Published online May 26, 2025. doi: 10.12998/wjcc.v13.i15.103239
Revised: December 19, 2024
Accepted: January 9, 2025
Published online: May 26, 2025
Processing time: 69 Days and 20.3 Hours
Immune checkpoint inhibitors (ICIs) are a new class of antitumor agents. They enhance antitumor effects by blocking inhibitory receptors and related ligands expressed on T cells. ICIs also modulate regular immune cell activity, affecting the immune system and causing immune-related adverse events. The renal system is sometimes affected by these adverse events. Currently, the literature on ICIs-related glomerular injuries is scarce.
We present a patient who developed granulomatosis with polyangiitis (GPA) 3 weeks after treatment with the anti-programmed cell death-1 inhibitor, tislelizumab. The patient experienced proteinuria, hematuria, and acute kidney injury without pulmonary hemorrhage and tested positive for anti-neutrophil cyto
Glucocorticoids plus cyclophosphamide are effective for treating GPA induced by tislelizumab. However, follow-up and patient education are needed.
Core Tip: Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) due to immune checkpoint inhibitors is extremely rare. AAV caused by the programmed cell death-1 inhibitor, tislelizumab, has not been reported. Our patient developed AAV and granulomatosis with polyangiitis after tislelizumab treatment. Glucocorticoids given in combination with cyclophosphamide effectively treated our patient. However, after the patient discontinued treatment without authorization, the vasculitis recurred. The glucocorticoid + cyclophosphamide regimen was continued, but the patient’s renal function deteriorated.
- Citation: Zhao JH, Wang JJ, Li YW. Granulomatosis with polyangiitis induced by the anti-programmed cell death-1 inhibitor tislelizumab: A case report. World J Clin Cases 2025; 13(15): 103239
- URL: https://www.wjgnet.com/2307-8960/full/v13/i15/103239.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i15.103239
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic small-vessel vasculitis characterized by ANCA detection in serum. It primarily involves small vessels, but medium-sized arterioles may also be affected. Classical AAV includes granulomatosis with polyangiitis (GPA), microscopic GPA (known as MPA), and eosinophilic granulomatosis with GPA (known as EGPA). Although the exact pathogenesis of AAV is unknown, studies have demonstrated that T cells and B cells, ANCA, complement alternative pathways, and neutrophil extracellular traps play various essential roles[1]. Antibody-induced AAV is rare, with only a few published studies. Due to the rarity of this disease, effective treatment options are inconclusive. We report herein a case of clinically diagnosed anti-programmed cell death (PD)-1 antibody-induced AAV renal damage and analyzed its pathogenesis, diagnosis, and treatment through a literature review.
A 73-year-old male was referred to the Department of Nephrology due to elevation of serum creatinine for more than 4 months, which had begun worsening more than 2 weeks prior.
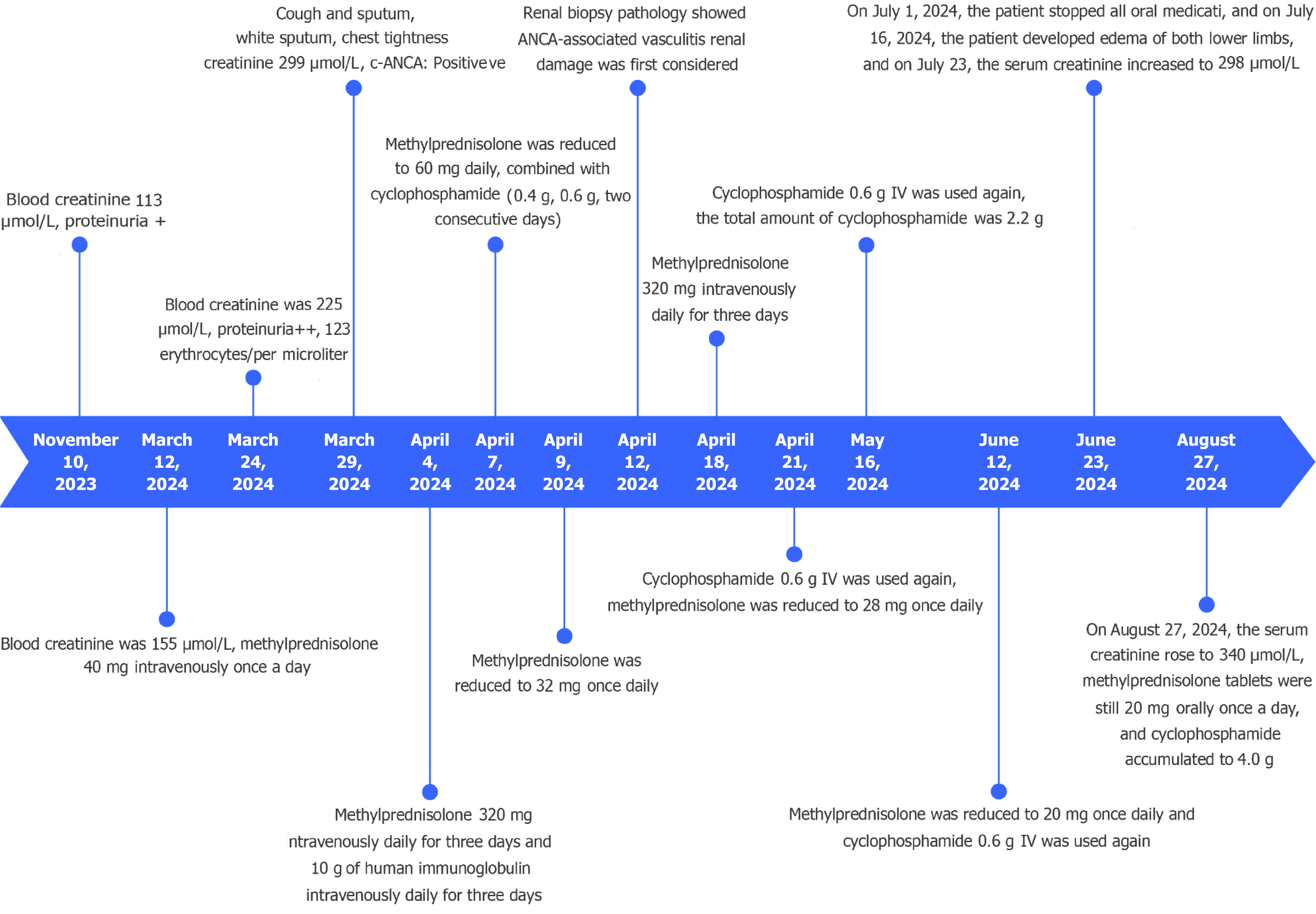
On November 10, 2023 (4 months prior to presentation), the patient’s renal function test showed a blood creatinine level of 113 μmol/L and positivity for proteinuria. Due to persistent renal insufficiency, however, the patient discontinued the tislelizumab [an anti-programmed death-1 (PD-1) inhibitor] treatment on February 20, 2024. Two weeks after the discontinuation, the blood creatinine had been found to have increased to 155 μmol/L and intravenous (IV) methylprednisolone injection (40 mg, once daily) was started. On March 24, 2024, the blood creatinine level had been found to have increased to 225 μmol/L. Moreover, the urine routine was suggestive of proteinuria++ and 123 erythrocytes/µL were detected. On March 29, 2024, the patient developed cough and sputum, white sputum, chest tightness and shortness of breath, chest pain, hemoptysis, fever, and chills. He was then transferred to the nephrology department for treatment.
On December 21, 2019, the patient had developed hemoptysis at home without obvious triggers. Computed tomography with contrast of the lungs had suggested paravalvular occupancy in the left lower lung, and lung cancer was considered. In addition, the mediastinal lymph nodes were found to be enlarged. On December 24, 2019, bronchoscopy had been performed and had revealed swelling and infiltration of the mucosa of the left main bronchus and all segments of the bronchus and narrowing of the opening of the left basal segment. The patient was then diagnosed with T4N3M0 stage IIIC. After exclusion of contraindications, the patient had received four cycles of chemotherapy with gemcitabine (1.4 g on day 1 and day 8) and cisplatin (110 mg on day 1). As a consequence, the hemoptysis improved, and the prominent cough sputum resolved.
After the chemotherapy was completed, the patient received palliative radiotherapy with intensity-modulated radiation therapy in the local hospital. In February 2022, the patient had been hospitalized due to a fever. He was then treated with docetaxel (130 mg on day 1) and tislelizumab (200 mg on day 1) for six cycles with satisfactory results. Tislelizumab (200 mg IV every 3 weeks) had then been started on July 20, 2022, for immune maintenance therapy.
The patient denied any family history of malignant tumors and AAV. The patient neither smoked nor consumed alcohol.
On physical examination, the vital signs were as follows: (1) Body temperature of 36.5 °C; (2) Blood pressure of 127 mmHg/80 mmHg; (3) Heart rate, 80 beats per minute; and (4) Respiratory rate of 20 breaths per minute. Breath sounds were coarse in both lungs, and dry rales were heard.
Urine automated analysis revealed urine red blood cell positivity and protein positivity. Blood biochemistry revealed: Urea nitrogen of 16.4 mmol/L (normal range: 3.1-8.0 mmol/L); creatinine of 299 μmol/L (normal range: 57-97 μmol/L); uric acid of 302 μmol/L (normal range: 208-428 μmol/L); total protein of 50.3 g/L (normal range: 65-85 g/L); albumin of 28.1 g/L (normal range: 40-55 g/L); and estimated glomerular filtration rate of 18.41 mL/minute/1.73 m2. The ANCA-cytoplasmic type was positive. The anti-glomerular basement membrane and anti-nuclear antibodies were negative. The 24-hour urine protein quantification revealed 2.35 g/24 hours (normal range < 0.15 g).
Thoracic ultrasound revealed bilateral pleural effusion (left 4.7 cm, right 3.6 cm). Renal ultrasound detected bilateral renal parenchymal echogenicity slightly enhanced, a suspicious stone in the right kidney, a cyst in the left kidney, an enlarged prostate with calcified foci, and abdominal effusion.
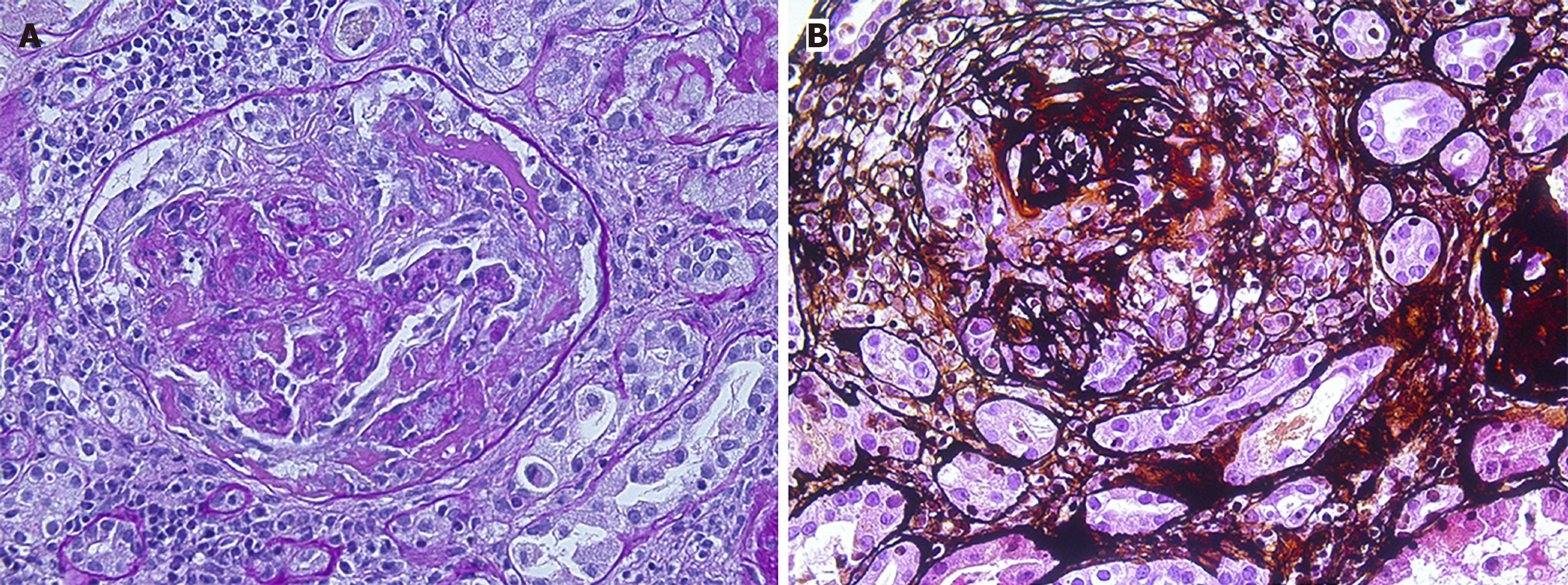
The patient developed acute kidney injury of unknown cause, and renal biopsy was undertaken immediately with the consent of the patient. Preliminary renal puncture pathology on April 4, 2024, showed AAV with crescent formation and massive inflammatory cell infiltration in the renal tubulointerstitial. A renal biopsy on April 12, 2024, showed AAV renal damage (Figure 1).
Per the 2022 edition of vasculitis classification criteria developed by the American College of Rheumatology and the European League Against Rheumatism[2-4], the patient scored 0 points for clinical criteria, 5 points for laboratory, imaging, and C-ANCA positive, and 1 point for renal biopsy-visible logo immune complex glomerulonephritis, with a total score of 6 points. Diagnostic criteria excluded MPA and EGPA, and the final diagnosis was GPA.
The patient was treated with glucocorticoids and cyclophosphamide. Methylprednisolone (320 mg once a day) was immediately administered on April 4, 2024. After 3 days of pulse therapy, methylprednisolone tablets (60 mg once a day) were given orally, and cyclophosphamide (1.0 g IV) was given. Pantoprazole was administered for gastric protection and sulfamethoxazole (one tablet once daily) to prevent Pneumocystis carinii pneumonia. Methylprednisolone was reduced to 32 mg once daily on April 9, 2024, but increased to 320 mg once a day for 3 days starting on April 18, 2024. Cyclophosphamide (0.6 g) was given intravenously every 3-4 weeks.
On July 1, 2024, against medical advice, the patient discontinued all oral medications including the methylprednisolone tablets. The disease relapsed. Given the high price of rituximab and the uncertainty of efficacy, the patient opted to continue the glucocorticoids + cyclophosphamide treatment.
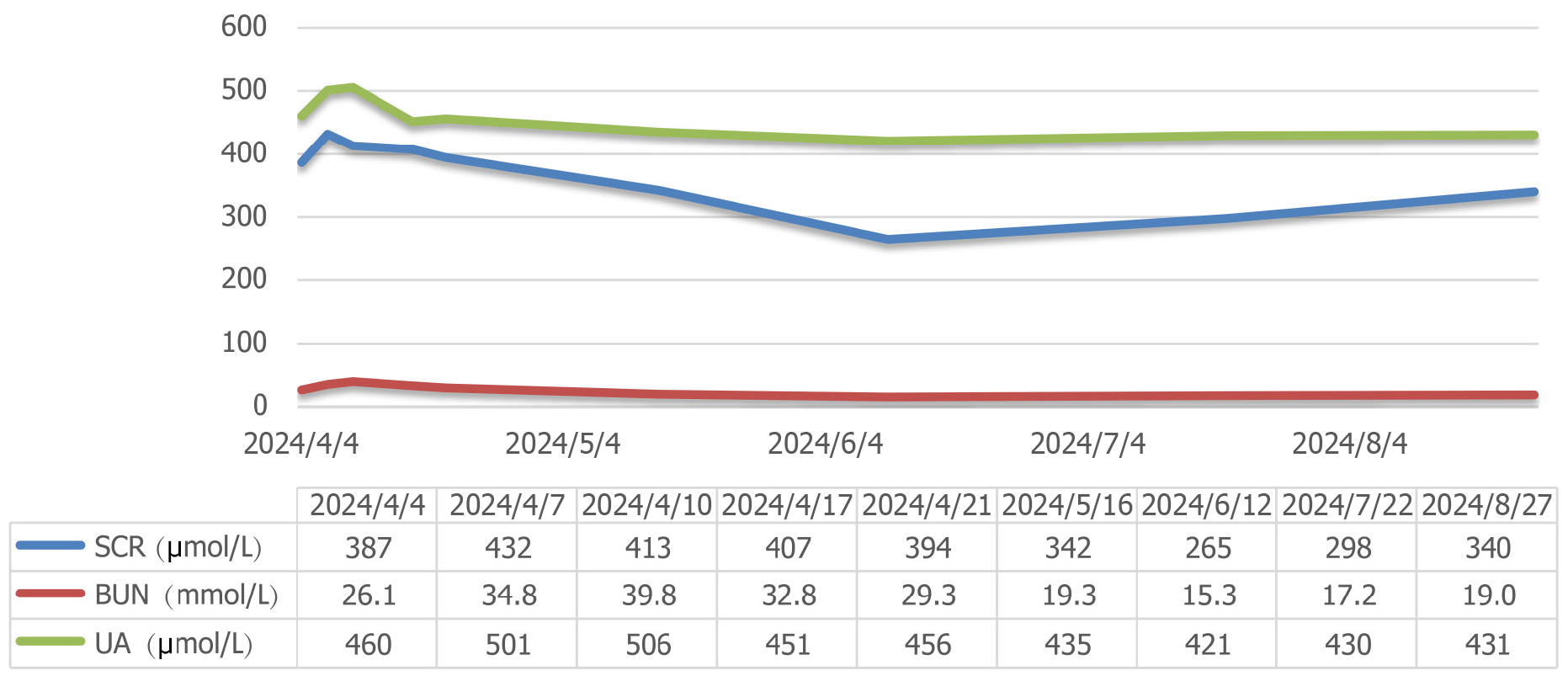
The patient’s serum creatinine (SCR) levels continued to rise, reaching 432 μmol/L on April 7, 2024, but then began to gradually decrease. The therapeutic effect was best when the cumulative dose of cyclophosphamide reached 2.8 g (oral methylprednisolone tablets 20 mg were taken daily). Subsequently, the SCR levels decreased to 255 μmol/L. However, the patient discontinued all oral medications on July 1, 2024.
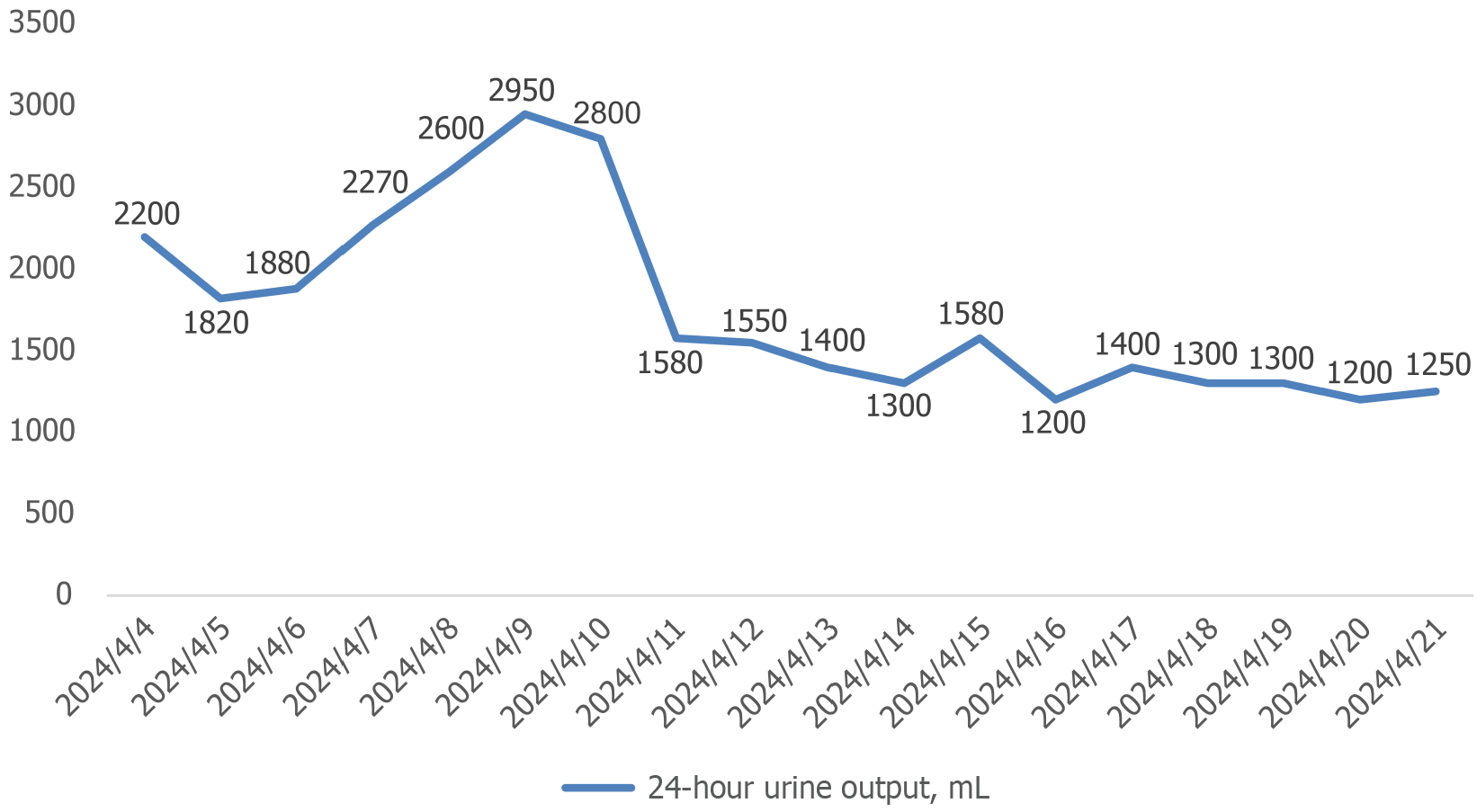
On July 16, 2024, the patient developed edema in both lower extremities and on July 23, 2024, the SCR levels rose to 298 μmol/L. Then, the SCR level gradually increased, reaching 340 μmol/L on August 2, 2024. The changes in the patient’s 24-hour urine output are shown in Figure 2, and the renal function is shown in Figure 3. The patient tolerated the treatment well, and no significant infectious complications were observed. The timeline graph for treatment is shown in Figure 4.
Immune checkpoints regulate the antigen recognized by the T cell receptor during an immune response and consist of a group of costimulatory and coinhibitory molecules expressed on the surface of T cells. Immune checkpoints maintain a balance between T cell activation and depletion. They can be divided into two categories: (1) Costimulatory immune checkpoints promote the immune process, e.g., CD28, inducible T-cell co-stimulator, and CD137; and (2) Coinhibitory immune checkpoints inhibit the immune process, e.g., PD-1, cytotoxic T-lymphocyte-associated molecule 4 (CTLA-4), T-cell immunoglobulin, and mucin domain-containing protein 3. Currently, three types of immune checkpoint inhibitors (ICIs) block the coinhibitory pathways. These are PD-1 (nivolumab, pembrolizumab, and cemiplimab), PD ligand 1, and CTLA-4. When coinhibitory signaling is blocked, T cells can be activated, thereby enhancing immunity against tumors[5,6]. However, T cell activation can also increase the inflammatory response, leading to immune-related adverse events.
Studies have also shown a causal relationship between the leukotriene receptor antagonist montelukast and EGPA[7]. In renal AAV, there is a decrease in PD-1 expression in renal tubular interstitial disease. The absence of renal tubular interstitial PD-1 is associated with active renal AAV. A negative correlation between renal interstitial PD-1 mRNA levels and complement factor B expression has been observed; specifically, complement factor B expression increases when renal interstitial PD-1 expression decreases. Since complement factor B is essential in the alternative complement pathway, anti-PD-1 antibody-induced AAV may be associated with activating the alternative complement pathway[8].
When anti-PD-1 antibodies block the co-inhibitory pathway, T cells are activated to assist in the conversion of B cells into plasma cells. Concurrently, neutrophils expressing protease-3 (PR3) and myeloperoxidase (MPO) are active and bind to ANCA secreted by plasma cells[9-11]. Activation of the alternative complement pathway produces the fifth complement fragment, which binds to the cognate receptor on neutrophils[12,13]. Combining these factors with neutrophils will further activate the neutrophils and promote their degranulation and production of neutrophil extracellular traps. All of this will exacerbate respiratory bursts, damage vascular endothelium, accelerate the inflammatory response, and ultimately contribute to the clinical damage of AAV[9,12,14,15].
AAV is generally treated with glucocorticoids in combination with immunosuppressants or rituximab. However, there is no standard treatment regimen for ICI-induced AAV. It was reported that 2 patients were successfully treated with glucocorticoids alone, while 1 patient treated with glucocorticoids alone had an unclear outcome[16-18]. Others have reported that corticosteroid combined with cyclophosphamide treatment was successful in 2 patients[19,20]. Another report found that a patient with a severe case of ICI-induced AAV treated with corticosteroid + cyclophosphamide + plasma exchange eventually progressed to chronic kidney disease[21]. Finally, glucocorticoid + rituximab treatment was successfully utilized for 2 patients[22,23].
AAV caused by anti-PD-1 inhibitors is limited to individual reports in literature. Unfortunately, the causal relationship between anti-PD-1 inhibitors and AAV remains unclear. The severity of ICI-associated glomerulopathies and the response to treatment varies with underlying conditions such as malignancy, autoimmune disease, and AAV-inducing drugs. However, the overall prognosis may be better than that of AAV. One case report showed that patients with GPA that is well controlled by immunosuppressive therapy and negative for ANCA antibodies can resume anti-PD-1 antibody treatment if non-small cell lung cancer recurs after surgery[24]. In our case, we recommend that the patient could resume tislelizumab if AAV remission is achieved or ANCA-cytoplasmic type is negative.
Another case report recorded AAV induced by nivolumab treatment. Three weeks after the last injection of nivolumab, the patient had developed proteinuria, hematuria, and acute kidney injury with pulmonary hemorrhage. ANCA was positive, and renal biopsy confirmed glomerular injury with crescents. The patient was treated with pulse glucocorticoid therapy, rituximab, and plasma exchange. Renal function and pulmonary hemorrhage improved, and the ANCA titer was eventually negative[21].
Despite the results of the PEXIVAS trial, plasma exchange has shown no significant benefit in idiopathic AAV[25]. However, the MEPEX study confirmed the efficacy of plasma exchange in AAV. Plasma exchange was associated with a higher rate of renal recovery and less need for dialysis compared to IV methylprednisolone in patients with blood creatinine > 500 μmol/L at the time of AAV diagnosis[26]. In our case, the patient’s blood creatinine reached a maximum of 432 μmol/L, thus not exceeding the 500 μmol/L threshold. The blood creatinine level gradually decreased, and renal function improved after aggressive anti-immunotherapy. For these reasons, plasma exchange therapy was not performed.
The advantages of this case included the complete clinical and pathological data, a detailed report of a rare case, and the analysis of the pathogenesis of AAV induced by an anti-PD-1 inhibitor. The limits of this study included the lack of analysis of ANCA-cytoplasmic type-associated antigens, such as PR3 and MPO. The detection of PR3 or MPO antigens improves the accuracy of GPA diagnosis and excludes EGPA and MPA. Second, it is difficult to determine the true effect of repeat glucocorticoids + cyclophosphamide because the follow-up period after the recurrence of AAV was only 1 month. Long-term follow-up is still needed.
Herein, we have reported a case of granulomatous vasculitis induced by an anti-PD-1 inhibitor. Treatment with glucocorticoids + cyclophosphamide was effective in this patient. The most pronounced decrease in SCR occurred when the cyclophosphamide accumulated to 2.8 g. After self-discontinuation of this treatment, the patient’s condition recurred. The original regimen was continued, but the patient’s condition deteriorated. For the recurrence of vasculitis, the use of glucocorticoids + rituximab requires further consideration. In addition, physicians must enhance patient follow-up and education to improve patient compliance.
| 1. | Pan M, Zhao H, Jin R, Leung PSC, Shuai Z. Targeting immune checkpoints in anti-neutrophil cytoplasmic antibodies associated vasculitis: the potential therapeutic targets in the future. Front Immunol. 2023;14:1156212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Suppiah R, Robson JC, Grayson PC, Ponte C, Craven A, Khalid S, Judge A, Hutchings A, Merkel PA, Luqmani RA, Watts RA; DCVAS INVESTIGATORS. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis. 2022;81:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 3. | Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Judge A, Khalid S, Hutchings A, Watts RA, Merkel PA, Luqmani RA; DCVAS Investigators. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 242] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 4. | Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, Khalid S, Hutchings A, Luqmani RA, Watts RA, Merkel PA; DCVAS Study Group. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann Rheum Dis. 2022;81:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 5. | Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ, Jacobs JF, Schalken JA, Oosterwijk E, van Eenennaam H, Boots AM. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 6. | Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, Choy EH, Benesova K, Radstake TRDJ, Cope AP, Lambotte O, Gottenberg JE, Allenbach Y, Visser M, Rusthoven C, Thomasen L, Jamal S, Marabelle A, Larkin J, Haanen JBAG, Calabrese LH, Mariette X, Schaeverbeke T. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. 2021;80:36-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 7. | Alexander G, Moore SA, Lenert PS. Eosinophilic granulomatosis with polyangiitis and its association with montelukast: a case-based review. Clin Rheumatol. 2024;43:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Hakroush S, Tampe B. Association between Loss of Immune Checkpoint Programmed Cell Death Protein 1 and Active ANCA-Associated Renal Vasculitis. Int J Mol Sci. 2023;24:2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990;87:4115-4119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 888] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 10. | London J, Dumoitier N, Lofek S, Dion J, Chaigne B, Mocek J, Thieblemont N, Cohen P, Le Jeunne C, Guillevin L, Witko-Sarsat V, Varin-Blank N, Terrier B, Mouthon L; French Vasculitis Study Group (FVSG). Skewed peripheral B- and T-cell compartments in patients with ANCA-associated vasculitis. Rheumatology (Oxford). 2021;60:2157-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Sharma RK, Lövström B, Gunnarsson I, Malmström V. Proteinase 3 Autoreactivity in Anti-Neutrophil Cytoplasmic Antibody-associated vasculitis-Immunological versus clinical features. Scand J Immunol. 2020;92:e12958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Brilland B, Garnier AS, Chevailler A, Jeannin P, Subra JF, Augusto JF. Complement alternative pathway in ANCA-associated vasculitis: Two decades from bench to bedside. Autoimmun Rev. 2020;19:102424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Mathern DR, Heeger PS. Molecules Great and Small: The Complement System. Clin J Am Soc Nephrol. 2015;10:1636-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Massicotte-Azarniouch D, Herrera CA, Jennette JC, Falk RJ, Free ME. Mechanisms of vascular damage in ANCA vasculitis. Semin Immunopathol. 2022;44:325-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Söderberg D, Segelmark M. Neutrophil extracellular traps in vasculitis, friend or foe? Curr Opin Rheumatol. 2018;30:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Roger A, Groh M, Lorillon G, Le Pendu C, Maillet J, Arangalage D, Tazi A, Lebbe C, Baroudjian B, Delyon J; PATIO group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) induced by immune checkpoint inhibitors. Ann Rheum Dis. 2019;78:e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Harada M, Naoi H, Yasuda K, Ito Y, Kagoo N, Kubota T, Ichijo K, Mochizuki E, Uehara M, Matsuura S, Tsukui M, Koshimizu N. Programmed cell death-1 blockade in kidney carcinoma may induce eosinophilic granulomatosis with polyangiitis: a case report. BMC Pulm Med. 2021;21:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Sibille A, Alfieri R, Malaise O, Detrembleur N, Pirotte M, Louis R, Duysinx B. Granulomatosis With Polyangiitis in a Patient on Programmed Death-1 Inhibitor for Advanced Non-small-cell Lung Cancer. Front Oncol. 2019;9:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Uner M, Alhasson B, Obhrai J, Bagnasco SM. ANCA-associated pauci-immune necrotizing glomerulonephritis during the treatment with pembrolizumab. Virchows Arch. 2021;478:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Nabel CS, Severgnini M, Hung YP, Cunningham-Bussel A, Gjini E, Kleinsteuber K, Seymour LJ, Holland MK, Cunningham R, Felt KD, Vivero M, Rodig SJ, Massarotti EM, Rahma OE, Harshman LC. Anti-PD-1 Immunotherapy-Induced Flare of a Known Underlying Relapsing Vasculitis Mimicking Recurrent Cancer. Oncologist. 2019;24:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Laamech R, Terrec F, Emprou C, Toffart AC, Pierret T, Naciri-Bennani H, Rostaing L, Noble J. Efficacy of Plasmapheresis in Nivolumab-Associated ANCA Glomerulonephritis: A Case Report and Pathophysiology Discussion. Case Rep Nephrol Dial. 2021;11:376-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Heo MH, Kim HK, Lee H, Ahn MJ. Antineutrophil Cytoplasmic Antibody-Associated Rapid Progressive Glomerulonephritis after Pembrolizumab Treatment in Thymic Epithelial Tumor: A Case Report. J Thorac Oncol. 2017;12:e103-e105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | van den Brom RR, Abdulahad WH, Rutgers A, Kroesen BJ, Roozendaal C, de Groot DJ, Schröder CP, Hospers GA, Brouwer E. Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology (Oxford). 2016;55:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Yamada T, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Miyamoto S, Nakashima T, Iwamoto H, Hirata S, Fujitaka K, Hamada H, Sugiyama E, Hattori N. Non-small Cell Lung Cancer Treated by an Anti-programmed Cell Death-1 Antibody without a Flare-up of Preexisting Granulomatosis with Polyangiitis. Intern Med. 2019;58:3129-3132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, Hawley CM, Khalidi N, Floßmann O, Wald R, Girard LP, Levin A, Gregorini G, Harper L, Clark WF, Pagnoux C, Specks U, Smyth L, Tesar V, Ito-Ihara T, de Zoysa JR, Szczeklik W, Flores-Suárez LF, Carette S, Guillevin L, Pusey CD, Casian AL, Brezina B, Mazzetti A, McAlear CA, Broadhurst E, Reidlinger D, Mehta S, Ives N, Jayne DRW; PEXIVAS Investigators. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N Engl J Med. 2020;382:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 499] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 26. | Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, Mirapeix E, Savage CO, Sinico RA, Stegeman CA, Westman KW, van der Woude FJ, de Lind van Wijngaarden RA, Pusey CD; European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 706] [Article Influence: 39.2] [Reference Citation Analysis (0)] |