Published online May 26, 2025. doi: 10.12998/wjcc.v13.i15.102343
Revised: December 23, 2024
Accepted: January 9, 2025
Published online: May 26, 2025
Processing time: 97 Days and 23.9 Hours
Pheochromocytoma (PHEO) is a type of tumor that originates from chromaffin cells in the adrenal medulla and is classified as an adrenal paraganglioma. PHEOs can secrete catecholamines, leading to a variety of symptoms. Accurate diagnosis and appropriate treatment selection are crucial for favorable outcomes in these cases.
The patient presented with unexplained chest tightness, palpitations, and pink sputum. Upon examination and analysis of laboratory results, a diagnosis of adrenal PHEO was established. The PHEO secreted high levels of catecholamines, causing sudden fluctuations in blood pressure and heart rate, leading to extre
The combination of extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation can enhance tissue perfusion, thus providing a solid foundation for the accurate diagnosis and effective surgical treatment of PHEO.
Core Tip: The clinical manifestation of adrenal pheochromocytoma was atypical and the hemodynamics was extremely unstable. If the treatment is not timely, life will be lost. Extracorporeal membrane oxygenation combined with intra-aortic balloon counterpulsation offers crucial time for diagnosing adrenal pheochromocytoma and provides a solid foundation for its surgical treatment. Surgery is the standard of care for the disease, and extracorporeal membrane oxygenation combined with intra-aortic balloon counterpulsation shortens the drug preparation time for surgery. This combination stabilizes blood flow and oxygenation, enhances systemic tissue perfusion, and protects vital organs such as the heart, brain, lungs, and kidneys.
- Citation: Zeng SY, Wu HH, Yu ZH, Zhang QQ. Extracorporeal membrane oxygenation combined with intra-aortic balloon counterpulsation for pheochromocytoma: A case report. World J Clin Cases 2025; 13(15): 102343
- URL: https://www.wjgnet.com/2307-8960/full/v13/i15/102343.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i15.102343
Pheochromocytoma (PHEO) is a type of tumor originating from chromaffin cells in the adrenal medulla and is classified as an adrenal paraganglioma[1,2]. PHEOs are capable of synthesizing, storing, and metabolizing catecholamines (CA), causing a range of symptoms due to CA release. PHEOs account for 0.1% to 0.6% of hypertension cases, with an annual incidence of 3 million to 4 million people and a population prevalence of 1:6500 to 1:250. Autopsy studies show a detection rate of about 0.09% to 0.25%, with 50% to 75% of PHEOs in the population going undiagnosed. Currently, approximately 25% of PHEO imaging findings are incidental, representing 4% to 5% of adrenal incidentalomas. The classic symptoms of PHEO include the triad of headache, palpitations, and excessive sweating (hyperhidrosis)[3]. Hypertension is the most common clinical manifestation, occurring in 80%-90% of cases, as the tumor releases varying proportions of adrenaline and norepinephrine (NE), which can cause either paroxysmal or persistent hypertension. Furthermore, CA can affect entire tissues and organs, leading to tissue and organ damage. If affecting the heart, CA can cause arrhythmias, heart failure, and cardiac hypertrophy. When acting on the digestive system, it may result in nausea and vomiting, and high levels of CA can impair gastrointestinal peristalsis, potentially leading to ileus over time. If CA impact the blood system, they can induce erythrocytosis, leukocytosis, and changes in coagulation function. Extracorporeal membrane oxygenation (ECMO) can enhance systemic tissue perfusion by maintaining stable blood flow and oxygenation, reducing tissue hypoxia and organ function damage caused by cardiopulmonary failure, and providing critical life support for patients with severely impaired cardiopulmonary function, thereby extending the treatment window[4]. Intra-aortic balloon counterpulsation (IABP) can increase coronary artery perfusion pressure during diastole, enhancing myocardial blood supply and effectively alleviating myocardial ischemia. During systole, it reduces the after-load of left ventricular ejection, facilitating easier blood ejection by the heart, reducing cardiac burden, and aiding in cardiac function recovery[5]. Given the rarity, diverse symptoms, and the harmful potential of PHEO, along with the therapeutic benefits of ECMO and IABP, we discuss a series of treatments combining ECMO and IABP for managing PHEO.
A 40-year-old woman. The patient suffered from intermittent chest tightness and shortness of breath for more than 10 months, which worsened for 6 hours. She was admitted to the emergency department and critical care department on April 11, 2024.
Since June 2023, the patient has experienced chest tightness and shortness of breath during sleep, which are accompanied by palpitations, difficulty breathing, and headaches, without an apparent cause. Symptoms improved when she was in a semi-recumbent position. No significant cough or edema was noted. She was treated at our hospital with traditional Chinese medicine and showed improvement after two weeks. On April 9, 2024, her shortness of breath increased during physical exertion, such as climbing stairs. On April 11, symptoms of chest tightness, palpitations, shortness of breath, sweating, chills, coughing, vomiting, and abdominal cramps reappeared, accompanied by the expulsion of pink mucus.
In 2018, she was diagnosed with subacute thyroiditis and treated with medication, but did not continue with regular follow-ups.
The patient has no relevant family history.
The patient had no enlargement of superficial lymph nodes, no jaundice, and pupils were bilaterally sized at 2 mm with a weak light reflex. Auscultation revealed coarse respiratory sounds in both lungs and scattered wet rales, but no lower limb edema or pathological reflexes were observed.
Before treatment, the following results were obtained from peripheral blood samples: Leukocyte count at 33.8 × 109/L, neutrophils at 79.8%, serum potassium at 2.75 mmol/L, D-dimer > 30 mg/L, high-sensitivity troponin I at 0.281 μg/L, N-terminal pro-B-type natriuretic peptide at 302 pg/mL, lactate at 10.1 mmol/L, blood pH at 7.316, and oxygenation at 192 mmHg (Table 1).
| Laboratory tests | Time | ||||||
| April 11 | April 12 | April 15 | April 18 | April 21 | April 24 | April 25 | |
| Blood | |||||||
| WBC (109/L) | 33.8 | 24.9 | 16.1 | 11.9 | 31.8 | 12.7 | 20.1 |
| Lymph (%) | 17 | 4.4 | 12.4 | 17.4 | 6.6 | 7.1 | 3.6 |
| Neut (%) | 79.8 | 93 | 81.1 | 72 | 89.6 | 88.6 | 93.5 |
| Hb (g/L) | 163 | 137 | 106 | 73 | 71 | 73 | 81 |
| Plt (109/L) | 198 | 102 | 58 | 65 | 120 | 203 | 250 |
| C-reactive protein | < 0.2 | 78.2 | 51.8 | 28.5 | 37.9 | 55.9 | 63.3 |
| PCT (ng/mL) | 0.03 | 3.949 | 1.268 | 0.585 | 0.578 | - | - |
| K (mmol/L) | 2.75 | 3.24 | 3.9 | 4.62 | 3.71 | 4.17 | 3.95 |
| Na (mmol/L) | 135.7 | 145.5 | 146.7 | 144.5 | 144.3 | 144.6 | 135.6 |
| Creatinine (μmol/L) | 97 | 78 | 65 | 60 | 45 | 43 | 42 |
| GFR (mL/minute) | 63 | 82 | 102 | 109 | 120 | 122 | 123 |
| D-D (mg/L) | > 30 | 6.06 | 13.83 | > 30 | 12.97 | 10.44 | 12.12 |
| APTT (second) | 23.6 | 60.6 | 48.2 | 35.9 | 38 | 21.9 | 24.7 |
| Blood gas analysis | |||||||
| PH | 7.326 | 7.385 | 7.513 | 7.399 | 7.441 | 7.432 | 7.449 |
| Lactic acid (mmoL/L) | 10.1 | 3.1 | 1.3 | 1.1 | 1 | 0.8 | 0.6 |
| HCO3- (mmoL/L) | 17.1 | 23 | 37.7 | 27.5 | 23.6 | 24.8 | 25.1 |
| P/F (mmHg) | 192 | 328 | 175 | 429 | 475 | 501 | 474 |
| Myocardial enzyme | |||||||
| CK (U/L) | 152 | 308 | 1255 | 2423 | 1534 | 716 | 357 |
| CK-MB (ng/mL) | 4.22 | 22.50 | 20.94 | 25.95 | 26.56 | 12.09 | 9.98 |
| CTnI (μg/L) | 0.281 | 4.287 | 1.119 | 0.73 | 0.614 | - | - |
| NT-proBNP (pg/mL) | 302 | 5665 | 7240 | 6566 | 5717 | - | - |
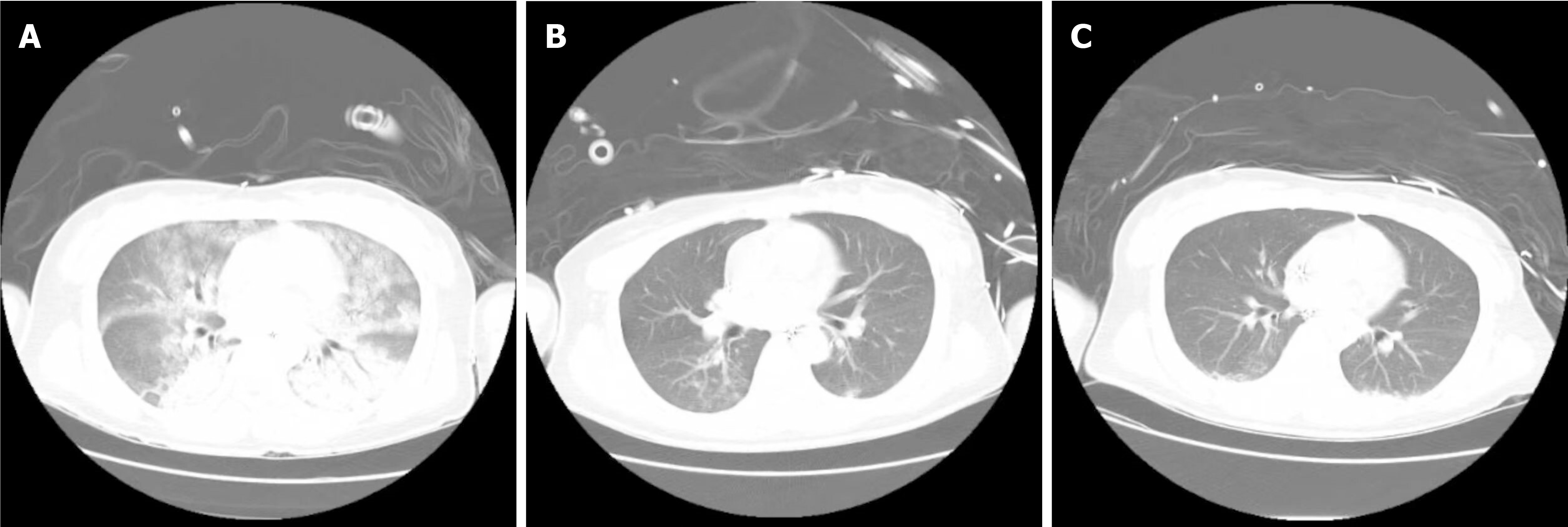
The patient’s whole abdomen computed tomography (CT) and chest CT are shown in Figures 1 and 2. Chest CT showed exudative changes in both lungs, suggestive of pulmonary edema (Figure 2A).
The patient was diagnosed with: (1) Decompensated acute heart failure; (2) Pulmonary edema; (3) Acute respiratory failure; (4) Hypokalemia; and (5) Abnormal coagulation function to be investigated.
Upon admission, the patient was irritable, sweating, and experiencing frequent nausea and vomiting. Her oxygenation level was initially 192 mmHg but decreased progressively. Tracheal intubation and mechanical ventilation were administered to improve oxygenation and reduce oxygen consumption. The patient’s blood pressure and heart rate were highly variable. Esmolol was used to manage heart rate, while NE, epinephrine (E), and pituitrin were administered alternately to maintain blood pressure. Levosimendan was used for cardiac support. Bedside ultrasound revealed left ventricular enlargement and diffuse weakening of left ventricular contraction, with an ejection fraction (EF) of 40% (Table 2). Despite high-dose vasoactive drugs, the patient’s circulation and oxygenation continued to worsen. The lowest recorded oxygenation was 128 mmHg, blood pressure was 40/20 mmHg, and heart rate ranged from 40-50 beats/minute (Table 3). Given the presence of cardiogenic shock and no significant contraindications, venous-arterial (VA)-ECMO was initiated after discussing with the family, with a rotational speed set at 2000 rpm and a flow rate of 2 L/minute. With VA-ECMO support, the lowest oxygenation improved slightly to 94 mmHg, though circulation remained unstable and blood lactate levels increased. Ultrasound evaluation showed no significant stenosis or plaque in the femoral artery. An IABP catheter was placed, and a bedside chest radiograph confirmed the tip position at the second intercostal space of the left anterior rib at a depth of 50 cm, with settings adjusted to the autonomous electrocardiogram mode for triggering. Heparin infusion via micropump was used for anticoagulation. The following day, oxygenation fluctuated between 300-500 mmHg and lactic acid levels decreased (Table 1). During treatment, the patient developed coagulation dysfunction; activated partial thromboplastin time (APTT) reached 120.4 seconds at admission, indicating a high risk of bleeding. Successive infusions of fresh frozen plasma were administered on April 12 and April 13, with a retested APTT of 39.6 seconds on April 14 (Table 1).
| Time | LVEF | Left ventricular outflow tract V-max (m/second) |
| First day | 40%-50% | - |
| Third day | 50% | - |
| Before withdrawing ECMO | 56%-62% | 1.7-1.9 |
| After withdrawing ECMO | 55%-60% | 2 |
| Before withdrawing IABP | 65% | 1.9 |
| After withdrawing IABP | 62% | 1.8 |
| Hemodynamics | April 12 | April 13 | April 14 | April 15 | April 16 | April 17 | April 18 | April 19 | April 20 | April 21 |
| NIBP (mmHg) | 152/87 | 104/61 | 115/63 | 72/62 | 181/93 | 170/89 | 158/122 | 130/82 | 134/82 | 121/70 |
| NIBPm (mmHg) | 109 | 77 | 80 | 65 | 138 | 116 | 146 | 99 | 101 | 87 |
| CVP | 11.7 | 6.8 | 6.5 | 7.3 | 6.8 | 7.0 | 12.9 | 12.2 | 5.2 | 12.8 |
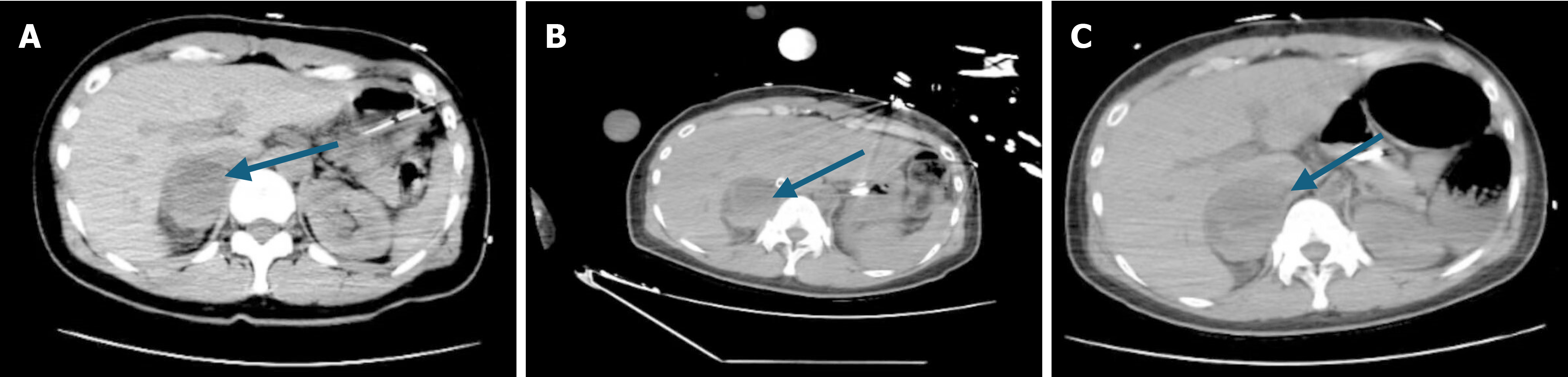
Upon admission, a whole abdominal CT scan (Figure 1A) revealed a mixed-density lesion, 47 mm in diameter, in the right adrenal gland. B-ultrasonography of the adrenal gland identified a mixed echoic mass with clear boundaries, a regular shape, uneven internal echoes, and anechoic areas, consistent with adrenal PHEO. Given the patient’s symptoms of pulmonary edema and acute heart failure, along with sudden intermittent fluctuations in heart rate and blood pressure, these findings aligned with the clinical manifestations of PHEO. Multidisciplinary consultations facilitated additional testing, including blood and urinary CA, 24-hour urinary cortisol, parathyroid hormone, and aldosterone levels (Table 4). The diagnosis of PHEO was confirmed on April 14, leading to an adjustment in therapy to include E and the alpha-receptor blocker phentolamine mesylate to manage the PHEO crisis (PCC).
| Laboratory tests | Time | |||
| The first time | The second time | The third time | ||
| Blood | 3-MT (pg/mL) | 144.1 | 23.9 | < 12 |
| DA (pg/mL) | 442.3 | < 18 | 23.4 | |
| NE (pg/mL) | 3697.6 | 1712.6 | 1242.9 | |
| E (pg/mL) | > 2000 | > 2000 | > 2000 | |
| NMN (pg/mL) | > 2000 | > 2000 | 424.6 | |
| MN (pg/mL) | > 1000 | > 1000 | > 1000 | |
| Urine | NMN (μg/24 hours) | - | 2027.9 | 1107.2 |
| MN (μg/24 hours) | - | > 2504 | 3842 | |
| DA (μg/24 hours) | - | 129.9 | 181.7 | |
| E (μg/24 hours) | - | > 250.4 | > 420 | |
| NE (μg/24 hours) | - | 3194.8 | 239.9 | |
| HVA (mg/24 hours) | - | 5.3 | 9.5 | |
| VMA/24 hours (mg/24 hours) | - | 42.88 | 22.58 | |
| 3-MT (μg/24 hours) | - | 144.9 | 301.3 | |
| PTH (Pmol/mL) | 58.410 | |||
| Blood cortisol (nmol/mL) | 8 am | 619.91 | ||
| 12 am | 687.53 | |||
| ACTH (pg/mL) | 8 am | 11.4 | ||
| 12 am | 18.52 | |||
| 24 hours urine cortisol (μg/24 hours) | 7857.56 | |||
| Aldosterone (pg/mL) | 174.44 | |||
Subsequent complications included recurrent fever and elevated inflammatory markers such as C-reactive protein and procalcitonin. A chest CT confirmed pulmonary edema indicative of heart failure, and a marked left shift in the patient’s leukocyte count suggested an infection (Figure 2A). Given the patient’s recent vomiting, aspiration pneumonia was considered. An anti-infection regimen with piperacillin sodium sulbactam sodium 4.5 g every 8 hours was administered from April 13 to April 18. A follow-up chest CT showed reduced inflammation (Figure 2B). Sputum culture on April 19 identified acinetobacter baumannii, with sensitivity testing indicating susceptibility to meropenem. Accordingly, the antibiotic was switched to meropenem 1 g every 8 hours to manage the infection, with ongoing monitoring of the patient’s temperature and inflammatory markers (Table 1).
On April 19, heart function assessments showed: Tricuspid annular plane systolic excursion at 22 cm/second, tricuspid annular plane systolic excursion at 9 cm/second, and EF at 55%-60% (Table 2), indicating normal cardiac structure and function. Despite hemodynamic instability linked to PCC, testing with an in-bed line pump suggested potential withdrawal from ECMO. On April 20, procedures including right iliac bone arteriography, right femoral artery balloon dilation, ECMO discontinuation, and right iliofemoral artery stent endovascular repair were successfully performed in the digital subtraction angiography room in collaboration with the vascular surgery department. Post-operation, the patient developed hyperthermia, likely due to bacteremia, leading to empirical coverage for gram-positive bacteria and administration of vancomycin, given normal creatinine levels. CT scans of the chest prior to the operation showed persistent inflammation (Figure 2C). By April 23, cardiopulmonary function had improved; heart systole and diastole were satisfactory, with an EF of approximately 60%. Arteriovenous blood flow in the left lower limb was good, though the upper segment of the superficial femoral artery in the right lower limb was narrow. Vital signs stabilized, and IABP was removed after a comprehensive evaluation. Post ECMO and IABP withdrawal, management included continued use of esmolol to control heart rate, phentolamine to counteract catecholamine secretion, and a combination of vancomycin and meropenem for infection control, along with heparin for anticoagulation and diuretics to reduce cardiac load. Offline training began on April 23, consisting of 2 hours per day, achieving retested oxygenation of 400 mmHg without symptoms of shortness of breath or sweating. Tracheal intubation was removed on April 24 Throughout the treatment, regular follow-ups with total abdominal CT were conducted to monitor the size of the adrenal PHEO (Figure 1B and C).
On April 25, she was transferred to a general ward and subsequently underwent surgical treatment at an external hospital after her condition stabilized.
In this case, the initial symptoms were chest tightness and shortness of breath, characterized by a sudden onset and rapid progression, with the abrupt appearance of pink frothy sputum, distinct rales, and audible lung sounds. Myocardial injury markers increased significantly, and a chest CT scan indicated pulmonary edema, suggestive of heart failure and respiratory failure. Treatment included cardiotonics, diuretics, and large doses of vasoactive agents, yet circulation remained highly unstable. Timely intervention with VA-ECMO and IABP led to increased oxygenation, decreased blood lactate levels, improved cardiac function, and alleviation of symptoms. During the treatment with VA-ECMO and IABP, active anticoagulation was essential. Given the patient’s prolonged APTT and significant bleeding risk, timely plasma infusions were administered to enhance coagulation. Despite mechanical assistance, fluctuations in blood pressure and heart rate persisted. A whole-abdominal CT scan revealed mixed-density foci, and after multidisciplinary consultation, a diagnosis of PHEO was confirmed. This tumor was releasing substantial amounts of CA, causing dramatic fluctuations in blood pressure and heart rate. The alpha-receptor antagonist phentolamine was effectively used to manage the PCC, with dynamic monitoring of CA levels. Subsequently, the patient’s circulation gradually stabilized, and as cardiac function improved, ECMO and IABP were promptly discontinued to prevent venous thrombosis in the lower extremities, which could impair blood supply. Following admission, the patient experienced recurrent fevers. Given a history of vomiting, aspiration pneumonia was initially suspected. An anti-infective treatment regimen was initiated, with dynamic monitoring of bacterial and fungal cultures from blood, urine, sputum, and catheter samples to identify the pathogen and determine sensitive antibiotics. Vancomycin combined with meropenem was accurately prescribed for infection control, with dynamic monitoring of blood drug concentrations.
This case report discusses the diagnosis and treatment of extremely unstable hemodynamics caused by PHEO. Beyond the symptoms previously described, the diagnosis of PHEO leverages specific biomarkers. Metanephrine and normetanephrine, which are metabolites of NE and E, are produced exclusively in adrenal medulla chromaffin cells or by PHEOs. These metabolites persist at high concentrations, have a longer half-life than CA, and are more stable, making them highly specific and sensitive markers. Vanilmandelic acid is the final metabolite of NE and E, with sensitivity ranging from 46% to 77% and specificity between 86% to 99%. Herpes virus allele, the final degradation product of dopamine, is primarily used in neuroblastoma screening and diagnostics. Detection of 3-methoxytyramine, an intermediate metabolite of dopamine, can enhance the screening of endocrine tumors in the head and neck region. Ultimately, the diagnosis of PHEO relies on 24-hour blood and urine catecholamine testing. Additionally, levels of renin, angiotensin, and aldosterone can assist in the diagnosis. Patients with PHEO often have increased CA secretion and reduced blood volume, which can activate the renin-angiotensin-aldosterone system, leading to elevated aldosterone secretion. Hypokalemia may also be present in some patients, and its detection can aid in assessing patient blood volume.
PCC is characterized by hemodynamic instability. E primarily targets the β1 receptor in the heart, accelerating heart rate, enhancing contractility, increasing cardiac output, and raising blood pressure. NE mainly activates the peripheral vascular α receptors, causing contraction of small arteries and veins, increasing peripheral resistance, and elevating blood pressure. NE’s effect on the β1 receptor is weaker, thus its impact on myocardial contraction and heart rate increase is less pronounced than that of E, and it has minimal effect on the β2 receptor. The pharmacological action of dopamine is dose-dependent; at low doses, it primarily activates the peripheral dopamine D1 receptor, selectively dilating renal, mesenteric, coronary, and cerebral vessels. Moderate doses of dopamine not only activate the dopamine D1 receptor but also stimulate the cardiac β1 receptor, producing a positive inotropic effect. High doses of dopamine can also activate peripheral vascular α receptors, leading to significant vasoconstriction and increased blood pressure. Based on such effects, PCC can be divided into type A crises[1]. When hypertensive crises occur, phentolamine, a short-acting alpha receptor blocker, should be administered promptly to reverse vasoconstriction, alleviate hypertension, and prevent arrhythmias. Nicardipine or magnesium sulfate, both calcium channel blockers, are used as vasodilators to correct hypertension. Urapidil, a piperazine-substituted uracil derivative, reduces peripheral and central vascular tension, thereby lowering blood pressure. In type B PCC cases, volume resuscitation and vasoactive agents such as NE, E, dopamine, and pituitrin should be administered, combined with IABP and ECMO for mechanical circulatory support.
The routine diagnosis and treatment of PHEO[3,6,7] involves preoperative drug preparation followed by surgical intervention. Preoperative drug preparation includes: (1) Control of hypertension: The most commonly used medication is a long-acting non-selective alpha-blocker such as phenoxybenzamine. Alpha-1-blockers like prazosin are also an option, in addition to calcium channel blockers, which can be used alone or in combination; (2) Arrhythmia Control: Beta-blockers are necessary for CA or α-blocker-mediated tachycardia (> 100-120 beats/minute) or for controlling supra
ECMO, an essential extracorporeal life support technology, is primarily utilized for patients with cardiac and respiratory failure who cannot maintain effective circulation through drug therapy alone due to acute or chronic cardiac insufficiency from various causes. The VA-ECMO mode is used for circulatory support. Its working principle involves draining venous blood from the body, reducing the afterload of the right heart, and using a membrane oxygenator to oxygenate the blood and remove carbon dioxide. The blood is then reinfused into the body with a centrifugal pump to facilitate gas exchange and circulation, thereby replacing damaged tissue and correcting internal environment disorders, ensuring adequate tissue perfusion. During ECMO use, Heparin is administered as an essential anticoagulant, while the dosage of positive inotropic drugs is gradually reduced for maintenance. ECMO can lead to several complications, such as bleeding (due to interference with blood clotting by the cardiopulmonary bypass system), thrombosis, infection, and limb ischemia (especially in VA-ECMO mode, where arterial blood transfusion can affect limb blood supply)[8]. ECMO does not address the primary disease of PHEO and should be withdrawn promptly after cardiac function improves. Continued drug therapy is necessary to manage heart failure, reduce infection risks from external tubing, prevent distal limb ischemia from femoral artery intubation, and facilitate conditions for surgical intervention. IABP is a pulsatile pump assist device suitable for acute myocardial infarction complicated by severe heart failure or cardiogenic shock, explosive myocarditis, and similar conditions. The principle of the IABP is to synchronize the inflation and deflation of the intra-aortic balloon with the cardiac cycle to aid circulation. Its hemodynamic effects include inflating the balloon immediately after the aortic valve closes during the early phase of ventricular diastole, which improves diastolic blood pressure and enhances blood perfusion to the brain, coronary arteries, kidneys, and peripheries. At the end of isovolumic systole, the balloon is rapidly deflated at the moment the aortic valve opens, creating a “hole” effect. This reduces cardiac afterload and ventricular wall tension, thereby decreasing myocardial oxygen consumption. After IABP treatment, cardiac output can increase by approximately 0.5-1.0 L/minute. Although IABP is generally safe, it can cause complications such as vascular injury (damage to blood vessels like the aorta may occur during balloon catheter implantation), thrombosis (a clot may form on the balloon’s surface, impacting circulation), and infection (from external devices like catheters)[9]. Overall, the combined benefits of ECMO and IABP can stabilize the preoperative vital signs of patients with PHEO, reduce the time needed for drug preparation, and facilitate earlier surgical intervention. However, prolonged use should be avoided to prevent complications like thrombosis or infection.
The symptoms of PHEO in this patient are atypical, and hemodynamics remain unstable despite the use of vasoactive substances. In such cases, ECMO and IABP should be promptly implemented to stabilize vital signs, ensure adequate tissue blood supply, and provide sufficient time for the diagnosis of PHEO. PHEO s are often complicated by cate
| 1. | Pacak K, Taïeb D. Pheochromocytoma (PHEO) and Paraganglioma (PGL). Cancers (Basel). 2019;11:1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Eisenhofer G, Pamporaki C, Lenders JWM. Biochemical Assessment of Pheochromocytoma and Paraganglioma. Endocr Rev. 2023;44:862-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 3. | Sbardella E, Grossman AB. Pheochromocytoma: An approach to diagnosis. Best Pract Res Clin Endocrinol Metab. 2020;34:101346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Hadaya J, Benharash P. Extracorporeal Membrane Oxygenation. JAMA. 2020;323:2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | White A, Fan E. What is ECMO? Am J Respir Crit Care Med. 2016;193:P9-P10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Parissis H, Graham V, Lampridis S, Lau M, Hooks G, Mhandu PC. IABP: history-evolution-pathophysiology-indications: what we need to know. J Cardiothorac Surg. 2016;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Farrugia FA, Martikos G, Tzanetis P, Charalampopoulos A, Misiakos E, Zavras N, Sotiropoulos D. Pheochromocytoma, diagnosis and treatment: Review of the literature. Endocr Regul. 2017;51:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Wrisinger WC, Thompson SL. Basics of Extracorporeal Membrane Oxygenation. Surg Clin North Am. 2022;102:23-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |