Published online Jun 6, 2025. doi: 10.12998/wjcc.v13.i16.100672
Revised: December 11, 2024
Accepted: January 17, 2025
Published online: June 6, 2025
Processing time: 171 Days and 16.9 Hours
Cryptococcus is a systemic opportunistic pathogenic fungus that can cause infe
Three male pediatric patients in good health were hospitalized because of chest pain without cough or fever. Chest computed tomography (CT) revealed pleural-based nodules and consolidation with cavitation. A lung biopsy was performed in one case, and Cryptococcus was cultured from the pathological tissues. Cryptococcus was detected in the alveolar lavage fluid, and serum Cryptococcus capsular
PC can also occur in immunocompetent children. When encountering children with chest pain only in the clinic, one should be vigilant about PC, promptly complete the relevant examinations, and avoid misdiagnosis.
Core Tip: Pulmonary cryptococcosis is a relatively rare pediatric pulmonary fungal infection. It usually occurs in immunocompromised children but may also occur in immunocompetent children. It can be complicated by central nervous system cryptococcosis or can occur alone. Pulmonary cryptococcal infection has no specific clinical features, and its diagnosis is difficult. If we do not pay sufficient attention to it, it can be easily missed or misdiagnosed, which can be life-threatening. Early diagnosis and timely initiation of antifungal therapy can improve disease prognosis.
- Citation: Li XN, Chen JH, Lu ZW. Pulmonary cryptococcosis in immunocompetent children presenting with chest pain: Three case reports. World J Clin Cases 2025; 13(16): 100672
- URL: https://www.wjgnet.com/2307-8960/full/v13/i16/100672.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i16.100672
Cryptococcus is an environmental saprophytic fungus with pigeons as its natural host and pigeon-dropping-contaminated soil as its main transmission medium. Other domestic animals and pets, such as cats and dogs, can also carry Cryptococcus spp. Pulmonary cryptococcosis (PC) is a fungal lung infection caused by the inhalation of cryptococcal spores in the air, which is common in immunocompromised individuals, especially those with human immunodeficiency virus (HIV) infection. Approximately 1 million HIV-related cryptococcal disease cases occur worldwide annually[1]. A Chinese study found that 60% of PC cases were diagnosed in immunocompetent non-HIV patients without obvious respiratory symptoms and signs[2]. A 17-year retrospective analysis conducted in Thailand found that the overall mortality rate of non-HIV PC patients was 27%[3]. A retrospective study from Beijing Children's Hospital showed that 96.2% of patients with cryptococcal infection without underlying diseases invaded the lungs, followed by the central nervous system (69.2%), with an overall mortality rate of 11.5%[4]. Currently, PC diagnosis poses significant challenges. Infections can be confirmed using various methods including culture, direct microscopy, histopathological examination, serological testing, and molecular detection. The primary treatments for PC are infection control and prevention of dissemination. Treatment strategies are tailored according to the patient's immune status and disease severity. Only few cases of PC with chest pain have been reported in immunocompetent patients. To raise clinicians' awareness of such patients at an early stage and reduce the mortality rate, this report describes three cases of children with PC who primarily presented with chest pain.
Case 1: A 15-year-old boy with a two-day history of chest pain admitted to our hospital.
Case 2: A 13-year-old boy with a one-day history of chest pain admitted to our hospital.
Case 3: A 6-year-old boy with a seven-day history of chest pain admitted to our hospital.
Case 1: The child experienced unprovoked chest pain for two days, without cough, dyspnea, fever, or profuse sweating. The child was admitted to the emergency department, and a chest computed tomography (CT) scan suggested the possibility of fungal infection.
Case 2: The child presented with non-provoked chest pain for one day, without cough, dyspnea, or fever. The child was admitted to the emergency department, and a chest CT scan revealed multiple nodules at the pleural base, with consolidation and visible cavitation.
Case 3: The child experienced chest pain without non-provoked for seven days, without cough, dyspnea, or fever for 1 day during the course of the disease.
Cases 1-3: All the patients were healthy and had no history of recurrent respiratory tract infections.
All family members were healthy. No pigeons were kept as pets at home, and the children had no prior contact with pigeons or other pet animals.
Cases 1-3: Physical examination was unremarkable.
Case 1: The bronchoalveolar lavage fluid (BALF) culture was positive for Cryptococcus neoformans (C. neoformans) and Cryptococcus capsular (C. capsular) polysaccharide antigens.
Case 2: The patient’s BALF culture and serological fungal antigen test results were negative, and the lung biopsy pathological tissue culture showed the presence of C. neoformans.
