Published online May 26, 2025. doi: 10.12998/wjcc.v13.i15.102691
Revised: December 23, 2024
Accepted: January 7, 2025
Published online: May 26, 2025
Processing time: 86 Days and 1.5 Hours
Mitochondrial myopathies are characterized by primary dysfunction of the mitochondrial respiratory chain; they typically present as chronic muscle weak
In this report, we present the case of a patient who developed postoperative hypoventilation after undergoing an uneventful administration of general anesthesia. A 34-year-old woman with no family history of myopathy underwent laparoscopic removal of a right-sided ureteric stone. Two days postoperatively, her oxygen saturation decreased rapidly, and blood gas analysis revealed hypercapnia. We promptly intubated and initiated the patient and initiated her on mechanical ventilation as she remained awake. Clinical examination findings were unremarkable; the results of laboratory investigations, including those for thyroid, hepatic, renal, and neuromuscular functions, were within normal limits. Muscle biopsy revealed muscle fibers of varying sizes as well as several dege
Our case highlights that mitochondrial myopathy should be considered in the differential diagnosis of patients with postoperative respiratory failure.
Core Tip: Mitochondrial myopathies typically present with chronic muscle weakness, and acute respiratory dysfunction is an uncommon symptom. We report the case of a 34-year-old woman who experienced postoperative hypoventilation after undergoing an uneventful administration of general anesthesia. Reports on mitochondrial myopathy-related respiratory failure in adults are limited. In this case, the patient did not exhibit typical signs and symptoms of mitochondrial myopathy and remained asymptomatic until she was exposed to a general anestheic. Mitochondrial myopathy should be considered in the differential diagnosis of postoperative respiratory failure, and the diagnosis should be confirmed with a muscle biopsy.
- Citation: Park SY, Hong SM, Lee HY, Kim MY, Lee HK, Han JY, Cho HJ, Oh SI, Lee H. Mitochondrial myopathy revealed postoperative acute respiratory failure: A case report. World J Clin Cases 2025; 13(15): 102691
- URL: https://www.wjgnet.com/2307-8960/full/v13/i15/102691.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i15.102691
Postoperative hypoventilation is a common complication associated with the administration of general anesthesia. Risk factors of postoperative hypoventilation include residual paralysis from neuromuscular blockade, anesthetic-related central nervous system depression, and dysfunction of ventilator muscle mechanics, as observed in cases of obesity and preexisting pulmonary disease[1]. While respiratory muscle weakness (typically in the context of severe and diffuse muscle weakness) manifests in several neuromuscular disorders present with respiratory muscle weakness, respiratory failure rarely occurs in adults[2,3]. Steele et al[4] reported on patients with respiratory failure associated with myopathies. However, sudden-onset motor axonal neuropathy rarely occurs in adults.
Mitochondrial myopathies constitute a large heterogeneous group of genetic neuromuscular diseases characterized by primary dysfunction of the mitochondrial respiratory chain[5,6]. Though multi-organ involvement is common, some mitochondrial disorders, characterized by primarily or exclusive skeletal muscle involvement without extraocular muscle involvement were previously considered distinct subtypes of mitochondrial myopathy[7]. However, it is a rare cause of postoperative hypoventilation, which can lead to death.
In this report, we present the case of a woman who developed postoperative hypoventilation after undergoing an uneventful administration of general anesthesia.
A 34-year-old woman developed postoperative hypoventilation after undergoing an uneventful administration of general anesthesia.
A 34-year-old woman underwent laparoscopic removal of a right-sided ureteric stone. Ten hours postoperatively, the patient was recovering well. However, her oxygen saturation decreased, and we initiated her on supplemental oxygen at 1 L/min. The patient’s muscular strength in all four limbs deteriorated to Medical Research Council (MRC)-grade 4. Two days postoperatively, her oxygen saturation levels decreased rapidly.
At this time point, the patient could no longer communicate with the medical staff, and her breathing was inadequate. We promptly intubated the patient and initiated her on mechanical ventilation as she remained awake. She was then transferred to the pulmonology department for further evaluation.
The patient reported no history of past illness, drugs use or exposure to toxic substances.
Parents and siblings are healthy.
The findings of mental status and neurological examinations performed shortly after the acute episode were normal. Neurological examination revealed normal extraocular muscle movements along with the absence of ptosis and cerebellar signs. Tendon reflexes were normal.
Blood-gas analysis before intubation revealed that PaCO2 was 128.1 mmHg, PaO2 was 72.9 mmHg, and the pH was 7.151 with oxygen supplementation at 1 L/min via a nasal cannula. Blood-gas analysis after intubation revealed that the PaCO2 was 46.5 mmHg, PaO2 was 107.8 mmHg, and pH was 7.49 on mechanical ventilation under the pressure-targeted, assist-control mandatory ventilation mode with a pressure of 12 cmH2O, respiratory rate of 24 breaths/min, positive end-expiratory pressure of 5 cmH2O, and fraction of inspired oxygen of 0.25.
At this stage, we suspected that the patient had familial periodic hypokalemic paralysis associated with hypokalemia. However, her serum potassium concentration was normal (4.7 mEq/L). Laboratory test results of thyroid, hepatic, and renal functions were within normal limits. We considered subclinical neuromuscular disease; however, the patient’s serum levels of creatine kinase and lactic acid were within normal limits (4.3 U/L and 1.7 mmol/L, respectively). Serum levels of autoantibodies against the acetylcholine receptor were within normal limits.
Echocardiography, chest radiography, and chest computed tomography (CT) findings were normal. The findings of brain CT and magnetic resonance imaging performed to rule out central nervous system pathology, were normal.
Tensilon test was normal. Needle electromyography (EMG) revealed mild fibrillation, sharp positive waves, and early recruitment of the right deltoid and biceps muscles. The results of repetive stimulation test (Jolley test) and nerve conduction study were normal.
Until the 17 days of hospitalization, only the minimum medication necessary to maintain the ventilator was used, while waiting to rule out the possibility of prolonged effects of anesthetics. She was emotionally stable, calm and cooperative. However, repeated attempts at a spontaneous breathing trial failed, leading to the decision to perform a muscle biopsy.
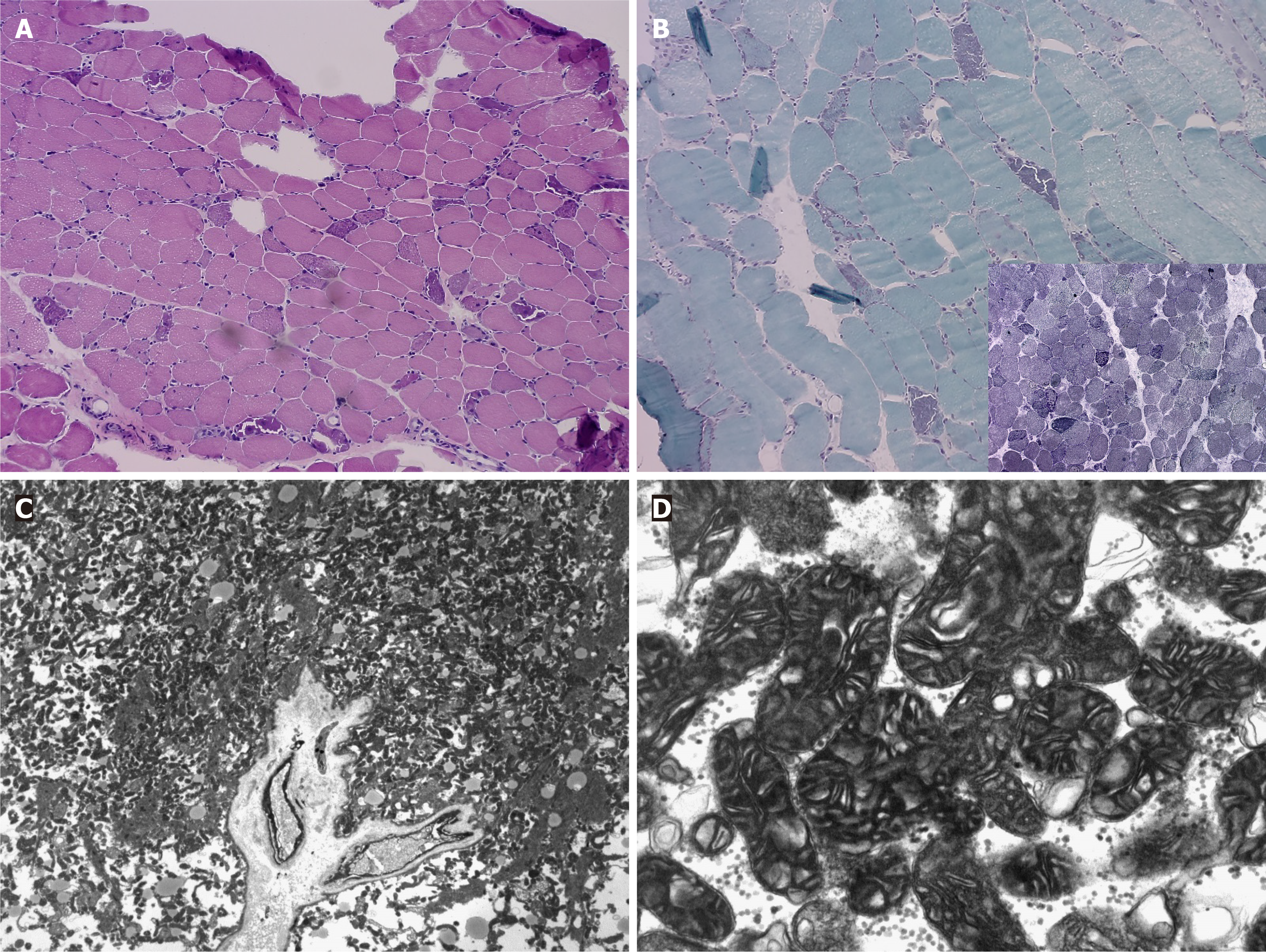
Biopsy of the right biceps bracii muscle revealed moderately varying muscle fiber size and several (circular/spherical), degenerated, and atrophic myofibers. Modified Gomori trichrome staining of the cross-sections revealed ragged red fibers (Figure 1A and B). Electron microscopy revealed a significant accumulation of mitochondria (Figure 1C and D).
