Published online Jun 6, 2025. doi: 10.12998/wjcc.v13.i16.102853
Revised: December 25, 2024
Accepted: January 18, 2025
Published online: June 6, 2025
Processing time: 82 Days and 20 Hours
Hypertension (HTN) is a prevalent chronic health condition that significantly increases the risk of cardiovascular diseases-associated mortalities. Despite the use of antihypertensive medications, numerous patients fail to achieve guideline-recommended blood pressure (BP) targets.
To evaluates the efficacy of catheter-based ultrasound renal denervation (uRDN) for the treatment of HTN.
Relevant studies were identified through searches in PubMed, Embase, the Cochrane Library, Web of Science, and China National Knowledge Infrastructure, with a cut-off date at April 1, 2024. A random-effects model was employed in this study to mitigate potential biases. The risk of bias for included studies was assessed using the Cochrane Risk of Bias assessment tool. Statistical analyses were conducted using Review Manager version 5.3. This meta-analysis incorporated four studies encompassing a total of 627 patients. The reporting bias of this study was deemed acceptable.
Compared to the Sham group, the uRDN group demonstrated a significant reduction in daytime ambulatory systolic BP (SBP) [mean difference (MD) -3.87 mmHg, 95% confidence interval (CI): -7.02 to -0.73, P = 0.02], office SBP (MD -4.13 mmHg, 95%CI: -7.15 to -1.12, P = 0.007), and home SBP (MD -5.51 mmHg, 95%CI: -8.47 to -2.55, P < 0.001). However, there was no statistically significant reduction observed in either 24-hour or nighttime ambulatory SBP levels. Subgroup analysis shows that uRDN can significantly reduce the SBP in patients with non-resistant HTN (MD -6.19 mmHg, MD -6.00 mmHg, MD -7.72 mmHg, MD -5.02 mmHg, MD -3.61 mmHg).
The current evidence suggests that uRDN may effectively reduce home, office, and daytime SBP in patients with HTN, particularly in those with non-resistant HTN.
Core Tip: This is the latest meta-analysis to date, and it confirms the clinical significance of ultrasound renal denervation (uRDN) for reducing systolic blood pressure (SBP). uRDN may effectively reduce daytime SBP in patients with hypertension (HTN), particularly in those with non-resistant HTN.
- Citation: Ou YC, Peng XY, Yang JX, Chen BY, Chen PF, Liu M. Efficacy of catheter-based ultrasound renal denervation in the treatment of hypertension. World J Clin Cases 2025; 13(16): 102853
- URL: https://www.wjgnet.com/2307-8960/full/v13/i16/102853.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i16.102853
Currently, approximately 1 billion individuals worldwide suffer from hypertension (HTN), contributing to more than 9 million annual fatalities due to various HTN-related complications[1]. Drug therapy remains the standard approach for managing HTN[2]. Nevertheless, poor medical compliance and drug tolerance can significantly impact patients’ ability to control blood pressure (BP)[3]. Therefore, exploring novel strategies for BP management is crucial. The renal sympathetic nerves play a key role in the persistence of HTN[4]. Inhibiting their activity has been shown to effectively reduce BP in patients with HTN. Although several interventions targeting renal sympathetic nerve activity have been explored[5], one of the most prominent methods is renal sympathetic denervation (RDN). Since the initial positive outcomes of renal nerve surgery were published, RDN has demonstrated significant promise in the treatment of HTN[6]. The more established technique involves the ablation of renal artery sympathetic nerves using high-focused radiofrequency energy. Meanwhile, clinical research is ongoing to investigate alternative ablation methods such as absolute ethanol ablation and ultrasound ablation. In recent years, ultrasound renal denervation (uRDN), a novel technique for treating HTN, has garnered increasing global attention. Unlike traditional radiofrequency-based approaches, uRDN employs ultrasound as the ablation energy source. Ultrasound is a superior energy source option, as it allows for deeper penetration, a larger ablation area, and minimal damage to the vascular intima[7]. Additionally, the hypotensive effects of uRDN are sustained over time. In recent years, two multi-center clinical trials have reported that uRDN is effective in treating HTN, despite some controversies arising from earlier studies. This article presents a comprehensive meta-analysis of the latest clinical trials to evaluate the efficacy of uRDN therapy for HTN.
This meta-analysis was conducted in accordance with the PRISMA 2020 guidelines for systematic reviews and meta-analyses[8]. This study was not registered.
Relevant literature on the effect of uRDN was queried from PubMed, Embase, the Cochrane Library, Web of Science, and China National Knowledge Infrastructure from their inception to April 1, 2024. The main search terms included: HTN, high BP, refractory HTN, resistant HTN, renal denervation, renal sympathetic, and sympathetic denervation.
All candidate articles were screened and extracted by two independent authors (Liu M and Ou YC). In case of discrepancies regarding data extraction, a third investigator (Peng XY) was consulted. The following information was extracted from each study: The first author’s name, country or region, sample size, gender, type of HTN in patients, uRDN system used, primary outcomes, number of anti-HTN medications, and follow-up duration.
The inclusion criteria were as follows: (1) Randomized controlled trial (RCT) design; (2) Studies involving patients with HTN or resistant HTN; and (3) Interventions including renal denervation (uRDN group) vs a sham procedure (Sham group). For a series of related studies, the article with the longest follow-up period was selected. Exclusion criteria included: (1) Abstracts, letters, case reports, reviews, or non-clinical studies; (2) Studies with incomplete or unusable data; and (3) Studies with duplicate data.
The risk of bias for the included studies was evaluated using the Cochrane Risk of bias assessment tool[9].
Statistical analyses were performed using Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). The heterogeneity among the included RCTs was assessed using the I2 test and the Q test. Heterogeneity was considered insignificant if the I2 value was < 50% and P > 0.10. A random-effects model was employed in this study to minimize the potential bias. Subgroup analyses were performed to explore sources of heterogeneity.
As this is a meta-analysis, ethical approval was not required.
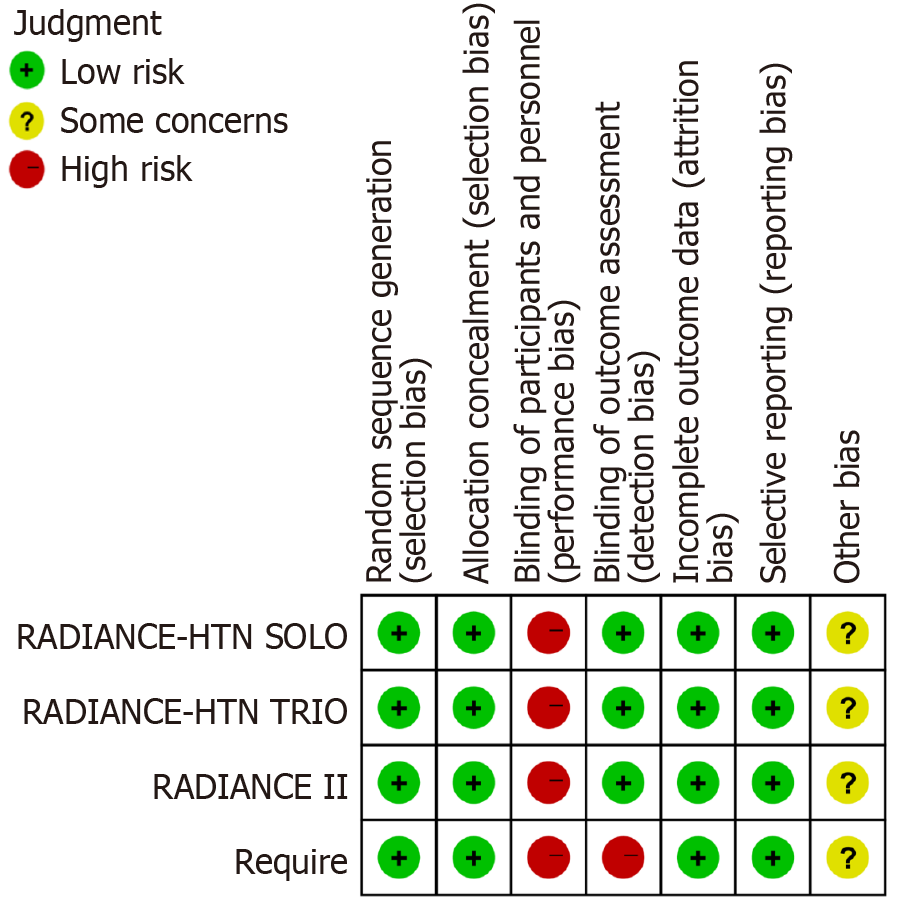
A total of 1587 studies were identified in the preliminary literature search. After reviewing the abstracts and full texts, only four studies were finally included[10-13] (Figure 1). One RCT involving the Kona Medical Surround Sound System was excluded to avoid systemic errors. The primary outcome was the change in daytime ambulatory systolic BP (SBP), with secondary outcomes including mean changes in 24-hour ambulatory, home, and office SBPs. Baseline characteristics were comparable between the uRDN and Sham groups in this meta-analysis (Table 1). According to the risk of bias graph (Figure 2), the reporting biases in this study was deemed acceptable.
| Ref. | Country or region | Sample | Mean age, years uRDN/sham | Female, % | Diabetes, % | eGFR, mL/min/1.73 m2 uRDN/sham | Type of HTN patients | uRDN system | Primary outcome | Number of AHT drugs uRDN/sham | Follow-up, months |
| Azizi et al[10] | United States, Europe | 146 | 54.4/53.8 | 37.8/45.8 | 2.7/6.9 | 84.7/83.2 | Aged 18-75 years; ABPM systolic BP/diastolic BP >/= 135/85 mmHg and < 170/105 mm Hg | ParadiseTM | Change in daytime ambulatory systolic BP at 2 months | 1.2/1.2 | 2 |
| Azizi et al[11] | United States, Europe | 129 | 51.9/53.0 | 18.5/20.3 | 29.2/26.6 | 86.8/82.5 | Aged 18-75 years; resistant hypertension; office systolic and diastolic BP >/= 140/90 mmHg, despite 3 or more AHT drugs including a diuretic | ParadiseTM | Changes in ambulatory, home, and systolic SBP | 4.0/3.9 | 6 |
| Kario et al[12] | Japan, South Korea | 143 | 50.5/55.6 | 30.4/20.9 | 26.1/29.9 | 74.2/69.6 | Aged 20-75 years; resistant hypertension; office BP >/= 150/90 mmHg and ABPM systolic BP > /= 140 mmHg; despite maximum tolerated dosages of at least three AHT drugs from different classes including a diuretic | ParadiseTM | Change in 24-hour ambulatory systolic BP from baseline at 3 months | 4.1/3.9 | 3 |
| Azizi et al[13] | United States, Europe | 224 | 55.1/54.9 | 31.3/23.0 | 6.0/6.8 | 81.4/82.3 | Aged 18-75 years; ABPM systolic BP/diastolic BP >/= 135/85 mmHg and < 170/105 mmHg | ParadiseTM | Mean change in daytime ambulatory systolic BP at 2 months | 0.9/1.0 | 2 |
Compared to the Sham group, the uRDN group had an effective reduction in daytime ambulatory SBP [mean difference (MD) -3.87 mmHg, 95% confidence interval (CI) -7.02 to -0.73, P = 0.02], office SBP (MD -4.13 mmHg, 95%CI: -7.15 to -1.12, P = 0.007), and home SBP (MD -5.51 mmHg, 95%CI: -8.47 to -2.55, P < 0.001) (Figure 3A-C). However, no statistically significant reductions were observed in the 24-hour ambulatory SBP (MD -2.95 mmHg, 95%CI: -5.94 to 0.04, P = 0.05) or nighttime ambulatory SBP reduction (MD -2.10 mmHg, 95%CI: -5.14 to 0.94, P = 0.18) (Figure 3D). The uRDN groups comprised 345, 345, 336, 352, and 351 participants, respectively, whereas the corresponding control groups comprised 275, 273, 264, 275, and 274 participants, respectively.
Subgroup analysis further revealed that uRDN exhibited particular effectiveness in reducing SBP among patients with non-resistant HTN. There are significant reductions in home SBP (MD -7.72 mmHg, 95%CI: -9.68 to -5.75, P < 0.00001), office SBP (MD -6.00 mmHg, 95%CI: -9.02 to -2.98, P < 0.0001), 24-hour ambulatory SBP (MD -5.02 mmHg, 95%CI: -7.08 to -2.97, P < 0.00001), nighttime ambulatory SBP (MD -3.61 mmHg, 95%CI: -7.13 to -0.09, P = 0.04), and daytime ambulatory SBP (MD -6.19 mmHg, 95%CI: -8.31 to -4.07, P < 0.00001). The only subgroups with low heterogeneity were the 24-hour ambulatory SBP subgroup (I² = 2%) and the nighttime ambulatory SBP subgroup (I² = 49%) (Supplementary Figures 1-5).
No statistical significant reductions were observed in home SBP (MD -2.20 mmHg, 95%CI: -5.73 to 1.33, P = 0.22), office SBP (MD -0.84 mmHg, 95%CI: -5.28 to 3.61, P = 0.71), 24-hour ambulatory SBP (MD 0.33 mmHg, 95%CI: -3.28 to 3.93, P = 0.86), nighttime ambulatory SBP(MD 0.93 mmHg, 95%CI: -3.57 to 5.44, P = 0.69), and daytime ambulatory SBP (MD -0.27 mmHg, 95%CI: -3.90 to 3.36, P = 0.88) of patients with resistant HTN (Supplementary Figures 1-5).
This meta-analysis included 627 patients from four randomized, sham-controlled trials. Our comprehensive review of the current clinical research, based on the latest and largest meta-analysis to date, confirms the clinical significance of uRDN in reducing SBP.
Increased activity of renal sympathetic nerves contributes to the development of HTN by excessively activating the renin-angiotensin-aldosterone system. This activation may lead to water and sodium retention, as well as the production of various vasoactive substances that elevate BP[14]. The sympathetic nervous system plays a crucial role in regulating perfusion and renal function by connecting afferent and efferent fibers from the aorta to the kidney via fibers located in the adventitia of the renal artery[15]. Renal denervation has been shown to effectively reduce renal artery overactivity, thereby blocking undesirable signals that contribute to high BP. A study published in the New England Journal of Medicine reported that RDN can reduce BP in patients with HTN through several mechanisms such as reducing renal norepinephrine overflow, halving renin activity, increasing renal plasma flow, and reducing central sympathetic drive. Additionally, it can reduce ventricular remodeling and the risk of adverse events[16].
Following the use of radiofrequency and chemical ablation, RDN has advanced with the introduction of ultrasound ablation technology. The uRDN procedure employs a balloon ablation catheter to generate ultrasound energy, which penetrates the renal artery at depths of 1 to 6 mm. This intervention effectively reduces renal sympathetic nerve activity, interrupting signals associated with HTN. The results of the RADIOSOUND-HTN trial demonstrated that the antihypertensive effect of ultrasonic RDN is superior to that of radiofrequency RDN[17]. Additionally, ultrasound ablation can achieve longer distances and more complete ablation[7]. These findings suggest that uRDN offers significant advantages. We conducted a comprehensive analysis of all current clinical RCTs using the ReCor Medical Paradise System to provide reliable evidence-based medical assessments for the future clinical application of uRDN.
Our study’s findings demonstrate that uRDN effectively reduces daytime ambulatory, office, and home SBPs (MD -3.87 mmHg, MD -4.13 mmHg, MD -5.51 mmHg, respectively). For patients with HTN, particularly those with resistant HTN, it effectively lowers SBP (MD -6.19 mmHg, MD -6.00 mmHg, MD -7.72 mmHg, repectively). This outcome aligns with previous meta-analyses exploring the variations in the efficacy of RDN in reducing SBP across different HTN patient profiles[18,19].
Patients with non-resistant HTN may be more influenced by sympathetic nervous system activity than those with resistant HTN. Evidence suggests that sympathetic nervous system activity is less pronounced in patients with resistant HTN than in patients with non-resistant HTN[20], potentially resulting in a less responsive reaction to renal denervation treatment. Additionally, the degree of vascular wall remodeling may influence the effectiveness of renal denervation. Patients with non-resistant HTN tend to be younger and have well-controlled BP, meaning vascular remodeling may still be reversible. In contrast, patients with resistant HTN often exhibit target organ damage such as renal fibrosis and vascular sclerosis, which are more challenging to reverse. Certain studies suggest that patients with abnormal endothelial function, inflammation, and extreme vascular stiffness may not be candidates for renal denervation for BP control[21,22]. Despite these concerns, our analysis found no significant difference in BP reduction between the groups. However, more patients in the uRDN group achieved controlled BP without additional antihypertensive medication compared to those in the Sham group. This indicates that uRDN can reduce the need for additional medications in patients with resistant HTN, which may improve medication adherence and reduce adverse reactions. This finding suggest that uRDN may serve as an adjunctive treatment to reduce BP for resistant HTN.
Our findings also suggest that the lack of significant disparities in BP reduction between the 24-hour ambulatory and nighttime SBP groups may be attributed to nocturnal vagal excitation and sympathetic inhibition, which help maintain relatively lower SBP levels during sleep, thus attenuating the antihypertensive effects of uRDN. Consequently, the observed differences between the groups were not pronounced. Although the variance between the 24-hour ambulatory and nighttime SBP did not reach statistical significance, prioritizing the reduction of daytime ambulatory SBP assumes paramount importance, as most adverse events in patients with HTN occur during this period. Our subgroup analysis also indicated that uRDN may be less effective in reducing refractory HTN, whereas it showed a positive antihypertensive effect on 24-hour ambulatory and nighttime SBP in patients with non-resistant HTN (MD -5.02 mmHg, and MD
Current guidelines recommend the addition of multiple antihypertensive medications, including spironolactone, for patients with resistant HTN, increasing the risk of side effects and poor medication adherence[23] -a major factor contributing to uncontrolled HTN management[24]. The continuous antihypertensive effect of uRDN eliminates concerns regarding medication adherence. Furthermore, our study determined that uRDN is safe and does not increase the risk of adverse events, which is consistent with other studies.
The limited scale of current clinical trials may reduce statistical power attributed to the novelty of uRDN technology. Despite utilizing a random-effects model in our analysis to minimize potential bias, the small sample size remains a constraint. Moreover, none of the included studies employed a double-blind design due to the nature of the intervention. Although uRDN had a positive effect in lowering BP in patients with non-resistant HTN, its development is limited by the invasive nature of the technique, its inability to achieve significant reduction of BP and its high cost, which present barriers to widespread application.
In summary, the available evidence suggests that uRDN can effectively lower home, office, and daytime SBP in patients with HTN and has a more pronounced effect on reduction of SBP in patients with non-resistant HTN. Larger sample sizes, higher-quality studies with longer follow-up periods are needed to further confirm these findings.
| 1. | Weber MA, Mahfoud F, Schmieder RE, Kandzari DE, Tsioufis KP, Townsend RR, Kario K, Böhm M, Sharp ASP, Davies JE, Osborn JW, Fink GD, Euler DE, Cohen DL, Schlaich MP, Esler MD. Renal Denervation for Treating Hypertension: Current Scientific and Clinical Evidence. JACC Cardiovasc Interv. 2019;12:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 3. | Simonetti F, Piccolo R, Esposito G. Renal denervation and long-term results. Eur Heart J Suppl. 2023;25:B85-B89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Lauder L, Azizi M, Kirtane AJ, Böhm M, Mahfoud F. Device-based therapies for arterial hypertension. Nat Rev Cardiol. 2020;17:614-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 6. | Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1538] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 7. | Sinelnikov Y, Mcclain S, Zou Y, Smith D, Warnking R. Renal denervation by intravascular ultrasound: Preliminary in vivo study. Am Inst Phys. 2012;. [DOI] [Full Text] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39315] [Article Influence: 9828.8] [Reference Citation Analysis (2)] |
| 9. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24648] [Article Influence: 1760.6] [Reference Citation Analysis (3)] |
| 10. | Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Lobo MD, Sharp ASP, Bloch MJ, Basile J, Wang Y, Saxena M, Lurz P, Rader F, Sayer J, Fisher NDL, Fouassier D, Barman NC, Reeve-Stoffer H, McClure C, Kirtane AJ; RADIANCE-HTN Investigators. Six-Month Results of Treatment-Blinded Medication Titration for Hypertension Control After Randomization to Endovascular Ultrasound Renal Denervation or a Sham Procedure in the RADIANCE-HTN SOLO Trial. Circulation. 2019;139:2542-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Azizi M, Mahfoud F, Weber MA, Sharp ASP, Schmieder RE, Lurz P, Lobo MD, Fisher NDL, Daemen J, Bloch MJ, Basile J, Sanghvi K, Saxena M, Gosse P, Jenkins JS, Levy T, Persu A, Kably B, Claude L, Reeve-Stoffer H, McClure C, Kirtane AJ; RADIANCE-HTN Investigators. Effects of Renal Denervation vs Sham in Resistant Hypertension After Medication Escalation: Prespecified Analysis at 6 Months of the RADIANCE-HTN TRIO Randomized Clinical Trial. JAMA Cardiol. 2022;7:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 12. | Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, Urata H, Cho JM, Kim CJ, Choi SH, Shinohara K, Mukai Y, Ikemoto T, Nakamura M, Seki S, Matoba S, Shibata Y, Sugawara S, Yumoto K, Tamura K, Yoshihara F, Nakamura S, Kang WC, Shibasaki T, Dote K, Yokoi H, Matsuo A, Fujita H, Takahashi T, Kang HJ, Sakata Y, Horie K, Inoue N, Sasaki KI, Ueno T, Tomita H, Morino Y, Nojima Y, Kim CJ, Matsumoto T, Kai H, Nanto S. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 13. | Azizi M, Saxena M, Wang Y, Jenkins JS, Devireddy C, Rader F, Fisher NDL, Schmieder RE, Mahfoud F, Lindsey J, Sanghvi K, Todoran TM, Pacella J, Flack J, Daemen J, Sharp ASP, Lurz P, Bloch MJ, Weber MA, Lobo MD, Basile J, Claude L, Reeve-Stoffer H, McClure CK, Kirtane AJ; RADIANCE II Investigators and Collaborators. Endovascular Ultrasound Renal Denervation to Treat Hypertension: The RADIANCE II Randomized Clinical Trial. JAMA. 2023;329:651-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 103] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 14. | McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Chinushi M, Izumi D, Iijima K, Suzuki K, Furushima H, Saitoh O, Furuta Y, Aizawa Y, Iwafuchi M. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 571] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 17. | Fengler K, Rommel KP, Blazek S, Besler C, Hartung P, von Roeder M, Petzold M, Winkler S, Höllriegel R, Desch S, Thiele H, Lurz P. A Three-Arm Randomized Trial of Different Renal Denervation Devices and Techniques in Patients With Resistant Hypertension (RADIOSOUND-HTN). Circulation. 2019;139:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Chen XH, Kim S, Zeng XX, Chen ZB, Cui TL, Hu ZX, Li Y, Fu P. Account for Clinical Heterogeneity in Assessment of Catheter-based Renal Denervation among Resistant Hypertension Patients: Subgroup Meta-analysis. Chin Med J (Engl). 2017;130:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Pappaccogli M, Covella M, Berra E, Fulcheri C, Di Monaco S, Perlo E, Burrello J, Monticone S, Rossato D, Rabbia F, Veglio F. Effectiveness of Renal Denervation in Resistant Hypertension: A Meta-Analysis of 11 Controlled Studies. High Blood Press Cardiovasc Prev. 2018;25:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Böhm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Dörr O, Liebetrau C, Möllmann H, Gaede L, Troidl C, Rixe J, Hamm C, Nef H. Soluble fms-like tyrosine kinase-1 and endothelial adhesion molecules (intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1) as predictive markers for blood pressure reduction after renal sympathetic denervation. Hypertension. 2014;63:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Ewen S, Ukena C, Linz D, Kindermann I, Cremers B, Laufs U, Wagenpfeil S, Schmieder RE, Böhm M, Mahfoud F. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1356] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 24. | Burnier M, Egan BM. Adherence in Hypertension. Circ Res. 2019;124:1124-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 446] [Article Influence: 89.2] [Reference Citation Analysis (0)] |