Case 3: The child tested positive for C. capsular polysaccharide antigen.
Cases 1-3: All children had normal humoral immunity [immunoglobulin (Ig) A, IgM, IgG, and IgE levels] and lymphocyte subset levels, and their HIV antibody test results were negative. All the children underwent tracheoscopy and BALF testing. BALF was sent for next-generation high-throughput sequencing and the etiology was negative. Acid-fast staining and bacterial culture of BALF were negative in all children. Two cases of cerebrospinal fluid examination were negative for ink staining and C. capsular polysaccharide antigen test.
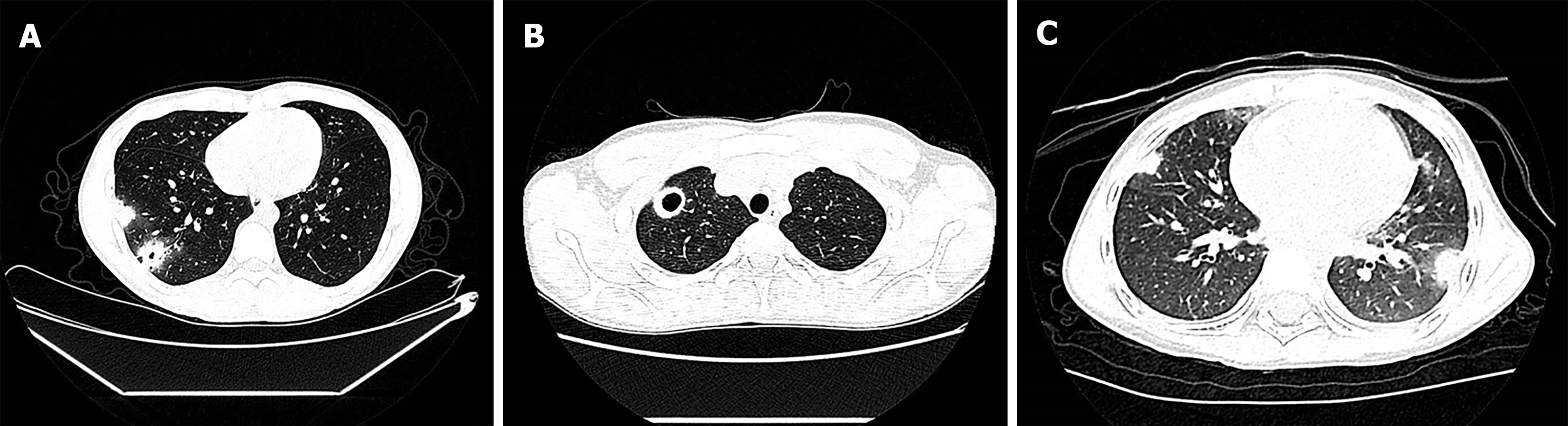
Case 1: Chest CT scan revealed blurred-boundary patchy high-density shadows and nodules in the lower lobe of the right lung, multiple cavities in the lesions, and halo signs (Figure 1A).
Case 2: Chest CT scan revealed multiple patchy high-density shadows and nodules close to the pleura with surrounding ground-glass shadows, partial liquefaction necrosis, and cavity formation (Figure 1B).
Case 3: Chest CT scan revealed multiple nodules and a clumpy solid shadow in both lungs of the child, distributed in the outer band close to the pleura, and a halo sign (Figure 1C).
The diagnosis of PC was confirmed based on the clinical manifestations, imaging, pathology, and etiology findings.
All three children were treated with fluconazole.
The child showed significant resolution of the lung lesion after 2 months of oral administration. On continuation of the oral administration for 6 months, only a small amount of ground-glass shadow persisted in the lungs. The medication was discontinued for one year, followed by a repeat lung CT scanning. The lesion remained largely unchanged, and the child remained asymptomatic.
The child showed complete resolution of the lesion on lung CT scan 6 months after oral administration.
The child showed significantly improved resolution of the lung lesion after 2 months of oral fluconazole dose and was transferred to another hospital for further treatment. A six-month follow-up phone call revealed that the lung lesion had resolved completely.
During the treatment process, all children showed good drug tolerance, and no obvious adverse reactions. All three patients were cured, with disappearance of symptoms and signs, and resolution of lesions, as observed on lung CT scans. All patients were followed-up for > 12 months and they showed no signs of recurrence.
In recent years, the incidence of PC and the number of non-HIV-infected patients have increased rapidly. The proportion of patients with normal immune function has also increased gradually[5]. A recent multicenter prospective study in China[6] found that 70.24% (321/457) of HIV-negative hosts with PC had no underlying diseases, and 87.75% (401/457) of the patients had no known immunodeficiency. Owing to the high rate of misdiagnosis and missed diagnoses, clinicians should pay attention to this disease. In addition, for healthy children with no obvious respiratory symptoms or lung involvement, PC should be suspected, and efforts should be made to avoid delays in diagnosis or excessive testing. Immune status can vary, presentation can be asymptomatic, and infection can be unexpectedly detected during routine examination or follow-up for other diseases. Studies have shown that in 67% of patients with normal immune function, PC disseminates to the central nervous system, leading to cryptococcal meningitis, increasing the difficulty of treatment and prognosis[7].
The clinical manifestations of PC are atypical, ranging from no clinical symptoms to acute respiratory distress syndrome, and mainly depend on the patient's immune status. In patients with PC and normal immune function, the clinical manifestations of Cryptococcus infection are relatively mild and mainly feature cough, expectoration, and low fever. Such patients may have no distinct clinical symptoms, and most of their lesions are confined to the lungs. These patients are prone to misdiagnoses and missed diagnoses. A few patients fail to receive timely treatment, resulting in infection dissemination[8] and acute respiratory failure[9]. However, in PC patients with compromised immune function, Cryptococcus spreads more rapidly within the lungs, and these patients present with more severe clinical symptoms, such as shortness of breath, hypoxemia, and even respiratory failure. The lesions are prone to dissemination, and the mortality rate is high. All three patients reported in this study presented with chest pain as the main symptom, without fever or cough. Such cases are rare in clinical practice. Lesions close to the periphery of the lung may cause pleurisy and pain due to nerve pulling. In addition, acute chest pain that occurs without respiratory symptoms requires complete electrocardiography and echocardiography to exclude the possibility of heart disease. Pain related to breathing is attributed to pleurisy or intercostal neuritis. Therefore, in clinical practice, lung imaging should be performed to rule out the possibility of lung lesions in patients with normal immune function and chest pain without obvious respiratory symptoms.
PC imaging is polymorphic and variable, such as multiple or solitary nodules, cavities, and consolidation, which can manifest as limited single or multiple nodules or as cavities and masses with a halo sign and can progress into consolidation shadows, often misdiagnosed as malignant lung tumors. Multiple Chinese articles have reported that the chest CT findings of adult patients with PC are mainly single or multiple nodules/masses that are easily misdiagnosed[10-12]. A retrospective study in China showed that PC imaging findings in children included hilar or mediastinal lymph node lesions (47%, 22/47) and nodules (45%, 21/47), including scattered nodules (57%, 12/21) or miliary nodules (43%, 9/25), mainly in the subpleural area[4]. Similar to the three pediatric cases reported in this study, lesions can coexist with nodules, cavities, and consolidation and occur at the pleural site. One of the three patients had multiple subpleural nodules, and the etiological examination was negative; the chest CT was re-examined one week after ceftriaxone infection, and no change was observed in the lesions. Further, a surgical thoracoscopic lung biopsy was performed, and histopathology revealed granulomatous inflammation and tissue culture was positive for Cryptococcus, confirming pulmonary cryptococcal disease.
The "gold standard" for PC diagnosis is the identification of Cryptococcus in sterile site specimens through pathogen smears, cultures, and histopathological examination. Cryptococcus is a non-normal respiratory bacterium. A positive culture or smear from the respiratory BALF is an important clinical reference for the diagnosis of PC. Compared to culture and pathological examination, the detection of the C. capsular polysaccharide antigen is rapid, simple, sensitive, and specific, which is helpful for early diagnosis, can be used to evaluate efficacy and prognosis, and has high clinical application value[13]. One study showed that the overall sensitivity and specificity of pulmonary cryptococcal serological detection technology for the diagnosis of cryptococcal infections were 97.6% and 98.1%[14]. In this study, two cases were confirmed using lung pathology and BALF culture, and the third case was confirmed using serum a immunology test, which provided an important etiological basis for diagnosis. Therefore, capsular antigen detection can assist in rapid clinical diagnosis. In recent years, metagenomic next-generation sequencing (mNGS) has been widely used to improve the detection rate of Cryptococcus and has several clinical advantages[15]. The three children we reported also underwent mNGS of BALF, but no pulmonary Cryptococcus was found, which may be due to the large genome of the Cryptococcus genus and its thick cell wall, leading to low efficiency of nucleic acid extraction. Even after the initial cell wall rupture treatment, the number of specific sequences detected was relatively small compared to that of other microorganisms. The sensitivity of mNGS for the diagnosis of cryptococcosis needs to be improved, and further exploration and research are needed.
The fundamental purpose of PC treatment is to control the infection status and prevent its spread. The treatment plan depends on the patient's immune status and the severity of the condition. The Infectious Diseases Society of America recommends that for mild to moderate non-immunosuppressed patients, oral fluconazole (400 mg/day) treatment be administered for 6 months to 12 months; severe cases are treated in the same manner as those of central nervous system infections[1]. The overall prognosis of PC was good, and the three children in this study showed significant improvement and resolution of lesions after fluconazole treatment, as shown by chest CT scan.
In conclusion, PC can develop in children with normal immune function. The clinical symptoms of PC lack specificity, and diagnosis is difficult. If we do not pay sufficient attention, it can easily disseminate systemically and endanger life. Early diagnosis and timely treatment can improve prognosis.
We thank the patient’s family members for providing detailed treatment information.
| 1. | Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50:291-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1988] [Cited by in RCA: 1791] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 2. | Liu K, Ding H, Xu B, You R, Xing Z, Chen J, Lin Q, Qu J. Clinical analysis of non-AIDS patients pathologically diagnosed with pulmonary cryptococcosis. J Thorac Dis. 2016;8:2813-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, Sungkanuparph S. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis. 2006;10:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Gao LW, Jiao AX, Wu XR, Zhao SY, Ma Y, Liu G, Yin J, Xu BP, Shen KL. Clinical characteristics of disseminated cryptococcosis in previously healthy children in China. BMC Infect Dis. 2017;17:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: A review of pathobiology and clinical aspects. Med Mycol. 2019;57:133-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Chen LA, She DY, Liang ZX, Liang LL, Chen RC, Ye F, Li YP, Zhou Y, Chen XH, Fang SF, Lai GX, Hu Q, Xie BS, Yao XJ, Shi Y, Su X, He LX, Zhou JY, Zhong SC, Zhang QL, Xiong SD, Qu JM, Tong ZH, Jiang SJ, Liu J, Xu F, He B, Li ER, Yuan YD, Zhang XY, Sun TY, Liu YN. [A prospective multi-center clinical investigation of HIV-negative pulmonary cryptococcosis in China]. Zhonghua Jie He He Hu Xi Za Zhi. 2021;44:14-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Suwatanapongched T, Sangsatra W, Boonsarngsuk V, Watcharananan SP, Incharoen P. Clinical and radiologic manifestations of pulmonary cryptococcosis in immunocompetent patients and their outcomes after treatment. Diagn Interv Radiol. 2013;19:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Li Y, Fang W, Jiang W, Hagen F, Liu J, Zhang L, Hong N, Zhu Y, Xu X, Lei X, Deng D, Xu J, Liao W, Boekhout T, Chen M, Pan W. Cryptococcosis in patients with diabetes mellitus II in mainland China: 1993-2015. Mycoses. 2017;60:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Vilchez RA, Linden P, Lacomis J, Costello P, Fung J, Kusne S. Acute respiratory failure associated with pulmonary cryptococcosis in non-aids patients. Chest. 2001;119:1865-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Mo Z, Li C, Liang Z, Cui J, Yu L, Chen L. Clinical Analysis of Non-AIDS Patients with Pulmonary Cryptococcosis and the Change in Their Clinical Features over 30 Years in a Tertiary Hospital in Beijing, China. Jpn J Infect Dis. 2022;75:476-483. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Wang T, Liu M, Zhang F. Clinical Diagnosis, Treatment, and Laboratory Detection of 50 Cases of Pulmonary Cryptococcosis. Comput Math Methods Med. 2022;2022:7981472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Deng H, Zhang J, Li J, Wang D, Pan L, Xue X. Clinical features and radiological characteristics of pulmonary cryptococcosis. J Int Med Res. 2018;46:2687-2695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Min J, Huang K, Shi C, Li L, Li F, Zhu T, Deng H. Pulmonary Cryptococcosis: comparison of Cryptococcal antigen detection and radiography in Immunocompetent and Immunocompromised patients. BMC Infect Dis. 2020;20:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Huang HR, Fan LC, Rajbanshi B, Xu JF. Evaluation of a new cryptococcal antigen lateral flow immunoassay in serum, cerebrospinal fluid and urine for the diagnosis of cryptococcosis: a meta-analysis and systematic review. PLoS One. 2015;10:e0127117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Dai Q, Wang Y, Ying Q, Ye Q. Cryptococcosis with pulmonary cavitation in an immunocompetent child: a case report and literature review. BMC Infect Dis. 2024;24:162. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |