Published online Jun 6, 2025. doi: 10.12998/wjcc.v13.i16.101665
Revised: December 6, 2024
Accepted: January 11, 2025
Published online: June 6, 2025
Processing time: 137 Days and 18.1 Hours
Pancreatic cancer (PC) is a highly malignant tumor that is resistant to chemo
We report a case of a 33-year-old male who was referred to our department with weight loss of 5 kg in 2 months, anorexia and abdominal pain. Imaging showed extensive lesions involving the pancreas, liver, bones, muscles and lymph nodes accompanied by elevated carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA). Biopsy yielded a diagnosis of PC. Treatment with gemci
BRAF alterations are infrequent in PC. This case highlights the significance of molecular profiling in patients with PC, especially in patients with a high tumor burden.
Core Tip: Pancreatic cancer (PC) is an extremely aggressive malignancy, characterized by a 5-year survival rate of merely 11% and is highly resistant to chemotherapy, radiotherapy and immunotherapy. PC is largely defined by core driver gene mutations. BRAF mutations are rare, but important because of the availability of targeted drugs. We report a rare case of PC with a high tumor burden with BRAF V600E mutation. Despite the use of chemotherapy and targeted therapy, the patient’s outcome remained poor. This case highlights the significance of molecular profiling in patients with pancreatic tumors, especially in young patients with aggressive characteristics.
- Citation: Li F, Shen F. Metastatic pancreatic cancer with activating BRAF V600E mutations: A case report. World J Clin Cases 2025; 13(16): 101665
- URL: https://www.wjgnet.com/2307-8960/full/v13/i16/101665.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i16.101665
Pancreatic cancer (PC) is an extremely aggressive malignancy with a 5-year survival rate of 11% and is highly resistant to chemotherapy, radiotherapy and immunotherapy[1-5]. Despite its high mortality, PC is uncommon, with an incidence of 8–12 cases per 100000 per year and a lifetime risk of 1.3%[6]. However, the global burden of PC has increased dramatically over the past few decades and is expected to remain a leading cause of cancer-related mortality (see Surveillance, Epidemiology, and End Results Program). The risk of death from PC rises significantly with age from < 2 deaths per 100000 person-years for individuals in the United States aged 35–39 years, to > 90 deaths per 100000 person-years for those aged > 80 years. As global health improves and life expectancy increases, the overall incidence of PC is also likely to rise[7].
Although genomic profiling is widely accessible, therapies approved by the United States Food and Drug Administration (FDA) for PC are primarily restricted to combination cytotoxic treatments, such as folinic acid, 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX)[8], gemcitabine with nab-paclitaxel[9], and nal-irinotecan with fluorouracil[10]. Tumor antigen markers for programmed death protein (PD)-1 inhibitors and Trk inhibitors are scarce in PC (< 1%)[11-13]. Olaparib is still the sole targeted treatment available for a specific molecularly defined group of PC[14].
The potential of precision oncology has been realized for some types of cancer, such as lung cancer and melanoma, and has gradually promoted the development of other tumors. Recently, specific actionable targets have been recognized in PC, which is primarily characterized by mutations in key driver genes; KRAS (90%), TP53 (64%) and CDKN2A (17%) are commonly seen in PC, and BRAF alterations are less frequent (2%)[15]. Patients who have actionable molecular alterations can derive considerable benefit from receiving matched therapy. The median overall survival (OS) of patients with advanced PC who have actionable alterations receiving matched therapy is 1 year longer than those with actionable alterations receiving unmatched therapy, or those without actionable alterations[16]. BRAF mutations are rare, but important because of the availability of targeted drugs. The NCI-MATCH basket trial subprotocol H tested dabrafenib plus trametinib for BRAF V600E mutation tumors, with a 38% objective response rate and 11.4 months progression-free survival (PFS). This was one of the most successful basket trials[17]. In this comprehensive study, two patients with PC were enrolled and both presented with encouraging responses. They demonstrated disease control and significant tumor volume reduction. This finding provides valuable insights into potential treatment approaches and paves the way for further research in this challenging field. Based on the positive results, the FDA approved dabrafenib plus trametinib combination in pretreated cancers with BRAF V600E mutation[18]. Because of its low incidence, a prospective randomized phase 3 clinical trial of a BRAF mutation in PC is not feasible. Therefore, it is crucial to share real-world experiences through case reports to ensure that new biomarkers are appropriately utilized in some rare tumors.
This case report presents a man aged 33 years who presented with PC with a high tumor burden. Combination chemotherapy was discontinued after < 2 months because of further disease progression with new brain metastasis. Molecular testing revealed the presence of BRAF mutation. Targeted therapy was started with the oral dabrafenib/trametinib combination, and the patient was on therapy for 2 months, with a decrease in carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) level, but he died after 2 months. This case highlights the importance of molecular profiling in patients with pancreatic tumors, especially in young patients with aggressive tumors.
A man aged 33 years with no history of illness was referred to our department with weight loss and anorexia for 2 months.
The patient’s appetite was abnormal, and he had a weight loss of 5 kg over 2 months, accompanied by abdominal pain. His stools were normal, with no history of constipation or diarrhea.
The patient denied any past illness.
The patient denied any family history of malignant tumors.
The patient’s height and weight were 172 cm and 54 kg, respectively. His body mass index was 18.25 kg/m2, and body surface area was 1.61 m2, and Eastern Cooperative Oncology Group performance status score was 2. Jaundice was not observed.
Laboratory results included elevated CA19-9 level of > 10000 U/mL, CEA of 82.1 ng/mL, elevated white blood cell count of 29.88 × 109/L, decreased hemoglobin of 108 g/L, elevated C-reactive protein of 145.3 mg/L, elevated serum creatinine of 138 μmol/L, elevated alanine aminotransferase of 51 U/L, elevated aspartate aminotransferase of 75U/L, and elevated total bilirubin of 28.3 μmol/L.
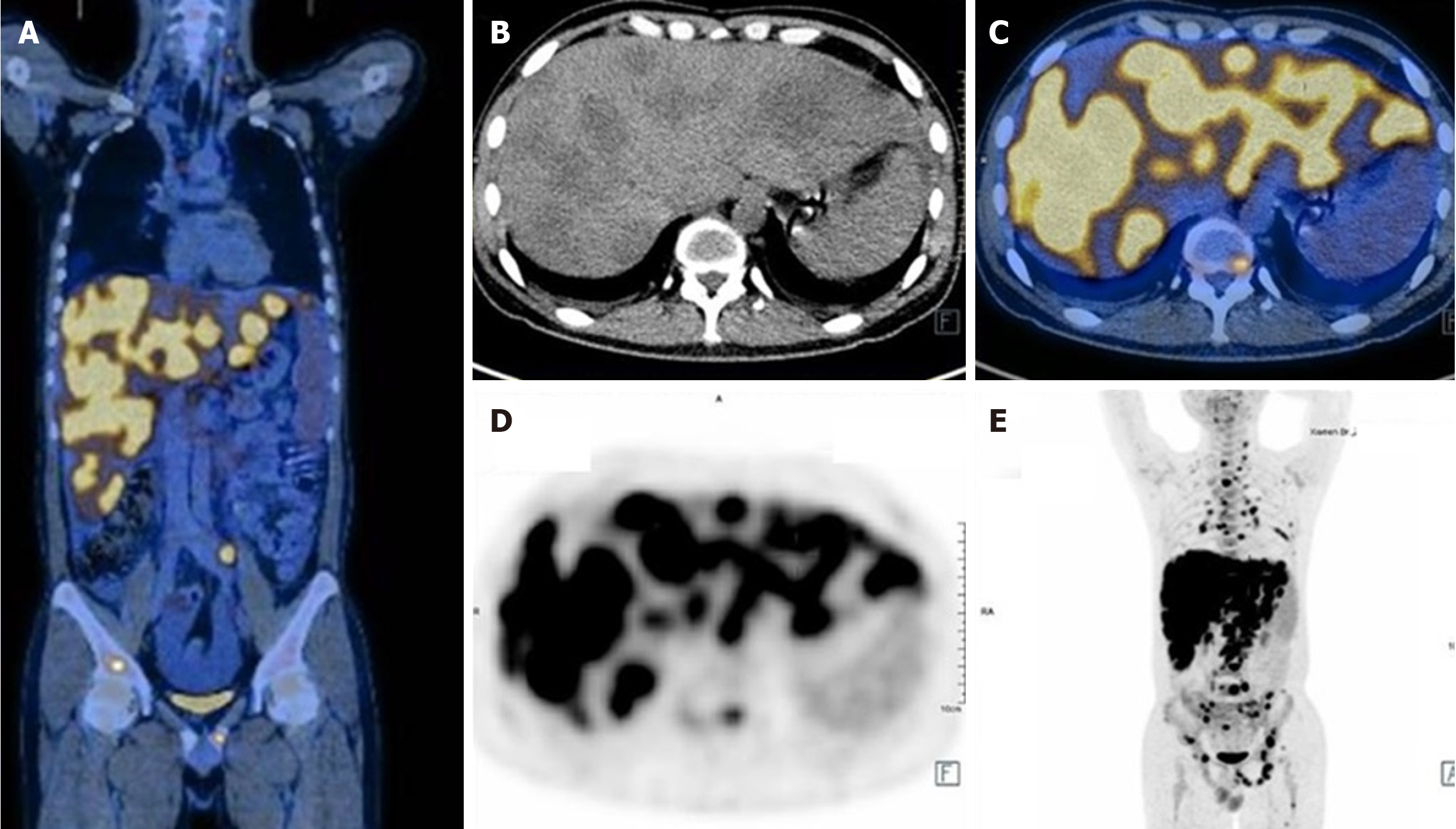
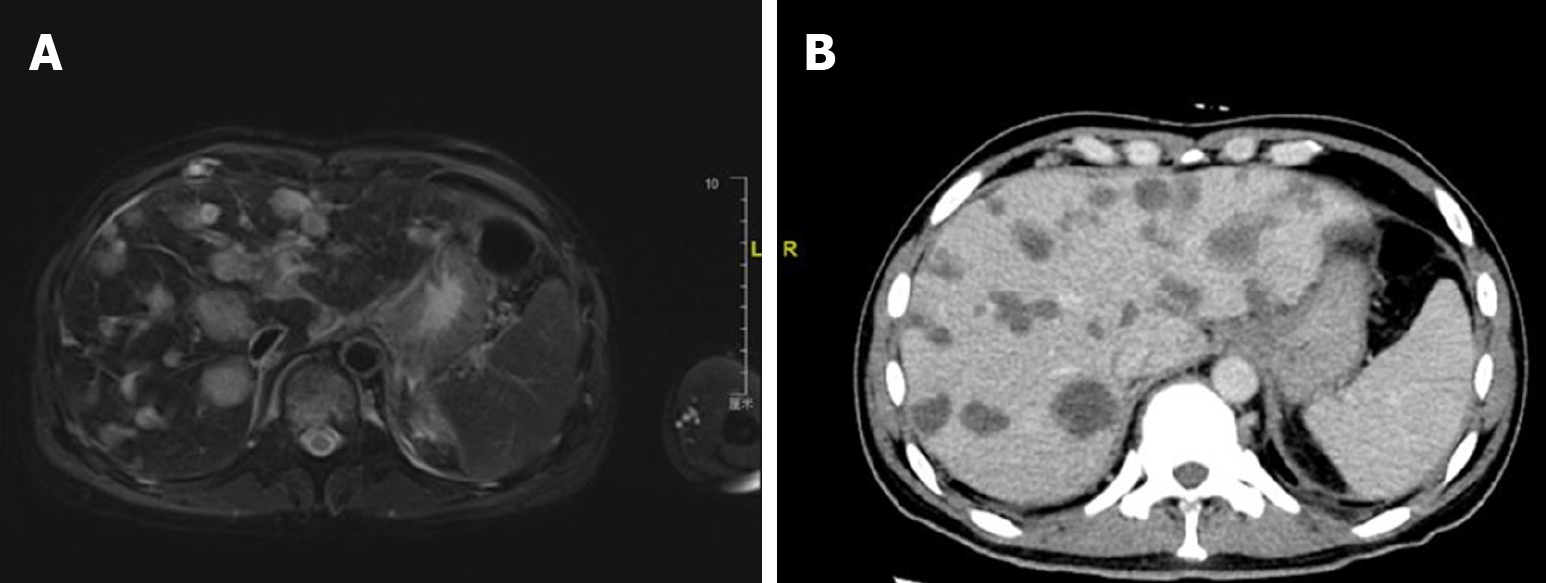
Ultrasonography revealed multiple masses in the liver. Positron emission tomography/computed tomography revealed a high tumor burden with malignant lesions involving the pancreas, liver, multiple bones, left supraspinatus, left adductor, multiple lymph nodes (bilateral neck, clavicle, mediastinum, bilateral hilum of the lung, abdomen, pelvis, and retroperitoneum), hydropericardium, hydrothorax, and splenomegaly. The tumor had infiltrated the liver diffusely, as shown in Figure 1. Computed tomography pulmonary angiography revealed a few emboli in the left lower lung. Liver biopsy detected adenocarcinoma. Immunohistochemical analysis revealed that the tumor was positive for cytokeratin (CK) 7, CK19, CK20, MutS Homolog (MSH) 2, MSH6, PMS1 Homolog 2, MutL Homolog 1 and Ki67 (60%) and negative for S100p, drosophila mothers against decapentaplegic protein 4, Thyroid Transcription Factor-1, NapsinA, Caudal type homeobox transcription factor 2, Cluster of Differentiation 20, PD-1 and PD-ligand 1, which suggested pancreatic origin.
The patient was diagnosed with cTxN + M1 (8th edition of the UICC-TNM classification) PC with liver, multiple bones, multiple lymph nodes and multiple muscle metastases, comorbid with pulmonary embolism, mild anemia, abnormal liver function, hydrothorax and hydropericardium.
The Gem-nabP regimen was initiated, which consisted of intravenous gemcitabine at 1 g/m2 on d 1 and 8 and nab-paclitaxel at 125 mg/m2 on day 1 and 8 of a 21-day cycle. After the first course, the patient presented with grade 4 leukopenia according to the Common Terminology Criteria for Adverse Events version 5.0. In the second course, the dose of the chemotherapeutic drugs was reduced by 25%. There was no recurrence of leukopenia and the biochemical tests did not reveal any side effects. Because of bone metastases, desuximab was administered subcutaneously at 120 mg/months. The pulmonary embolism was treated with rivaroxaban at 20 mg once daily.
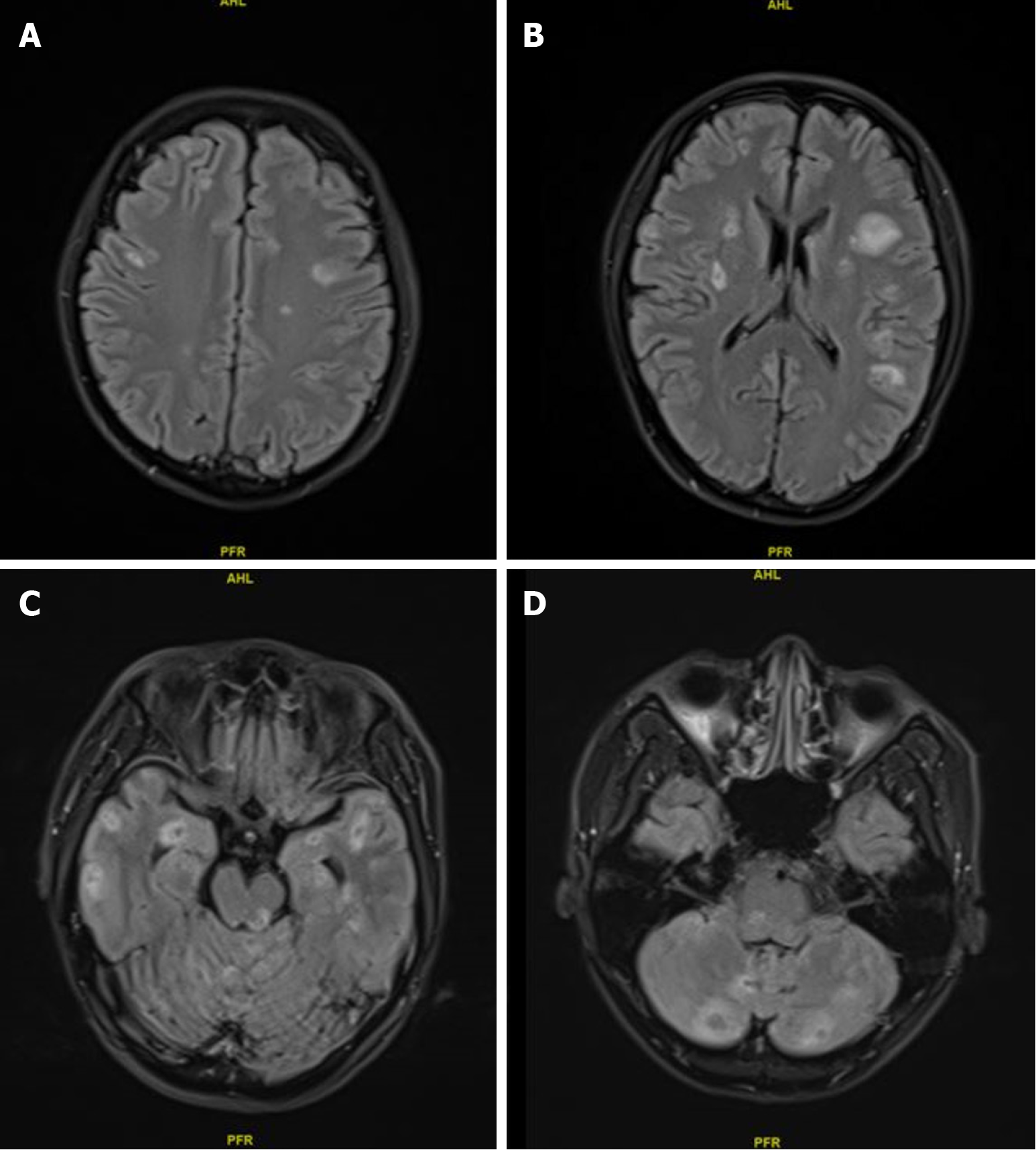
After two courses of chemotherapy, the patient’s general condition improved, and his transaminase levels returned to normal and CEA level decreased from 82.1 to 10.5 ng/mL, but CA19-9 remained > 10000 U/mL. Gem-nabP regimen was stopped after three cycles because of further disease progression, when a seizure occurred, and new multiple intracranial metastases (Figure 2), hydrothorax and ascites, peritoneal metastasis, and carcinomatous lymphangitis were identified on imaging, while the metastatic liver and lymph node lesions were reduced, as shown in Figure 3. Comparing Figure 1 and Figure 3, it can be observed that both the volume and the number of metastatic liver lesions were reduced. Thoracentesis was performed to drain the hydrothorax. Exfoliative cytology of the hydrothorax suggested an adenocarcinoma.
Genomic testing (AmoyDx Company@, Xiamen, China) revealed a BRAF V600E mutation in exon 15 with an abundance of 61.62% as class 1, Myc gene amplification with 55.93 copies as class 2, tumor protein 53 gene mutation in exon 7 as class 2, BRCA 1/2 wild type, and microsatellite-stable status by next-generation sequencing.
Targeted therapy was initiated with oral dabrafenib 150 mg twice daily and trametinib 2 mg once daily. Oral antiepileptic drugs were introduced, which consisted of levetiracetam (0.5 g) twice daily and sustained-release valproic acid (500 mg) twice daily, and no seizures occurred. Whole-brain palliative radiotherapy was performed using volumetric modulated arc therapy. The whole brain was used as the clinical target volume, and 3000 cGy/10 fractions was planned, during which mannitol dehydration was administered. After dabrafenib/trametinib combination treatment, the patient presented with grade 1 vomiting and fatigue. Routine blood and biochemical tests did not reveal any side effects. One and a half months after dabrafenib/trametinib combination treatment, CA19-9 level decreased from > 10000 to 9284 ng/mL and CEA from 10.5 to 3.4 ng/mL. Unfortunately, due to the patient's death, no images were obtained after the targeted therapy.
After 2 months of treatment, the patient died at home during his sleep. His family members refused an autopsy. The OS was 4 months.
PC is highly malignant and has limited treatment options. Combination chemotherapy regimens, such as Gem-nabP and FOLFIRINOX, are the standard first-line regimens for metastatic disease, with a median survival < 12 months. To tailor therapy effectively, it is essential to clarify the molecular subtypes and comprehend both intertumoral and intratumoral heterogeneity. In a retrospective analysis of The Know Your Tumor program, patients receiving molecularly matched therapy had a 6-mo extension in median PFS and a 1-year OS benefit compared with those who only received unmatched therapies[16]. These real-world outcomes suggest that the adoption of molecularly guided treatments can have a substantial effect on the survival of patients with PC. It is advised that all patients with locally advanced or metastatic disease undergo somatic molecular testing using tumor biopsies or cell-free DNA if a biopsy is not possible. Additionally, germline testing for hereditary genetic mutations is recommended for all patients who have recently been diagnosed with PC[18]. However, one real-world study showed a gap in the implementation of National Comprehensive Cancer Network (NCCN) guideline-directed genetic testing in PC patients, as only a third underwent testing, suggesting the need for systematic processes to facilitate testing[19].
PC is largely defined by core driver gene mutations. KRAS mutations are present in 90% of cases. KRAS wild-type status is found in 10% of PC cases and can be as high as 20% in younger patients. This status is associated with various targetable alterations, such as high microsatellite instability (MSI-high), high tumor mutational burden, BRAF mutations, ERBB2 amplification, and fusions involving NRG1, FGFR1-3, ALK, ROS, RET, and NTRK1-3[12]. KRASG12C inhibitors have demonstrated effectiveness in cancers with G12C mutations, and new pan-RAS and G12D inhibitors are currently undergoing clinical trials (NCT03785249, NCT04185883). Abnormalities in germline or somatic DNA damage repair are found in 5% to 10% of patients with PC and these patients are likely to respond positively to treatments involving DNA-damaging agents and maintenance therapy using poly-ADP ribose polymerase inhibitors such as olaparib[14]. Fewer than 1% of PC exhibit MSI-high status and are responsive to immune checkpoint inhibitors. While BRAF V600E mutations, as well as RET and NTRK fusions, are present in less than 1% of patients with KRAS wild-type PC, these alterations can be effectively treated using FDA-approved targeted therapies.
Accumulating data suggest that BRAF V600E-positive PC may benefit from a range of treatment strategies, including single-agent BRAF inhibitors, combined BRAF and MEK inhibitors, and MEK inhibitors combined with chemotherapy. A Phase 2 basket study evaluating vemurafenib in solid tumors harboring BRAF V600E mutations demonstrated that vemurafenib resulted in an approximately 20% reduction in the diameter of target lesions and disease stability for > 6 months in one of two patients with PC[20]. In another phase 2a basket study, four PC patients with BRAF alteration received vemurafenib monotherapy, but only one patient with a BRAF fusion achieved an objective response[21]. The specific BRAF variants in the other three patients and detailed outcome data have not been published. One study indicated that a patient with BRAF V600E-mutated PC exhibited a sustained response to treatment with dabrafenib and trametinib[22]. Furthermore, in a larger cohort, two patients with advanced BRAF V600E-mutated PC achieved prolonged partial responses following treatment with dabrafenib and trametinib[23]. Several additional case reports document clinical benefits from MAPK pathway inhibitors in BRAF V600E-driven PC[24-26]. Most of these case reports have noted disease sustained remission lasting > 6 months. Inn a comparable case report to ours, the patient exhibited a reduction in CA19-9 levels following 1 week of targeted therapy; however, the patient ultimately succumbed to intestinal perforation, which precluded the possibility of conducting an imaging assessment[27]. All these reports on BRAF alterations in PC strongly suggest the potential benefits of targeted therapy, but more data are needed to better define the extent of efficacy and the optimal treatment strategies[28]. Further preclinical investigations and collaborative, multicenter clinical trials are essential to enhance our understanding of this rare subtype of PC and to determine the most effective therapeutic strategies.
The patient in this case was only 33 years old and had a high tumor burden and rapid disease progression. Genetic testing suggested the presence of a BRAF V600E mutation. The disease progressed rapidly after the first-line Gem-nabP regimen despite an improvement in the patient’s general condition; the PFS of first-line treatment was only 1.8 months. The dabrafenib/trametinib combination was introduced as the second-line regimen; even though the CA19-9 level decreased, the patient died within 2 months, and the OS was 4 months. The patient’s treatment strategy was consistent with the NCCN guideline[29] recommendation of combination chemotherapy, such as Gem-nabP and FOLFIRINOX, as first-line regimens for PC with BRAF mutations, and BRAF/MEK inhibitors as second-line treatment when the disease progresses. However, combination chemotherapy and dabrafenib/trametinib did not result in long-term survival in our patient, possibly because of the high tumor burden and multiple intracranial metastases or the outgrowth of BRAF V600E-negative and/or -resistant clones. PC features a prominent stromal microenvironment with marked cellular and spatial heterogeneity that meaningfully affects disease biology and treatment resistance[30]. BRAF gene mutations lead to sustained cell proliferation and uncontrolled growth, and the specific isoform and tumor context influence sensitivity to targeted therapeutics[28]. At present, the mechanism of drug resistance in PC has not been fully elucidated, and more studies are needed to explore the mechanism of drug resistance. It is not known whether a better outcome will be achieved if dabrafenib/trametinib treatment is initiated as the first-line treatment. With the development of precision medicine and increasing medical evidence for targeted drugs, the use of targeted therapies in first-line therapy may be considered in the future.
This report describes a 33-year-old PC patient with a BRAF gene mutation and a high tumor burden. Combination chemotherapy and BRAF/MEK inhibitors were introduced as first-line and second-line treatment regimens, respectively; however, they did not result in long-term survival. This case study highlights the importance of identifying targetable genetic alterations in PC, specifically in young patients with high tumor burden.
| 1. | Hruban RH, Gaida MM, Thompson E, Hong SM, Noë M, Brosens LA, Jongepier M, Offerhaus GJA, Wood LD. Why is pancreatic cancer so deadly? The pathologist's view. J Pathol. 2019;248:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Motoi F, Unno M. Neoadjuvant treatment for resectable pancreatic adenocarcinoma: What is the best protocol? Ann Gastroenterol Surg. 2020;4:100-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (1)] |
| 4. | Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019;156:2024-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (1)] |
| 5. | Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP. Chemotherapy for pancreatic cancer. Presse Med. 2019;48:e159-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 6. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55666] [Article Influence: 7952.3] [Reference Citation Analysis (132)] |
| 7. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 668] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 8. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5609] [Article Influence: 400.6] [Reference Citation Analysis (1)] |
| 9. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4857] [Article Influence: 404.8] [Reference Citation Analysis (0)] |
| 10. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 824] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 11. | Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, Lowery MA, Diaz LA Jr, Mandelker D, Yu KH, Zervoudakis A, Kelsen DP, Iacobuzio-Donahue CA, Klimstra DS, Saltz LB, Sahin IH, O'Reilly EM. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin Cancer Res. 2018;24:1326-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 12. | Singhi AD, George B, Greenbowe JR, Chung J, Suh J, Maitra A, Klempner SJ, Hendifar A, Milind JM, Golan T, Brand RE, Zureikat AH, Roy S, Schrock AB, Miller VA, Ross JS, Ali SM, Bahary N. Real-Time Targeted Genome Profile Analysis of Pancreatic Ductal Adenocarcinomas Identifies Genetic Alterations That Might Be Targeted With Existing Drugs or Used as Biomarkers. Gastroenterology. 2019;156:2242-2253.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 13. | Gupta M, Sherrow C, Krone ME, Blais EM, Pishvaian MJ, Petricoin EF, Matrisian LM, DeArbeloa P, Gregory G, Brown A, Zalewski O, Prinzing G, Roche C, Kanehira K, Mukherjee S, Iyer R, Fountzilas C. Targeting the NTRK Fusion Gene in Pancreatic Acinar Cell Carcinoma: A Case Report and Review of the Literature. J Natl Compr Canc Netw. 2021;19:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1614] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 15. | Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, Choti MA, Yeo CJ, McCue P, White MA, Knudsen ES. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 16. | Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 17. | Salama AKS, Li S, Macrae ER, Park JI, Mitchell EP, Zwiebel JA, Chen HX, Gray RJ, McShane LM, Rubinstein LV, Patton D, Williams PM, Hamilton SR, Armstrong DK, Conley BA, Arteaga CL, Harris LN, O'Dwyer PJ, Chen AP, Flaherty KT. Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J Clin Oncol. 2020;38:3895-3904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 18. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 680] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 19. | Ghosh AK, Bhushan S, Abidoye O, Rynarzewska AI, Robinson S, Sampat D. Evaluating Implementation of NCCN Guideline-Directed Genetic Screening Recommendations for Patients with Pancreatic Ductal Adenocarcinoma. 2023 preprint. [DOI] [Full Text] |
| 20. | Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1220] [Cited by in RCA: 1375] [Article Influence: 137.5] [Reference Citation Analysis (0)] |
| 21. | Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, Burris H, Bose R, Yoo B, Stein A, Beattie M, Kurzrock R. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol. 2018;36:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 22. | Guan M, Bender RJ, Pishvaian MJ, Halverson DC, Tuli R, Klempner SJ, Wainberg ZA, Singhi AD, Petricoin E, Hendifar AE. Molecular and clinical characterization of BRAF mutations in pancreatic ductal adenocarcinomas (PDACs). J Clin Oncol. 2018;36:214-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Hendifar A, Blais EM, Wolpin B, Subbiah V, Collisson E, Singh I, Cannon T, Shaw K, Petricoin EF 3rd, Klempner S, Lyons E, Wang-Gillam A, Pishvaian MJ, O'Reilly EM. Retrospective Case Series Analysis of RAF Family Alterations in Pancreatic Cancer: Real-World Outcomes From Targeted and Standard Therapies. JCO Precis Oncol. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Ardalan B, Azqueta JI, England J, Eatz TA. Potential benefit of treatment with MEK inhibitors and chemotherapy in BRAF-mutated KRAS wild-type pancreatic ductal adenocarcinoma patients: a case report. Cold Spring Harb Mol Case Stud. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Cramer S, Marcus MA, Ramkissoon S, Szabo S, Pressey JG. Pediatric BRAF (V600E)-Mutated Pancreatic Acinar Cell Carcinoma With Complete and Durable Response to Dabrafenib and Trametinib. JCO Precis Oncol. 2020;4:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Sasankan S, Rebuck L, Darrah G, Harari Turquie M, Rabinowitz I. Metastatic Pancreatic Cancer with BRAF and P53 Mutations: Case Report of Therapeutic Response to Doublet Targeted Therapy. Case Rep Oncol. 2020;13:1239-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Grinshpun A, Zarbiv Y, Roszik J, Subbiah V, Hubert A. Beyond KRAS: Practical Molecular Targets in Pancreatic Adenocarcinoma. Case Rep Oncol. 2019;12:7-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Ciner AT, Jiang Y, Hausner P. BRAF-Driven Pancreatic Cancer: Prevalence, Molecular Features, and Therapeutic Opportunities. Mol Cancer Res. 2023;21:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 29. | Pajewska M, Partyka O, Czerw A, Deptała A, Cipora E, Gąska I, Wojtaszek M, Sygit K, Sygit M, Krzych-Fałta E, Schneider-Matyka D, Cybulska AM, Grochans E, Asendrych-Woźniak A, Romanowicz A, Drobnik J, Bandurska E, Ciećko W, Maciuszek-Bartkowska B, Curyło M, Wróbel K, Kozłowski R, Marczak M. Management of Metastatic Pancreatic Cancer-Comparison of Global Guidelines over the Last 5 Years. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Sherman MH, Beatty GL. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu Rev Pathol. 2023;18:123-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 200] [Article Influence: 100.0] [Reference Citation Analysis (0)] |