We diagnosed the patient with mitochondrial myopathy. The patient refused to undergo genetic evaluation. The patient reported no family history upon admission, but was unaware of the illnesses in her relatives. After we diagnosed for her with the disease, her mother remembered that her brother (maternal uncle of patient) undergone a biopsy abroad for similar symptoms. Since a muscle biopsy performed on a maternal relative with a similar clinical manifestation is consistent with the finding of mitochondrial myopathy, we assumed that the muscle biopsy results of our patient are consistent with the related symptoms.
Up to 20 days postoperatively, all attempts at weaning the patient off of mechanical ventilation failed owing to repeat episodes of sleep-associated hypoventilation. On postoperative day 21, we performed a tracheostomy, and the patient was placed under mechanical ventilation only during sleep.
The patient’s condition improved, and we closed the tracheostomy 35 days postoperatively. On the 73rd postoperative day, she was discharged on a home ventilator in biphasic positive airway pressure mode, with inspiratory positive pressure of 8 cmH2O and expiratory positive pressure of 4 cmH2O. The patient followed-up 5 months after discharge, and her muscular strength of all limbs recovered to MRC-grade 5. Moreover, she was able to perform her day-to-day activities without using the home ventilator. After seven years, we confirmed that she performs daily activities with no recurrence of symptoms.
Mitochondrial myopathies constitute a group of genetic neuromuscular diseases characterized by the disruption of energy production. This results in clinical disorders of the muscle and other tissues, which exhibit high energy demands[5]. Defective mitochondrial metabolism causes increased energy requirements, which leads to tissue dysfunction manifesting as fatigue, muscle weakness, and exercise intolerance[8]. Reports on mitochondrial myopathy-related respiratory failure are limited. Sweeney et al[9] have described respiratory failure and sudden death in young adults. Bruno[10] reported the case of a 16-year-old boy with myopathy who died due to progressive respiratory failure. However, respiratory failure rarely occurs in adults.
Postoperative hypoventilation following the administration of general anesthesia is common. The risk factors associated with postoperative hypoventilation include residual paralysis from neuromuscular blockade, anesthetic drug-related central nervous system depression, and dysfunction of ventilator muscles as observed in obesity, acid-base balance, and pre-existing pulmonary disease[1]. Several neuromuscular diseases, such as Guillain–Barré syndrome and myasthenia gravis, present with respiratory failure. Mahfouz et al[11] reported the case of a patient who experienced late-onset central hypoventilation syndrome after receiving general anesthesia. However, mitochondrial myopathy is a rare cause of postoperative hypoventilation that may cause death.
Our patient was at risk of being misdiagnosed because she did not present with the typical signs and symptoms of mitochondrial myopathy. She remained asymptomatic until she received a general anesthetic. The patient had no external ophthalmoplegia. In the immediate postoperative period, we waited to rule out any abnormal responses or residual effects of the perioperative anesthetic. Additionally, we performed several examinations to rule out any primary cardiac, pulmonary, hepatic, renal, or central nervous system pathologies. The results of both EMG test to exclude the possibility of other motor-neuron-disease and myositis and Jolly test to exclude myasthenia gravis were normal. The most common neuromuscular causes of acute respiratory dysfunction, including Guillain–Barré syndrome and myasthenia gravis, were ruled out based on the findings of testing for autoantibodies against the acetylcholine receptor, multiple nerve conduction studies, and the Tensilon test.
Muscle biopsy is the diagnostic method of choice for mitochondrial myopathy. The biopsy findings revealed moderate variations in fiber size along with several degenerating and regenerating myofibers. The sections should be stained with the modified Gomori trichrome stain to easily identify the ragged red fibers[12]. The diagnostic values of other laboratory tests are limited. Serum levels of creatinine kinase and lactic acid were normal. We did not check serum pyruvate levels on admission. Performing polysomnography during an episode of acute respiratory insufficiency is not always possible. Our case suggests that when the common causes of neurogenic respiratory failure have been excluded, and mitochondrial myopathy is suspected, muscle biopsy should be performed to confirm the diagnosis.
In patients with mitochondrial myopathy, respiratory failure could be caused by dysfunctioning respiratory centers in the brain stem that leads to abnormal respiratory drive or fatigued inspiratory muscles. Carroll et al[13] observed a decreased ventilator response to hypoxia and hypercapnia in patients with mitochondrial disease. Barohn et al[14] reported recurrent respiratory insufficiency in several patients with mitochondrial myopathies. Patients with mitochondrial disease frequently exhibit decreased preoperative cardiac function, conduction disorders, and metabolic dysfunction-all of which take longer to recover from general anesthesia[15]. However, few reports have discussed patients with postoperative hypoventilation who exhibited no typical signs and symptoms of mitochondrial myopathy and remained asymptomatic until they received general anesthesia. In our case, we suspect that muscle fatigue was the cause of respiratory failure; therefore, mechanical ventilation enabled the muscles to recover. Eventually, our patient recovered her full muscle strength.
Mitochondrial myopathy is a rare cause of postoperative hypoventilation. However, this case highlights that mitochondrial myopathy should be considered in the differential diagnosis of postoperative respiratory failure, and emphasizes that a muscle biopsy should be performed to confirm the diagnosis.
| 1. | Kervin MW. Residual neuromuscular blockade in the immediate postoperative period. J Perianesth Nurs. 2002;17:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Polkey MI, Lyall RA, Moxham J, Leigh PN. Respiratory aspects of neurological disease. J Neurol Neurosurg Psychiatry. 1999;66:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Bennett DA, Bleck TP. Diagnosis and treatment of neuromuscular causes of acute respiratory failure. Clin Neuropharmacol. 1988;11:303-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Steele HE, Harris E, Barresi R, Marsh J, Beattie A, Bourke JP, Straub V, Chinnery PF. Cardiac involvement in hereditary myopathy with early respiratory failure: A cohort study. Neurology. 2016;87:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Milone M, Wong LJ. Diagnosis of mitochondrial myopathies. Mol Genet Metab. 2013;110:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Pfeffer G, Chinnery PF. Diagnosis and treatment of mitochondrial myopathies. Ann Med. 2013;45:4-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Montano V, Lopriore P, Gruosso F, Carelli V, Comi GP, Filosto M, Lamperti C, Mongini T, Musumeci O, Servidei S, Tonin P, Toscano A, Primiano G, Valentino ML, Bortolani S, Marchet S, Ricci G, Modenese A, Cotti Piccinelli S, Risi B, Meneri M, Arena IG, Siciliano G, Mancuso M. Primary mitochondrial myopathy: 12-month follow-up results of an Italian cohort. J Neurol. 2022;269:6555-6565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Scaini G, Rezin GT, Carvalho AF, Streck EL, Berk M, Quevedo J. Mitochondrial dysfunction in bipolar disorder: Evidence, pathophysiology and translational implications. Neurosci Biobehav Rev. 2016;68:694-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Sweeney MG, Bundey S, Brockington M, Poulton KR, Winer JB, Harding AE. Mitochondrial myopathy associated with sudden death in young adults and a novel mutation in the mitochondrial DNA leucine transfer RNA(UUR) gene. Q J Med. 1993;86:709-713. [PubMed] |
| 10. | Bruno C, Sacco O, Santorelli FM, Assereto S, Tonoli E, Bado M, Rossi GA, Minetti C. Mitochondrial myopathy and respiratory failure associated with a new mutation in the mitochondrial transfer ribonucleic acid glutamic acid gene. J Child Neurol. 2003;18:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Mahfouz AK, Rashid M, Khan MS, Reddy P. Late onset congenital central hypoventilation syndrome after exposure to general anesthesia. Can J Anaesth. 2011;58:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Vogel H. Mitochondrial myopathies and the role of the pathologist in the molecular era. J Neuropathol Exp Neurol. 2001;60:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Carroll JE, Zwillich C, Weil JV, Brooke MH. Depressed ventilatory response in oculocraniosomatic neuromuscular disease. Neurology. 1976;26:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Barohn RJ, Clanton T, Sahenk Z, Mendell JR. Recurrent respiratory insufficiency and depressed ventilatory drive complicating mitochondrial myopathies. Neurology. 1990;40:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zheng Q, Wei P, Zhou J, Zhou H, Ji F, Tang W, Li J. Case report: perioperative management of caesarean section for a parturient with mitochondrial myopathy. BMC Anesthesiol. 2017;17:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |