Published online May 26, 2025. doi: 10.12998/wjcc.v13.i15.99212
Revised: December 20, 2024
Accepted: January 7, 2025
Published online: May 26, 2025
Processing time: 188 Days and 5.7 Hours
Brush cytology is the most commonly used technique for tissue acquisition during endoscopic retrograde cholangiopancreatography for the evaluation of biliary strictures. Nonetheless, brush cytology is limited by its low sensitivity due to insufficient cellular yield.
To evaluate the impact of the sheath-rinse technique on improving the cellularity yield.
A total of 112 patients with suspected malignant biliary strictures were enrolled at two tertiary centers in South Korea. The sample cellularity and diagnostic accuracy of brush-wash and sheath-rinse specimens were compared.
A significantly increased number of total cell clusters per representative 20 × field was recorded in the sheath-rinse compared with the brush-wash specimens (median: 12 vs 3, P < 0.001). This trend persisted when large (> 50 cells) clusters (median: 8 vs 3, P < 0.001), medium (6-49 cells) (median: 7 vs 3, P < 0.001), and small (2-5 cells) clusters (median: 9 vs 3, P < 0.001) were evaluated. Diagnostic accuracy and sensitivity for differentiating malignancy were superior with sheath-rinsing than with the brush-wash method (72.3% vs 62.5%, P < 0.001 and 69.9% vs 59.2%, P < 0.001, respectively).
Incorporating sheath-rinse specimens significantly improved the yield and diagnostic accuracy of biliary brush cytology. Sheath-rinsing should be integrated into routine clinical practice to improve diagnostic performance for biliary strictures.
Core Tip: Brush cytology is commonly used to collect tissue samples during endoscopic retrograde cholangiopancreatography for the evaluation of biliary strictures, but the diagnostic sensitivity of the method is limited because of an insufficient cellular yield. We evaluated whether sheath rinsing affects cellular yield. Our findings showed that the yield and diagnostic accuracy of biliary brush cytology improved significantly when sheath-rinsed specimens were assessed. This technique should be included in routine clinical practice to improve diagnostic performance for biliary strictures.
- Citation: So H, Jang SI, Ko SW, Yoon SB, Lee YS, Bang S, Kim M, Choi HJ. Effect of brush rinse on the diagnostic accuracy of biliary stricture evaluation: A multicenter trial. World J Clin Cases 2025; 13(15): 99212
- URL: https://www.wjgnet.com/2307-8960/full/v13/i15/99212.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i15.99212
Diagnosing and managing biliary strictures and malignancies pose a considerable clinical challenge. Despite advances in imaging techniques, certain biliary lesions remain elusive or indeterminate, thus requiring direct cellular examination for a definitive diagnosis[1]. Biliary brush cytology is the most commonly used technique for tissue acquisition during endoscopic retrograde cholangiopancreatography (ERCP)[2] and is considered a reliable initial sampling method owing to its high specificity, simplicity, safety, and cost-effectiveness[3]. Nevertheless, brush cytology has limitations, such as a low sensitivity and increased negative predictive value (NPV), because of low specimen cellularity[4]. A systematic review and meta-analysis reported that the pooled sensitivity of brush cytology for cholangiocarcinoma was 56.0% (95%CI: 48.8-63.1)[5]. Intraductal sampling with biopsy forceps is another option for tissue sampling; however, this method is less commonly used because of the difficulty in advancing the forceps in various biliary lesions, especially in the intrahepatic duct[6]. Single-operator cholangioscopy (SOC) enables visually directed biopsy and has superior diagnostic accuracy compared to conventional ERCP-guided biopsy[7]. Nonetheless, its high cost and lack of widespread availability limit their use in routine clinical practice[6].
In this evolving landscape, improving the diagnostic yield of more accessible techniques, such as biliary brush cytology, remains a priority. A recent study suggested that incorporating brush-rinse techniques improves diagnostic accuracy by increasing the cellular yield, and reported that the addition of a sheath rinse to the standard brushing procedure increased the overall specimen cellularity[8]. However, the study included a limited number of patients (n = 13). Therefore, the current multicenter prospective study aimed to validate the effect of brush-rinsing on diagnostic accuracy for biliary strictures in a sufficient number of patients.
This study involved prospective data collection and a retrospective cohort review at two tertiary referral centers (Ulsan University Hospital and Eunpyeong St. Mary’s Hospital) in South Korea from September 2022 to October 2023. Patients aged ≥ 18 years with suspected malignant biliary strictures on computed tomography (CT) and/or magnetic resonance imaging (MRI) that required histological confirmation through ERCP were included in the study. Patients were deemed ineligible for enrollment if they met any of the following exclusion criteria: (1) Coagulopathy characterized by an international normalized ratio greater than 1.5; (2) A platelet count of < 50000/mm3; (3) Current use of anticoagulants that could not be temporarily discontinued; (4) Cardiopulmonary disease that could render sedation unsafe; (5) Anatomic changes from previous surgery or gastric outlet obstruction; (6) Inability or unwillingness to provide informed consent; and (7) Ongoing pregnancy. The study protocol was approved by the Institutional Review Board of each center, and written informed consent was obtained from all enrolled patients.
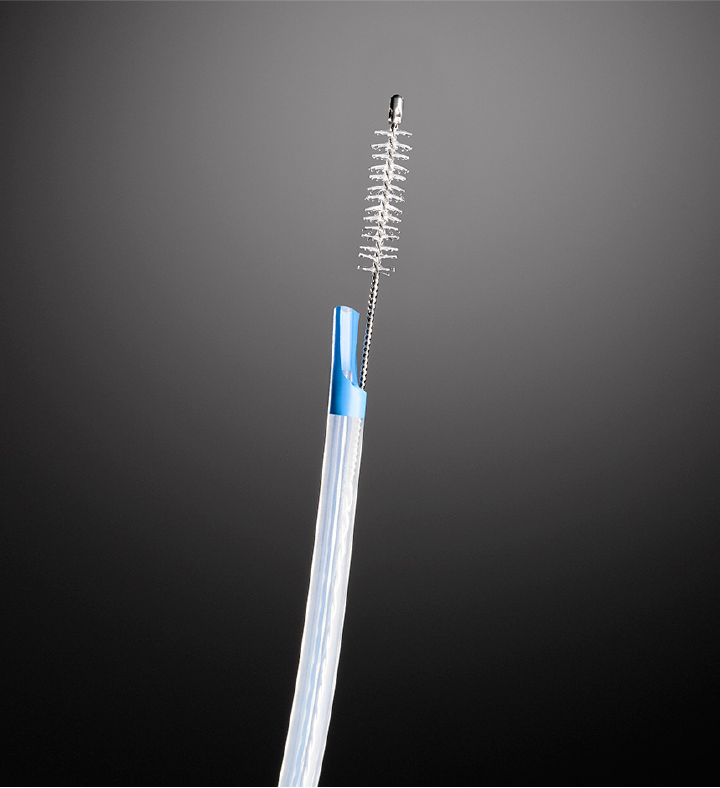
All procedures were performed by three experienced therapeutic endoscopists from each center (So H, Ko SW, and Yoon SB). After selective biliary cannulation, a contrast medium was injected into the stricture site through a catheter. The location and length of the strictures were determined using fluoroscopy. A 0.025-inch guidewire (VisiGlide; Olympus Medical, Tokyo, Japan or Jagwire; Boston Scientific, Natick, MA, United States) was placed over the ductal stricture area. Following the removal of the catheter, a cytology brush (RX Cytology Brush; Boston Scientific, Natick, MA, United States) with an 8-Fr-diameter sheath and a 2.1-mm-diameter brush integrating a two-part lumen (one for the guidewire and the other for the brush) (Figure 1) was inserted along the guidewire. Once the sheath was advanced to the distal part of the stricture, the brush was pushed out of the sheath and withdrawn through the stricture with 10 in-and-out movements under fluoroscopic guidance. Subsequently, the brush was retracted into the sheath and simultaneously withdrawn from the duodenoscope, along with the sheath catheter, leaving the guidewire across the stricture.
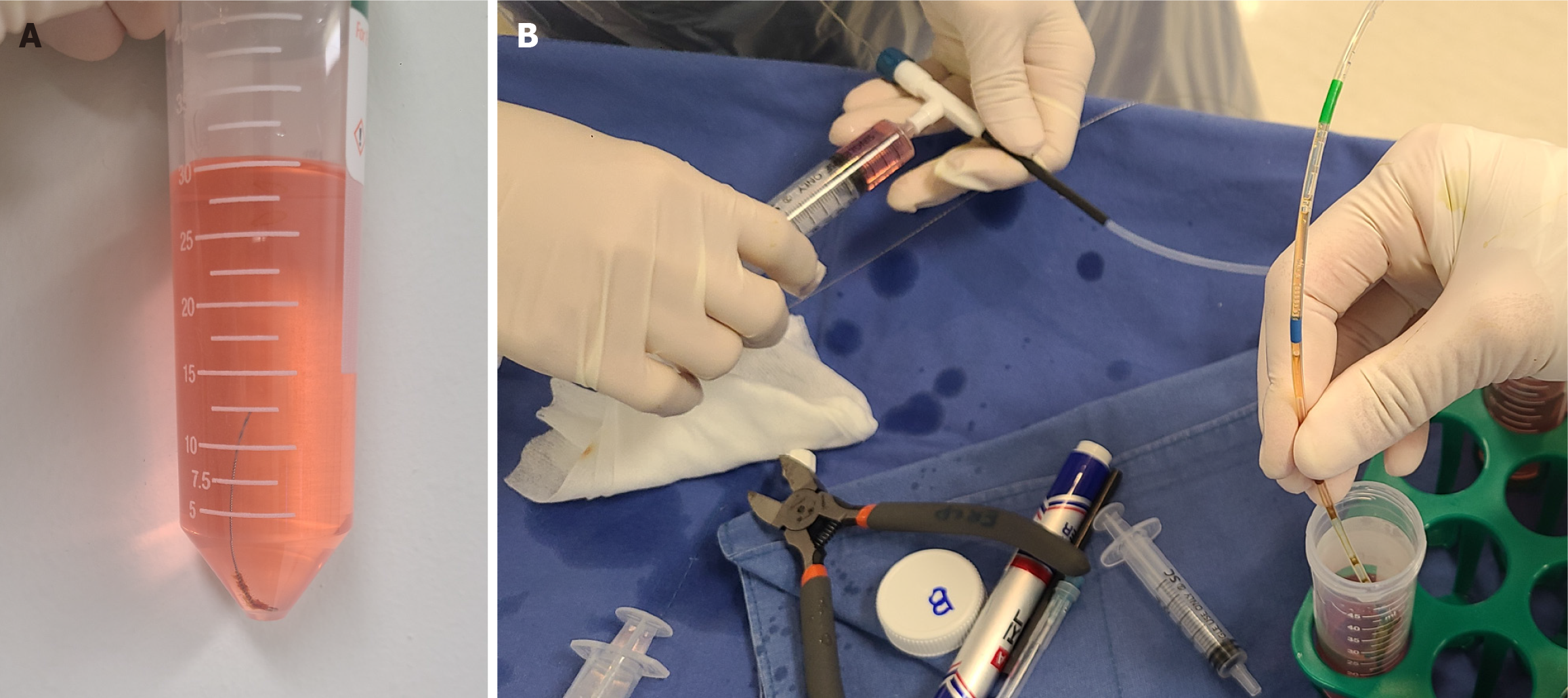
For all patients, brush-wash and sheath-rinse specimens were prepared in pairs (Figure 2). For the brush-wash, SurePath liquid-based cytology (LBC) specimens were prepared as follows: After retrieving the brush, it was cut and placed in CytoRich Red solution (Becton Dickinson, Franklin Lakes, NJ, United States) for processing. The sample was immediately delivered to the pathology laboratory and centrifuged at 3000 r/m for 1 minute. Following the removal of the brush, a second centrifugation was performed to isolate the cell pellet. Subsequently, the collected pellet was smeared onto a glass slide, fixed with 95% ethanol, and stained with the papanicolaou stain.
For the sheath-rinse, SurePath LBC specimens were prepared as follows: (1) The contents in the sheath were rinsed in a separate container using a 10-cc syringe filled with CytoRich Red solution (Becton Dickinson); (2) The specimen was centrifuged at 3000 r/m for 1 minute to isolate the cell pellet, which was then smeared onto a glass slide; and (3) The subsequent steps for the sheath-rinse samples were the same as those for the brush-wash samples.
The primary outcome measure was a comparison of cellular yield between the brush-wash and sheath-rinse specimens. Cellular yield was determined by counting cell clusters as follows: (1) First, each slide was evaluated to identify a representative microscopic field of 20 ×, which corresponded to approximately 4% of the total area of the slide; (2) Upon selection of this field, a detailed evaluation was performed to quantify epithelial cellularity by categorizing the cell clusters into large (> 50 cells), medium (6-49 cells), small (2-5 cells), or single-cell clusters[4]; (3) A subgroup analysis of cellular yield was conducted according to stricture location (proximal vs distal); and (4) The hepatic hilum and intrahepatic bile duct were defined as the proximal bile ducts, whereas the common hepatic and bile ducts were defined as the distal bile ducts.
The secondary outcome measure was the diagnostic accuracy of the specimens for detecting malignancy. A gold standard diagnosis of malignancy was established based on clear histological evidence of malignancy in specimens obtained through ERCP or surgical resection, the appearance of metastatic lesions, or a decline in clinical status at follow-up. Lesions were classified as benign if malignancy was absent in the surgical pathology reports or if the patients exhibited continued well-being and showed no evidence of radiological progression, as determined in CT and/or MRI, for at least 6 months after the initial procedure. According to current standards, cytopathologic diagnoses of “malignant” or “suspicious for malignancy” were considered positive, whereas “atypical” and “negative for malignancy” were considered negative[9]. Diagnostic accuracy was determined by calculating the ratio of true positives and true negatives to the total number of cases evaluated. The cytological specimens were evaluated by board-certified cytopathologists (Lee YS and Choi HJ) at each center, who were blinded to the sample source.
Normally distributed continuous variables are presented as means ± SD and were analyzed using Student’s t-test. Non-normally distributed continuous variables are expressed as medians and interquartile range (IQR) and were analyzed using the Wilcoxon rank-sum test. Categorical variables are reported as counts and percentages and were compared using the χ² test or Fisher’s exact test, as appropriate. Comparisons between pairs of samples from the same patients were performed using McNemar’s test. Statistical analyses were performed using R software version 4.3.0 as part of RStudio, with statistical significance set at P < 0.05.
A total of 112 patients with biliary strictures were enrolled at two tertiary centers in South Korea from September 2022 to October 2023. Table 1 summarizes the baseline characteristics of the participants. The mean age of the cohort was 71.2 years ± 9.6 years, with men accounting for 58.9% (n = 66) of all patients. The locations of biliary brushing were the common bile duct in 66 (58.9%) patients, common hepatic duct in 8 (7.1%) patients, hepatic hilum in 36 (32.1%) patients, and the intrahepatic bile duct in 2 (1.9%) patients.
| Demographic factors | N = 112 |
| Age (years) | 71.2 ± 9.6 |
| Male sex | 66 (58.9) |
| Baseline bilirubin level (mg/dL) | 6.8 (2.1-13.4) |
| Baseline cancer antigen 19-9 level (IU/L) | 220 (37-628.5) |
| Location of brushing | |
| Common bile duct | 66 (58.9) |
| Common hepatic duct | 8 (7.1) |
| Hepatic hilum | 36 (32.1) |
| Intrahepatic duct | 2 (1.9) |
| Diagnosis | |
| Malignancy | |
| Pancreatic adenocarcinoma | 32 (28.6) |
| Hilar cholangiocarcinoma | 32 (28.6) |
| Distal cholangiocarcinoma | 29 (25.9) |
| Gallbladder adenocarcinoma | 7 (6.3) |
| Intrahepatic cholangiocarcinoma | 2 (1.8) |
| Hepatocellular carcinoma | 1 (0.9) |
| Benign | |
| Benign common bile duct stricture | 8 (7.1) |
| Immunoglobulin G 4-related sclerosing cholangitis | 1 (0.9) |
In the entire cohort, 103 (92.0%) patients and 9 (8%) patients had malignant and benign strictures, respectively. Among the 103 patients with malignancy, the most common diagnoses were pancreatic cancer (n = 32, 28.6%) and hilar cholangiocarcinoma (n = 32, 28.6%), followed by distal cholangiocarcinoma (n = 29, 25.9%), gallbladder cancer (n = 7, 6.3%), and other malignancies (n = 3, 2.7%). Of the nine patients with benign diagnoses, eight had benign biliary strictures, whereas one had immunoglobulin G 4-related cholangitis. None of the patients developed any adverse events associated with cytological brushing.
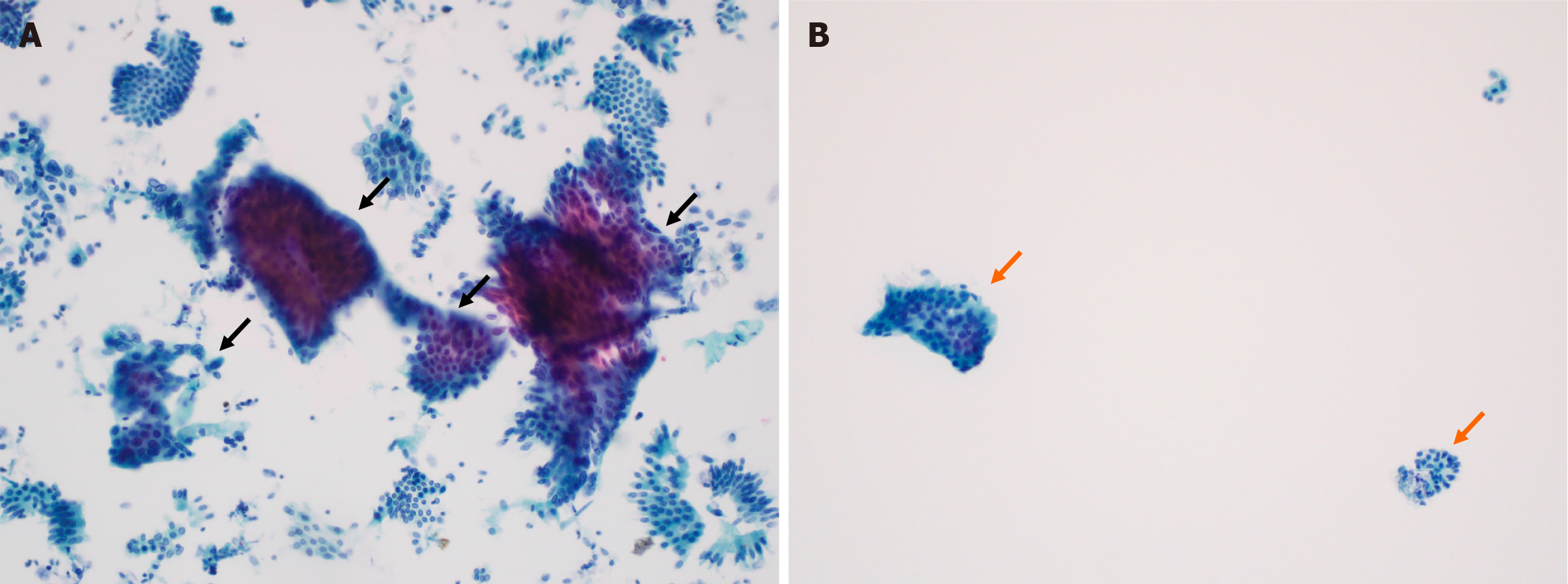
The cellular yield of the brush-wash vs sheath-rinse specimens was evaluated based on the median number of cell clusters per 20 × microscopic fields. The median number of total cell clusters was significantly higher in sheath-rinse specimens than in brush-wash specimens [median (IQR): 12 (4-21) vs 3 (1-6), P < 0.001] (Figure 3). This pattern was consistent across specimens with different-sized clusters: (1) Large (> 50 cells) [8 (3-38) vs 3 (1-13), P < 0.001]; (2) Medium (6-49 cells) [7 (3-27) vs 3 (1-9), P < 0.001]; (3) Small (2-5 cells) [9 (2-36) vs 3 (1-14), P < 0.001]; and (4) Single-cell clusters [20 (4-111) vs 6 (0-41), P < 0.001], as presented in Table 2. Subgroup analysis according to bile duct location revealed that the sheath-rinse specimens consistently yielded a significantly higher number of cell clusters than the brush-wash specimens, irrespective of location (proximal vs distal) (Table 3).
| Brush-wash (n = 112) | Sheath-rinse (n = 112) | P value1 | |
| Total cell clusters | 3 (1-6) | 12 (4-21) | < 0.001 |
| Large (> 50 cells) clusters | 3 (1-13) | 8 (3-38) | < 0.001 |
| Medium (6-49 cells) clusters | 3 (1-9) | 7 (3-27) | < 0.001 |
| Small (2-5 cells) clusters | 3 (1-14) | 9 (2-36) | < 0.001 |
| Single-cell clusters | 6 (0-41) | 20 (4-111) | < 0.001 |
| Proximal bile duct | Brush-wash (n = 38) | Sheath-rinse (n = 38) | P value1 |
| Total cell clusters | 3 (1-7) | 14 (8-23) | < 0.001 |
| Large (> 50 cells) clusters | 5 (1-19) | 12 (4-77) | < 0.009 |
| Medium (6-49 cells) clusters | 5 (1-13) | 8 (3-53) | < 0.019 |
| Small (2-5 cells) clusters | 5 (1-19) | 13 (3-54) | < 0.017 |
| Single-cell clusters | 18 (1-70) | 54 (9-207) | < 0.0021 |
| Distal bile duct | Brush-wash (n = 74) | Sheath-rinse (n = 74) | P value1 |
| Total cell clusters | 3 (2-6) | 10 (3-19) | < 0.001 |
| Large (> 50 cells) clusters | 2 (1-11) | 7 (2-28) | < 0.001 |
| Medium (6-49 cells) clusters | 2 (1-6) | 7 (3-18) | < 0.001 |
| Small (2-5 cells) clusters | 3 (1-9) | 8 (2-22) | < 0.001 |
| Single-cell clusters | 4 (0-28) | 18 (3-82) | < 0.001 |
Table 4 presents the results regarding the diagnostic performance of brush-wash and sheath-rinse specimens, as well as their combined use. The performance measures were diagnostic accuracy, sensitivity, specificity, NPV, and positive predictive value (PPV). The sheath-rinse specimens were associated with significantly higher diagnostic accuracy [72.3% vs 62.5%; difference 9.8% (95%CI: 3.5-22.5), P < 0.001] and sensitivity [69.9% vs 59.2%; difference 10.7 (95%CI: 12.6-26.0), P < 0.001] than the brush-wash specimens. When used together, a higher accuracy of 74.1% and sensitivity of 71.8% were achieved, which were statistically superior to those of brush-wash specimens (P < 0.001). No significant differences in specificity, NPV, or PPV were identified between the methods.
| Brush-wash (n = 112) | Sheath-rinse (n = 112) | Combined (n = 112) | P value | |
| Accuracy | 62.5 (52.9-71.5) | 72.3 (63.1-80.4) | 74.1 (65.0-81.9) | < 0.0011 |
| < 0.0012 | ||||
| 0.1573 | ||||
| Sensitivity | 59.2 (49.1-68.8) | 69.9 (60.1-78.5) | 71.8 (62.1-80.3) | < 0.0011 |
| < 0.0012 | ||||
| 0.1573 | ||||
| Specificity | 100 (66.3-100) | 100 (66.4-100) | 100 (66.3-100) | NA |
| Positive predictive value | 100 (94.1-100) | 100 (95.0-100) | 100 (95.1-100) | NA |
| Negative predictive value | 17.6 (8.4-30.9) | 22.5 (10.8-38.5) | 23.7 (11.4-40.2) | 0.5644 |
| 0.4835 | ||||
| 0.9016 |
Various strategies involving the use of different sampling techniques[6], newly developed devices[4], and molecular methods such as fluorescence in situ hybridization (FISH)[10] have been evaluated to improve diagnostic accuracy in assessing biliary strictures. Multimodal sampling is technically challenging, time-consuming, and expensive. While the usefulness of rapid on-site evaluation (ROSE) for biliary cytology specimens has been reported[11,12], ROSE may not be cost-effective or available in most endoscopic suites[13]. SOC, a relatively new diagnostic method for pancreaticobiliary strictures, provides a more accurate diagnosis than conventional ERCP-guided brush cytology according to the results of a recent study by Gerges et al[7]. However, its clinical utility is constrained by the need for advanced technical expertise, significant procedural costs, and the platform's design limitation, which accommodates only dedicated small-caliber forceps[6]. Therefore, there exists a need to develop sampling techniques that can be widely adopted, regardless of the endoscopic equipment or personnel infrastructure available.
Previous studies have shown the usefulness of sheath-rinse specimens in enhancing cellularity. Wakasa et al[14] evaluated the diagnostic accuracy of brush-wash and sheath-content specimens obtained from 141 patients with biliary strictures and reported that conventional smears yielded an accuracy rate of 68.8%, which increased to 78.7% when brush-wash and sheath-content specimens were combined. Notably, the sheath was not rinsed but was directly cut and centrifuged to isolate cells, which differs from the methodology adopted in our study. Furthermore, the sheath-content specimens yielded a higher cellularity than the brush-wash specimens, although the assessment method was semiquantitative. Amog-Jones et al[8] also investigated the effect of sheath rinsing on cellular yield in biliary brush cytology by conducting a prospective study at a tertiary care center in the United States, which included 13 patients who underwent ERCP for biliary strictures. The methodology used for sheath rinsing was similar to that used in our study. They showed that the sheath-rinse technique, when used in combination with standard brush cytology, improved the overall cellularity of the specimens. Specifically, overall cellularity was rated as moderate or high in 77% of the sheath-rinse specimens compared with 62% of the brush-wash specimens alone. A notable limitation of that study was the small sample size (26 paired biliary specimens), which may have affected the statistical power and generalizability of the results.
The current study demonstrated the superiority of sheath-rinse specimens with respect to cellularity and diagnostic accuracy in a sufficient number of patients with biliary strictures in a multicenter setting. Quantitative evaluation was performed by board-certified cytopathologists using a scoring system, and the sheath-rinse specimens were found to have a significantly higher total number of cell clusters. Additionally, the sensitivity and diagnostic accuracy for distinguishing malignant lesions from benign lesions increased significantly compared to those obtained with the brush specimens. The theoretical mechanism underlying the higher cellularity of sheath-rinse specimens is as follows: (1) It is hypothesized that cells are dislodged from the brush into the protective sheath during the brushing process; and (2) The sheath scrapes the bile duct wall in the stricture area, potentially dislodging additional cellular material into the sheath[8]. Furthermore, scraping through the stricture likely increases the cellular yield by exposing the subepithelial tissue[15]. This is critical because some cellular components of biliary lesions are located in the submucosal tissue, and simple brushing may not effectively extract these deeper cells.
A further point for consideration is the LBC methodology, which includes two primary methods: (1) SurePath; and (2) ThinPrep. ThinPrep involves collecting a sample, preserving it in a solution, and using filtration to produce a thin and uniform layer of cells for microscopic examination[16]. This approach minimizes sample artifacts such as blood and mucus and allows for a clearer diagnostic evaluation. To date, no studies have directly compared these two methods when assessing biliary tract lesions. However, researches have demonstrated that compared to ThinPrep, SurePath is associated with superior cellularity when evaluating uterine cervical[17,18] and urine specimens[19]. This advantage is likely due to the distinct processing techniques employed. SurePath utilizes an ethanol-based preservative and a centrifugation-based cell enrichment process, which more effectively separates diagnostic cells from contaminants such as blood or mucus[17]. Further studies are needed to compare the diagnostic performance of the two methods in biliary lesions.
Biliary brush cytology is considered the cornerstone technique used during ERCP owing to its high specificity, simplicity, safety, and cost-effectiveness. However, its utility has been hampered by its low sensitivity and increased NPV, largely because of the low cellularity of specimens. This study proposes that sheath-rinse specimens, which have higher cellularity than conventional brush-wash specimens, might be a potential solution for overcoming the drawbacks of biliary brush cytology. This technique does not require additional accessories or procedures and appears to be cost-effective and safe, with no adverse events associated with brush cytology. Using the same brush catheter requires the endoscopist to insert the sheath into the stricture and perform at least 10 back-and-forth movements of the brush.
This study had some limitations. First, ancillary techniques were not used for evaluation of the specimens. Coupling routine cytology specimens with FISH and next-generation sequencing appears to improve diagnostic sensitivity[10], and the application of these techniques to sheath-rinse specimens rich in cells may improve performance. Additionally, novel molecular approaches, including flow cytometry for DNA analysis of aneuploidy, morphometry for assessing the nuclear area, nuclear DNA content, as well as measurements of tumor markers in the bile fluid may be warranted to enhance the diagnostic performance of brush cytology[20]. Second, while this was a multicenter study, only two institutions that used identical processes in preparing the cytological specimens participated in this study. Future studies should include institutions employing different processes to validate the effectiveness of sheath rinsing in various settings. Finally, there was a lack of centralized pathology readings for all specimens, which may have introduced measurement bias in the assessment of cellularity and diagnostic accuracy. However, we minimized this bias by using a validated scoring system for cytological specimens.
This multicenter prospective study showed that sheath-rinse specimens, when combined with conventional brush-wash specimens, significantly improved the diagnostic accuracy for biliary strictures by increasing the cellular yield. The efficacy of this method highlights its potential as a valuable adjunct to ERCP-guided cytology, offering enhanced diagnostic capabilities without additional procedural requirements. This approach may refine diagnostic strategies and improve the outcomes of biliary stricture management.
| 1. | Nabi Z, Reddy DN. Multidisciplinary Approach to Indeterminate Biliary Strictures. Gastrointest Endosc Clin N Am. 2022;32:411-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Korc P, Sherman S. ERCP tissue sampling. Gastrointest Endosc. 2016;84:557-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 4. | Bank JS, Witt BL, Taylor LJ, Adler DG. Diagnostic yield and accuracy of a new cytology brush design compared to standard brush cytology for evaluation of biliary strictures. Diagn Cytopathol. 2018;46:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Yoon SB, Moon SH, Ko SW, Lim H, Kang HS, Kim JH. Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis. Dig Dis Sci. 2022;67:3284-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Ko SW, Lee SS, So H, Hwang JS, Song TJ, Lee SK, Kim MH. A novel method of biopsy for indeterminate pancreaticobiliary strictures: tube-assisted biopsy. Endoscopy. 2020;52:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Gerges C, Beyna T, Tang RSY, Bahin F, Lau JYW, van Geenen E, Neuhaus H, Nageshwar Reddy D, Ramchandani M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: a prospective, randomized, multicenter trial (with video). Gastrointest Endosc. 2020;91:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Amog-Jones GF, Chandra S, Jensen C, Johlin FC. Including the Sheath Rinse to Improve Cellular Yield in Biliary Brushing Cytology. Clin Endosc. 2017;50:614-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Pitman MB, Centeno BA, Ali SZ, Genevay M, Stelow E, Mino-Kenudson M, Fernandez-del Castillo C, Max Schmidt C, Brugge W, Layfield L; Papanicolaou Society of Cytopathology. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol. 2014;42:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Nanda A, Brown JM, Berger SH, Lewis MM, Barr Fritcher EG, Gores GJ, Keilin SA, Woods KE, Cai Q, Willingham FF. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therap Adv Gastroenterol. 2015;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Varadarajulu S, Bang JY, Hasan MK, Navaneethan U, Hawes R, Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? (with video). Gastrointest Endosc. 2016;84:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Archibugi L, Mariani A, Ciambriello B, Petrone MC, Rossi G, Testoni SGG, Carlucci M, Aldrighetti L, Falconi M, Balzano G, Doglioni C, Capurso G, Arcidiacono PG. High sensitivity of ROSE-supported ERCP-guided brushing for biliary strictures. Endosc Int Open. 2021;9:E363-E370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Kong F, Zhu J, Kong X, Sun T, Deng X, Du Y, Li Z. Rapid On-Site Evaluation Does Not Improve Endoscopic Ultrasound-Guided Fine Needle Aspiration Adequacy in Pancreatic Masses: A Meta-Analysis and Systematic Review. PLoS One. 2016;11:e0163056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Wakasa T, Inayama K, Honda T, Shintaku M, Okabe Y, Kakudo K. Brushing cytology of the biliary tract: bile juice from the ERCP sheath tube provides cell-rich smear samples. Acta Cytol. 2014;58:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Curcio G, Traina M, Mocciaro F, Liotta R, Gentile R, Tarantino I, Barresi L, Granata A, Tuzzolino F, Gridelli B. Intraductal aspiration: a promising new tissue-sampling technique for the diagnosis of suspected malignant biliary strictures. Gastrointest Endosc. 2012;75:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Davey E, d'Assuncao J, Irwig L, Macaskill P, Chan SF, Richards A, Farnsworth A. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. BMJ. 2007;335:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Zhao FH, Hu SY, Bian JJ, Liu B, Peck RB, Bao YP, Pan QJ, Frappart L, Sellors J, Qiao YL. Comparison of ThinPrep and SurePath liquid-based cytology and subsequent human papillomavirus DNA testing in China. Cancer Cytopathol. 2011;119:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Rozemeijer K, Penning C, Siebers AG, Naber SK, Matthijsse SM, van Ballegooijen M, van Kemenade FJ, de Kok IM. Comparing SurePath, ThinPrep, and conventional cytology as primary test method: SurePath is associated with increased CIN II+ detection rates. Cancer Causes Control. 2016;27:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Okuda C, Kyotake A, Nakamura A, Itoh T, Kamoshida S, Ohsaki H. Quantitative cytomorphological comparison of SurePath and ThinPrep liquid-based cytology using high-grade urothelial carcinoma cells. Cytopathology. 2021;32:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Gress TM. Molecular diagnosis of pancreatobiliary malignancies in brush cytologies of biliary strictures. Gut. 2004;53:1727-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |