 3 criteria present w/n first 48 hours of presentation qualifies for SAP |
| BISAP | 5 | BUN > 25 mg/dL (8.92 mmol/L); Impaired mental status; ≥ 2 SIRS criteria; Age > 60 years; Pleural effusion present on CT | Score 0 mortality 0.1%; Score 1 mortality 0.4%; Score 2 mortality 1.6%; Score 3 mortality 3.6%; Score 4 mortality 7.4%; Score 5 mortality 9.5% | |
| APACHE II; Assess disease severity in Intensive Care unit patients (age > 16 years old) | 15 | History of severe organ failure or immunocompromised, age, temperature, mean arterial pressure, pH, heart rate/pulse, respiratory rate, serum sodium Serum potassium, serum creatinine, presence of acute renal failure, hematocrit, WBC count, glasgow coma scale, FiO2 | Total score of > 30 increases non-operative and post-operative status to approximately 73%; total score: 0 to 4; non-operative status: 4% mortality; post-operative status: 1% mortality; total score: 5 to 9; non-operative status: 8% mortality; post-operative status: 3% mortality; total score: 10-14; non-operative status: 15% mortality; post-operative status: 7% mortality; total score: 15-19; non-operative status: 24% mortality; post-operative status: 12% mortality; total score: 20-24; non-operative status: 40% mortality; post-operative status: 30% mortality; total score: 25-29; non-operative status: 55% mortality; post-operative status: 35% mortality; total score: 30-34; non-operative status: 73% mortality; post-operative status: 73% mortality; total score: 35-100; non-operative status: 85% mortality; post-operative status: 88% mortality | |
| CTSI (Balthazar Score + Necrosis Score) | Balthazar Score (CT Score: Grading); Score 0 Normal Pancreas (Grade A); Score 1 Edematous Pancreas (Grade B); Score 2 Edematous Pancreas and mild extrapancreatic changes (Grade C); Score 3 Severe extrapancreatic changes AND one fluid collection (Grade D); Score 4 Multiple or extensive fluid collections (Grade E). Necrosis Score (Extent of Necrosis), Score 0 No necrosis; Score 2 Necrosis < 33%; Score 4 Necrosis 33% to 50%; Score 6 Necrosis 50% or more | CTSI > 5 is associated with: Longer length of hospitalization; Mortality increased 15 fold |
The Ranson criteria is the generally used scoring system to determine the severity of pancreatitis based on accuracy and population generability. Our patient had a Ranson score of 2 which suggests an approximately mortality rate of 1%. Even with mortality of 1%, our patient’s course of disease rapidly progressed to SAP ultimately resulting in distributive shock. More investigation should be conducted on scoring criteria that is inclusive of end-organ damage in combination with imaging studies in the initial stages of scoring disease severity. As a result, more aggressive management can be initiated earlier in the disease process.
The treatment course of PAP is analogous to AP with fluid resuscitation, enteral nutrition, analgesic and antibiotic[5,12]. Analgesics and surgical intervention are usually unnecessary in PAP due to the lack of nociception and antibiotics are reserved in cases of sepsis. In the rare cases that PAP progresses to SAP, aggressive management such as pressors, antibiotics, and/or mechanical ventilation may be utilized[5,12]. Fluid resuscitation is one of the most important steps in the management of SAP. Lactated ringer has shown to be the preferred fluid of choice in fluid resuscitation via its anti-inflammatory effects[5,12]. On the contrary, NS is not recommended secondary to triggering hyperchloremic metabolic acidosis when administered in large volumes. Adequate fluid resuscitation can be indicated by a decrease in blood urea nitrogen and hematocrit with appropriate urine output[12].
Enteral feeding is a recommendation as a treatment modality for SAP as shown in meta-analysis studies by Qi et al[13] and Li et al[14]. However, limitations exist when SAP causes distributive shock to which multiple pressors such as Levophed and phenylephrine are required. When a patient requires multiple pressors, all feedings are stopped due to risk of bowel ischemia. Thus early feeding is not a viable treatment option in patients with SAP and distributive shock requiring pressors[15].
A propensity-score matching analysis and meta-analysis on glucocorticoid use in pancreatitis showed preliminary beneficial effects of low dose glucocorticoids[9,15,16]. The studies showed reduced length in hospitalization, need for surgery, and mortality rate[10,16]. However, more studies need to be conducted to prove the efficacy of early steroid use in AP management.
In conclusion, PAP is a rare presentation of AP. Although atypical, it is imperative for clinicians to be aware of early signs of organ failure in the earlier stages of the disease. Further studies should be conducted on the incidence of PAP and the likelihood of disease progressing to SAP.
The authors thank the patient’s family for their cooperation.
| 1. | Bhullar FA, Faghih M, Akshintala VS, Ahmed AI, Lobner K, Afghani E, Phillips AE, Hart PA, Ramsey ML, Bick BL, Kuhlmann L, Drewes AM, Yadav D, Olesen SS, Singh VK; P-QST Consortium. Prevalence of primary painless chronic pancreatitis: A systematic review and meta-analysis. Pancreatology. 2022;22:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Xu L, Pandya N, Basit A, Kausar R. S3455 Atypical Acute Pancreatitis in Elderly: A Fatal Near-Miss. Am J Gastroenterol. 2021;116:S1424-S1424. [DOI] [Full Text] |
| 3. | Dite P, Precechtelova M, Bojkova M, Lovecek M, Ambroz R, Martinek A, Dolina J. Painless form of chronic pancreatitis - multicentre study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2023;167:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Lin NH, Chen C, Yang HW, Su YJ. Clinical presentation of painless pancreatitis: a systematic review. Signa Vitae. 2023;19:30-35. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Bank S, Singh P, Pooran N, Stark B. Evaluation of factors that have reduced mortality from acute pancreatitis over the past 20 years. J Clin Gastroenterol. 2002;35:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, Dixit VK. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013;2013:367581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Evrimler Ş, Çakmakçı M, Karaibrahimoğlu A, Kayan M. The prognostic value of fat necrosis deposits on CT imaging in acute pancreatitis. Turk J Med Sci. 2021;51:749-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Luo X, Wang J, Wu Q, Peng P, Liao G, Liang C, Yang H, Huang J, Qin M. A modified Ranson score to predict disease severity, organ failure, pancreatic necrosis, and pancreatic infection in patients with acute pancreatitis. Front Med (Lausanne). 2023;10:1145471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Wang M, Jiang Z, Liang H. Glucocorticoids in acute pancreatitis: a propensity score matching analysis. BMC Gastroenterol. 2021;21:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Chauhan R, Saxena N, Kapur N, Kardam D. Comparison of modified Glasgow-Imrie, Ranson, and Apache II scoring systems in predicting the severity of acute pancreatitis. Pol Przegl Chir. 2022;95:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Li Y, Zhang J, Zou J. Evaluation of four scoring systems in prognostication of acute pancreatitis for elderly patients. BMC Gastroenterol. 2020;20:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Siregar GA, Siregar GP. Management of Severe Acute Pancreatitis. Open Access Maced J Med Sci. 2019;7:3319-3323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Qi D, Yu B, Huang J, Peng M. Meta-Analysis of Early Enteral Nutrition Provided Within 24 Hours of Admission on Clinical Outcomes in Acute Pancreatitis. JPEN J Parenter Enteral Nutr. 2018;42:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Li W, Liu J, Zhao S, Li J. Safety and efficacy of total parenteral nutrition versus total enteral nutrition for patients with severe acute pancreatitis: a meta-analysis. J Int Med Res. 2018;46:3948-3958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Dong LH, Liu ZM, Wang SJ, Zhao SJ, Zhang D, Chen Y, Wang YS. Corticosteroid therapy for severe acute pancreatitis: a meta-analysis of randomized, controlled trials. Int J Clin Exp Pathol. 2015;8:7654-7660. [PubMed] |
| 16. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 90.0] [Reference Citation Analysis (0)] |