Published online May 26, 2025. doi: 10.12998/wjcc.v13.i15.101272
Revised: November 3, 2024
Accepted: January 7, 2025
Published online: May 26, 2025
Processing time: 133 Days and 17.5 Hours
Hypertrophic cardiomyopathy (HCM) is one of the most prevalent inherited myocardial disorders and is characterized by considerable genetic and phenotypic heterogeneity. A subset of patients with HCM progress to a dilated phase of HCM (DPHCM), which is associated with a poor prognosis; however, the underlying pathogenesis remains inadequately understood.
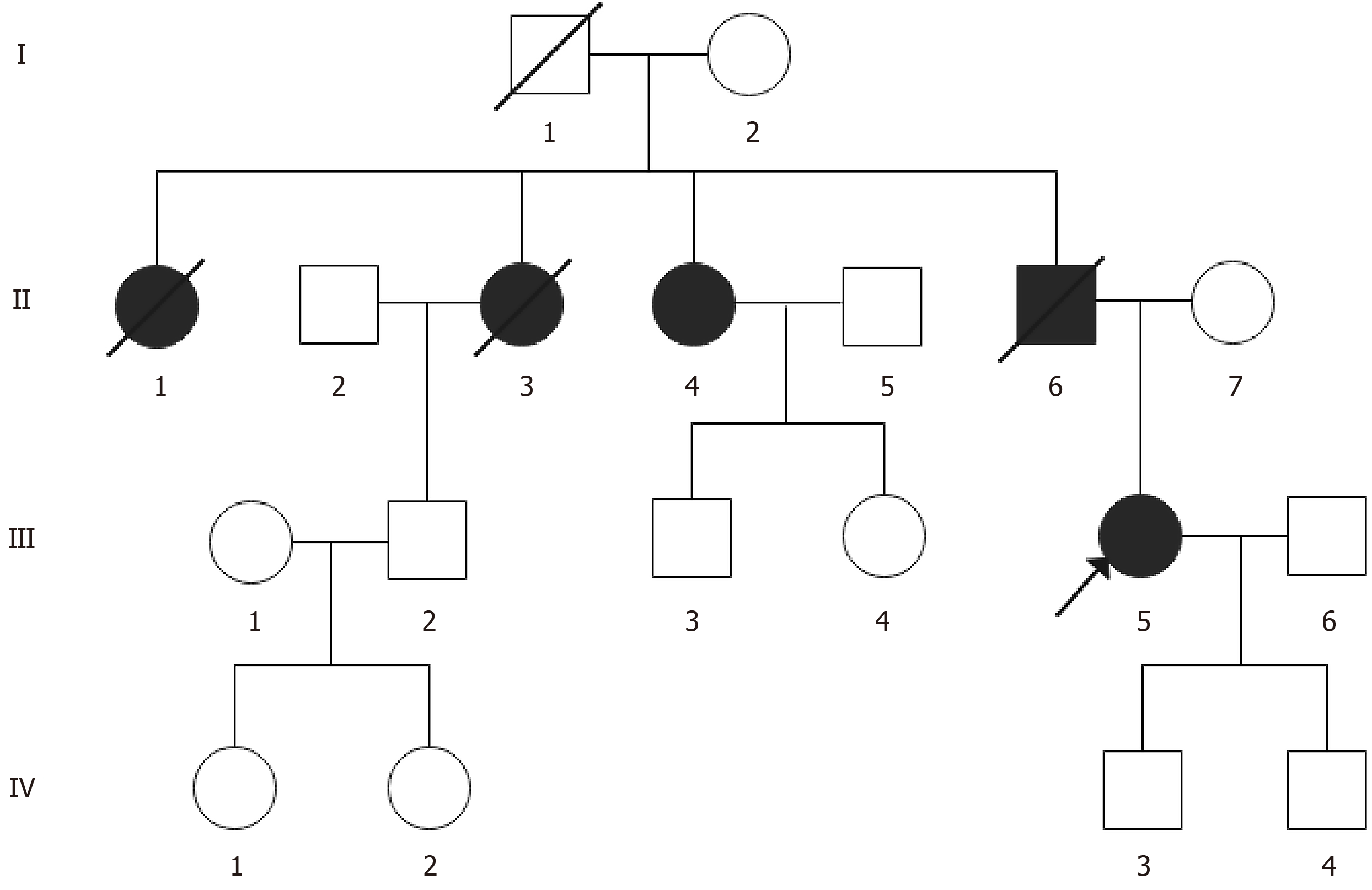
In this study, we present a case involving a pedigree with familial DPHCM and conduct a retrospective review of patients with DPHCM with identified gene mutations. Through panel sequencing targeting the coding regions of 312 genes associated with inherited cardiomyopathy, a heterozygous missense mutation (c.746G>A, p.Arg249Glu) in the MYH7 gene was identified in the proband (III-5). Sanger sequencing subsequently confirmed this pathogenic mutation in three additional family members (II-4, III-4, and IV-3). A total of 26 well-documented patients with DPHCM were identified in the literature. Patients with DPHCM are commonly middle-aged and male. The mean age of patients with DPHCM was 53.43 ± 12.79 years. Heart failure, dyspnoea, and atrial fibrillation were the most prevalent symptoms observed, accompanied by an average left ventricular end-diastolic size of 58.62 mm.
Our findings corroborate the pathogenicity of the MYH7 (c.746G>A, p.Arg249Glu) mutation for DPHCM and suggest that the Arg249Gln mutation may be responsible for high mortality.
Core Tip: A subset of patients with hypertrophic cardiomyopathy (HCM) progress to a dilated phase of HCM (DPHCM), which is associated with a poor prognosis. Our findings corroborate the pathogenicity of the MYH7 (c.746G>A, p.Arg
- Citation: Hong Y, Fan Z, Guo Y, Ma HH, Zeng SZ, Xi HT, Yang J, Luo K, Luo R, Li XP. MYH7 mutation in a pedigree with familial dilated hypertrophic cardiomyopathy: A case report and review of literature. World J Clin Cases 2025; 13(15): 101272
- URL: https://www.wjgnet.com/2307-8960/full/v13/i15/101272.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i15.101272
The incidence of hypertrophic cardiomyopathy (HCM) varies widely around the world, ranging from 0.02% to 0.2% in Western countries and Asian countries[1]. HCM is a primary myocardial disease characterized by left ventricle (LV) and asymmetric septal hypertrophy[2]. In most patients with HCM, the systolic function is normal, and most patients are asymptomatic for a long period of time[3]. However, a subset of patients with HCM may experience a lifelong process of left ventricular remodelling and progressive dysfunction. This stage, referred to as the dilated phase of HCM (DPHCM), has been associated with a poor prognosis[4,5]. Currently, due to unknown disease mechanisms, the clinical remedies for HCM and DPHCM are limited and serve to relieve symptoms. HCM is caused by mutations in sarcomere genes, with MYH7, which encodes the β myosin heavy chain, being one of the most common pathogenic genes. Approximately 40% of patients with HCM exhibit mutations in the MYH7 gene[6].
The patient (III-5) was a 31-year-old female with more than a 26-year history of fatigue after physical activity.
She was diagnosed with HCM at the age of 26 in 2018. Patient complained of more than a 26-year history of fatigue after physical activity. At that time, a general physical examination revealed no abnormalities and no evidence of cardiac dysfunction.
There is a family history of HCM and sudden death (Figure 1). The patient’s grandfather (I-1) and aunt (II-1) died suddenly at the ages of 18 and 40, respectively. The patient reported that another aunt (II-3) was definitively diagnosed with DPHCM by a local hospital and suffered from SCD at the age of 50. The patient’s youngest aunt (II-4) was also diagnosed with DPHCM and presented with symptoms of fatigue after activity and occasional oedema in her lower extremities. Laboratory data revealed elevated levels of high-sensitivity troponin I (hsTnI) (15.8 ng/L), creatine kinase MB (5.78 ng/L), and B-type natriuretic peptide (BNP) (462.5 pg/mL). Electrocardiographic findings included a widened P wave in the limb leads and an increased P wave terminal force in lead V1. Echocardiographic assessment revealed a markedly enlarged left atrium (45 mm), thickened ventricular septum (14 mm), and significantly reduced left ventricular ejection fraction (LVEF) (45%). The proband's father (II-6) suffered from DPHCM characterized by fatigue and dyspnoea, along with dilation in the LV diameter (71 mm) and a decreased LVEF (38%). He died out of the hospital at the age of 43 with a diagnosis of cardiac dysfunction. The patient’s 9-year-old cousin (III-4) and 8-year-old son (IV-3) currently do not exhibit any clinical symptoms and have normal electrocardiogram (ECG) results, possibly due to their young ages.
She is an outpatient and no obvious positive signs were found during the visit.
Laboratory tests revealed mildly elevated levels of hsTnI: 75.8 ng/L and BNP: 209.4 pg/mL).
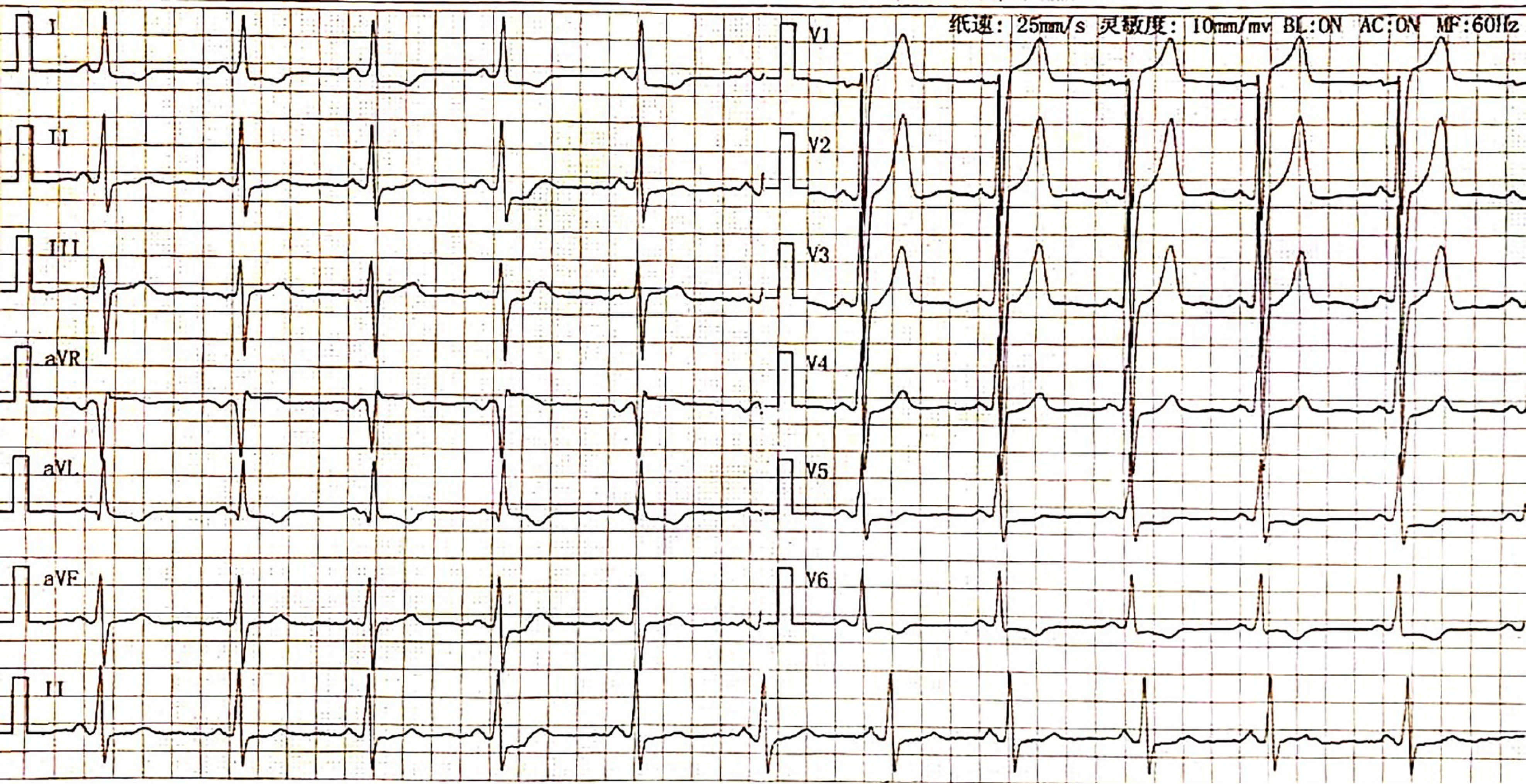
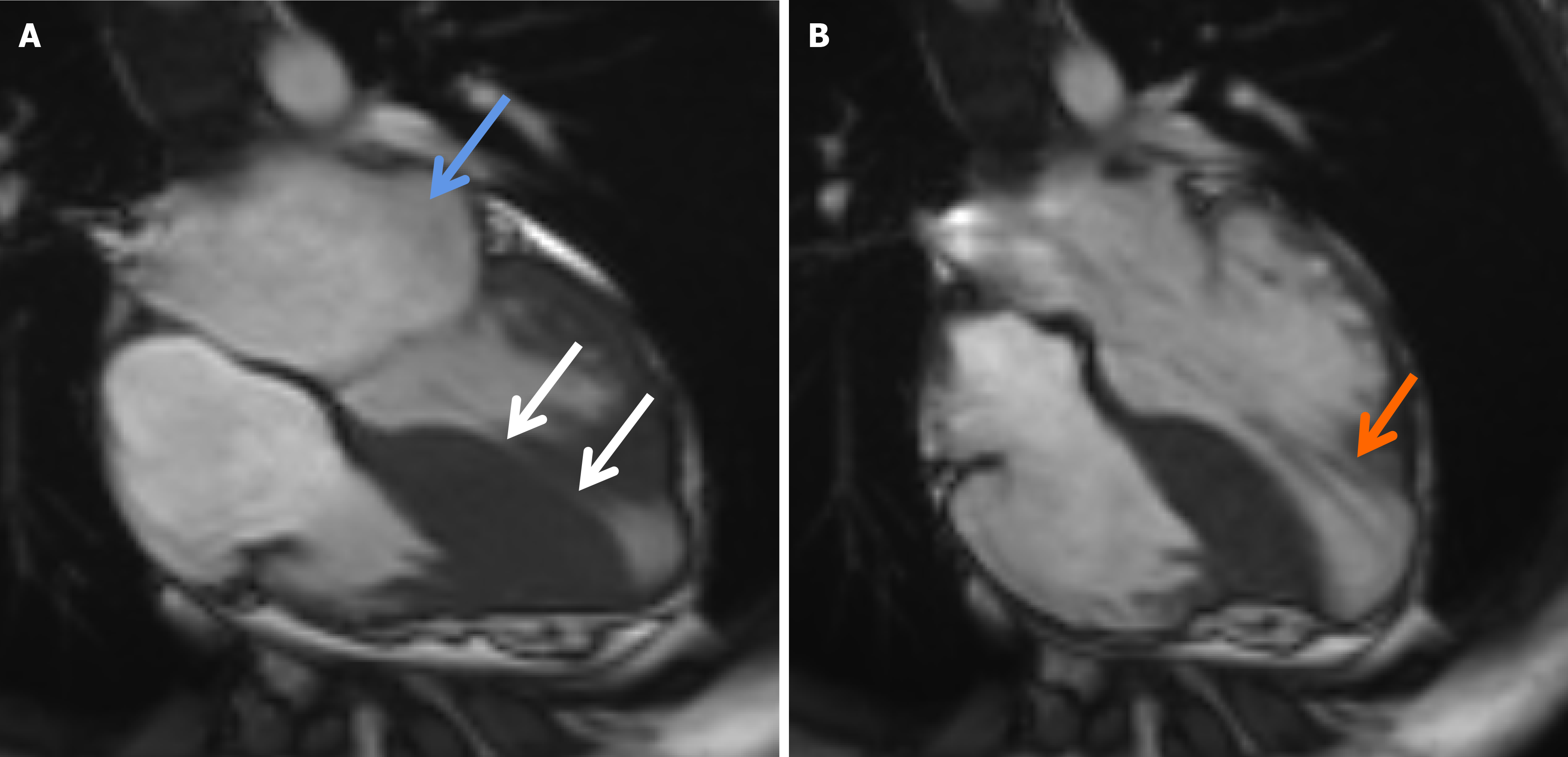
The ECG indicated a depressed S-T segment and an inverted T wave in leads I, aVL, and V6 (Figure 2). Echocardiographic assessment revealed asymmetric thickening of the wall of the LV, with a thickened ventricular septum (21 mm), a normal LV diameter (37 mm), and a LVEF of 72% (Figure 3A). Five years later, echocardiography revealed an increase in the LV diameter from 37 mm to 43 mm accompanied by a reduction in the thickness of the ventricular septum from 21 mm to 18 mm (Figure 3B). Additionally, cardiac magnetic resonance imaging (CMR) revealed thinning at the base of the ventricular septum and the LV free wall, with a measured thickness of approximately 3.4 mm at end-diastole. The LVEF decreased to 63%. Furthermore, an apical ventricular aneurysm measuring approximately 24.6 mm × 29.8 mm was observed. Late gadolinium enhancement on CMR demonstrated characteristic patchy enhancement of the ventricular septum (Figure 4).
The diagnostic criteria for DPHCM are based on previous literature reports[7,8]. DPHCM is defined as left ventricular systolic dysfunction LVEF < 50%, accompanied by one of the following conditions: (1) Left ventricular hypertrophy unexplained by other factors; (2) Left ventricular hypertrophy documented on a previous echocardiogram, unexplained by other factors; or (3) A confirmed diagnosis of familial HCM (with at least one relative having been diagnosed). Based on a family history of HCM, clinical manifestations, dynamic changes in echocardiography (Table 1), and CMR, the final diagnosis was DPHCM.
Considering that the patient currently has no significant hemodynamic changes, beta blockers were used for treatment, and clinical observation and follow-up were conducted.
The patient's fatigue has improved after daily activities and requires regular outpatient follow-up.
A comprehensive literature review was conducted utilizing the PubMed and Medline databases. All English publications from January 1997 to February 2023 documenting cases of DPHCM were evaluated. A total of 35 articles were initially selected for review. Following the removal of duplicates and noncompliant cases, 22 articles containing recorded cases of DPHCM were ultimately identified for analysis[9-30]. In this study, we reviewed 26 patients diagnosed with DPHCM, as reported in 22 articles (Table 2). Among all patients diagnosed with DPHCM, the number of male patients was approximately two times greater than the number of female patients. The mean age was 53.43 ± 12.79 years, ranging from 10 to 67 years. The most common clinical manifestations included heart failure, dyspnoea, and atrial fibrillation (AF). Some patients exhibit sustained ventricular tachycardia (VT), nonsustained VT, mitral regurgitation, chest pain, or aneurysmal changes, among other symptoms. Of the 22 articles, 15 provided data regarding LV dilation size, reporting an average LV end-diastolic dimension of 58.62 mm.
| Ref. | Sex | Age of onset or diagnosis (year) | Size of LV (mm) | Clinical symptoms or signs | Number of cases |
| Morimoto et al[9] | Male | 30 | No available | AF, dyspnea, heart failure | 1 |
| Takemura et al[10] | Female | 55 | No available | Heart failure, paroxysmal AF | 1 |
| Kuno et al[11] | Male | 40 | 54 | Appetite loss, hearing loss | 1 |
| Kitahara et al[12] | Female | 19 | 63 | No obvious symptoms | 1 |
| Higashi et al[13] | Female | 53 | No available | Chest pain, dyspnea, and MR | 1 |
| Miyoshi et al[14] | Male | 55 | No available | Sustained VT, chronic AF | 1 |
| Kolekar et al[15] | Male | 61 | No available | Congestive heart failure, stroke, scar epilepsy, AF and VT | 1 |
| Kitamura et al[16] | Male | 56 | 65 | Congestive heart failure and sustained VT | 1 |
| Sato et al[17] | Female | 27 | 56 | Dyspnea, heart failure | 1 |
| Tanaka et al[18] | Female | 53 | No available | Heart failure | 1 |
| Sato et al[19] | Female | 43 | 69.7 | VT, severe heart Failure, shortness of breath and dyspnea, aneurysmal changes from the mid-septal region to the apex and NSVT | 1 |
| Canpolat et al[20] | Male | 10 | 62 | Exertional dyspnea and limited exercise capacity | 1 |
| Ueda et al[21] | Male | Mean age 67 | Mean 57 | Heart failure, sustained monomorphic VT | 5 |
| Fukuzawa et al[22] | Male | 48 | 55 | Dyspnea, palpitation, atrial flutter, NSVT, diastolic dysfunction | 1 |
| Matsushita et al[23] | Female | 16 | No available | Severe mid-ventricular obstruction | 1 |
| Ueno et al[24] | Male | 57 | 68 | Heart failure, MR | 1 |
| Matsuo et al[25] | Male | 57 | 60 | Dyspnea and NYHA functional class III heart failure | 1 |
| Benezet-Mazuecos et al[26] | Male | 48 | 59 | Congestive heart failure, LBBB | 1 |
| Kawai et al[27] | Male | 38 | 52 | AF, NSVT, premature ventricular contractions | 1 |
| Suzuki et al[28] | Male | 60 | 44 | AF, tricuspid regurgitation | 1 |
| Matsuda et al[29] | Male | 47 | 69 | Congestive heart failure | 1 |
| Akazawa et al[30] | Female | 38 | 56 | Fatigue and dyspnoea on exertion, heart failure | 1 |
HCM is an autosomal-dominant cardiomyopathy. This disease is typically undiagnosed or is diagnosed at a late stage. Up to 5% of patients with HCM experience progressive myocardial remodelling and systolic dysfunction, culminating in a dramatic final stage known as the DPHCM. A recent study by Marstrand et al[5] estimated that 7.5% of individuals with HCM will progress to DPHCM within 15 years. During this phase, DPHCM demonstrates the most fundamental pathological characteristics of HCM, including myocardial cell elongation, disarray, and luminal stenosis. Research indicates that patients with DPHCM experience poor long-term survival, and AF, SCD, NYHA classification of III-IV, and an LVEF < 50% are identified as independent risk factors impacting individual survival status[31,32]. In this study, we identified a mutation in the MYH7 gene within a pedigree affected by DPHCM. This mutation is believed to contribute to the high mortality in this family.
HCM is primarily attributed to various mutated or dysregulated genes that are responsible for the synthesis of the myostatin protein in cardiac tissue. Previous research has identified more than 1400 mutations across 11 or more genes encoding proteins of the cardiac sarcomere. Among these, the most prevalent mutations are those in MYH7, MYBPC3), TNNT2, TNNI3, and TPM1[33,34]. Through panel sequencing, a mutation in the MYH7 gene, potentially responsible for HCM, was identified in this study. The MYH7 gene encodes the β-cardiac myosin heavy chain, is located on the long arm of chromosome 14, and comprises 40 exons. The Human Gene Mutation Database (http://www.hgmd.cf.ac.uk) has documented more than 350 pathogenic variants in the MYH7 gene. Mutations in the MYH7 gene are associated with a more malignant phenotype characterized by early onset, high penetrance, increased left ventricular hypertrophy, a high incidence of SCD, and poor prognosis[35,36].
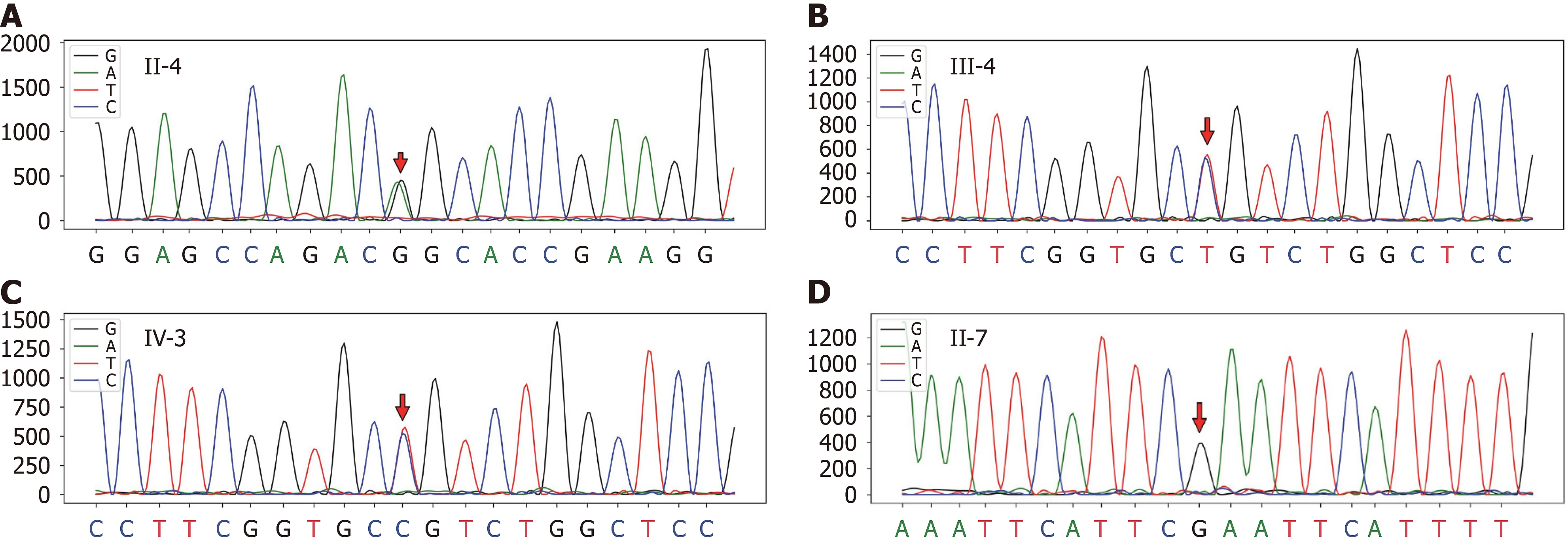
Some patients with HCM develop systolic dysfunction termed DPHCM, which is associated with increased morbidity and mortality. Previous studies characterized DPHCM as an LV ejection fraction < 50% at rest (reflecting global systolic dysfunction) at study entry or during follow-up using two-dimensional echocardiography[37]. In the present study, the proband exhibited a progressive increase in LV size, a reduction in the LV ejection fraction, the formation of an LV apical aneurysm (Table 1), and a history of familial HCM, all of which are consistent with the diagnostic criteria for DPHCM. The proband's aunt and father also presented characteristics consistent with DPHCM. Furthermore, panel sequencing revealed missense mutations (c.746G>A, p.Arg249Glu) in the proband. Sanger sequencing subsequently validated this mutation in three other family members (II-4, III-4, and IV-3). Among them, the sample sequences of the two family members (III-4, IV-3) presented G/A base heterozygosity at the target mutation position of the MYH7 gene (displayed in reverse as C/T base heterozygosity) (Figure 5). A specific mutation (c.746G>A, p.Arg249Gln) in MYH7 has been documented in individuals with HCM in at least 15 studies (https://www.ncbi.nlm.nih.gov/clinvar/variation/14088/). These studies generally suggest that the Arg249Gln mutation is a malignant missense mutation of the MYH7 gene that is characterized clinically by early onset, a family history of SCD, and poor prognosis[4,38,39]. However, there is also evidence that the Arg249Gln mutation in MYH7 does not invariably predict a poor prognosis. Posen et al[40] and Woo et al[41] reported that patients with HCM patients who have the Arg249Gln mutation did not have significantly increased LV wall thickness and had no occurrences of SCD. Furthermore, Watkins et al[38] reported that the life expectancy of patients harbouring the Arg249Gln mutation was significantly longer than that of patients with other mutations in MYH7. Our study reports a pedigree with an average onset age of 38 years and a significant incidence of SCD, with 4 out of 19 individuals affected (21%). To our knowledge, this is the first time that a mutation (c.746G>A, p.Arg249Gln) in MYH7 has been reported to cause DPHCM, which differs from previously reported phenotypes of the same mutation, possibly due to race, the incorporation of common or rare variants of other genes, and epigenetic factors.
Previous studies have reported that the incidence of DPHCM ranges from 3.5% to 5.7%[4]. On average, it takes approximately 14 years from the onset of HCM to the diagnosis of DPHCM, with 66% of patients experiencing progression to death due to progressive heart failure, SCD, or required transplantation within an average period of 2.7 years[42]. Predictive factors for DPHCM include a younger age at the initial visit; a family history of HCM, DPHCM, or SCD; and greater myocardial wall thickness[4,37]. Compared with patients with DCM, those with DPHCM presented more pronounced symptoms, were more likely to be male, and presented higher incidences of prior stroke, AF, and VT/fibrillation. Furthermore, the risk of mortality was also elevated in this cohort[43,44]. Our study demonstrated that a younger age, the presence of heart failure and dyspnoea, AF, VT, chest pain, and aneurysmal changes are significantly associated with the progression of DPHCM.
Patients with HCM may progress to the dilated phase, referred to as DPHCM, which is associated with increased morbidity and mortality. However, the mechanisms underlying the transition from HCM to DPHCM remain poorly understood. In this study, we identified an MYH7 (c.746G>A, p.Arg249Glu) mutation in a pedigree with DPHCM, which may be associated with the pathogenesis and high mortality of DPHCM.
| 1. | Bai Y, Zheng JP, Lu F, Zhang XL, Sun CP, Guo WH, Zou YX, Lip GYH, Shi XB. Prevalence, incidence and mortality of hypertrophic cardiomyopathy based on a population cohort of 21.9 million in China. Sci Rep. 2022;12:18799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (33)] |
| 2. | Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail. 2012;5:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Biagini E, Coccolo F, Ferlito M, Perugini E, Rocchi G, Bacchi-Reggiani L, Lofiego C, Boriani G, Prandstraller D, Picchio FM, Branzi A, Rapezzi C. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol. 2005;46:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Marstrand P, Han L, Day SM, Olivotto I, Ashley EA, Michels M, Pereira AC, Wittekind SG, Helms A, Saberi S, Jacoby D, Ware JS, Colan SD, Semsarian C, Ingles J, Lakdawala NK, Ho CY; SHaRe Investigators. Hypertrophic Cardiomyopathy With Left Ventricular Systolic Dysfunction: Insights From the SHaRe Registry. Circulation. 2020;141:1371-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (33)] |
| 6. | Wang B, Wang J, Wang LF, Yang F, Xu L, Li WX, He Y, Zuo L, Yang QL, Shao H, Hu D, Liu LW. Genetic analysis of monoallelic double MYH7 mutations responsible for familial hypertrophic cardiomyopathy. Mol Med Rep. 2019;20:5229-5238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (33)] |
| 7. | Kitaoka H, Tsutsui H, Kubo T, Ide T, Chikamori T, Fukuda K, Fujino N, Higo T, Isobe M, Kamiya C, Kato S, Kihara Y, Kinugawa K, Kinugawa S, Kogaki S, Komuro I, Hagiwara N, Ono M, Maekawa Y, Makita S, Matsui Y, Matsushima S, Sakata Y, Sawa Y, Shimizu W, Teraoka K, Tsuchihashi-Makaya M, Ishibashi-Ueda H, Watanabe M, Yoshimura M, Fukusima A, Hida S, Hikoso S, Imamura T, Ishida H, Kawai M, Kitagawa T, Kohno T, Kurisu S, Nagata Y, Nakamura M, Morita H, Takano H, Shiga T, Takei Y, Yuasa S, Yamamoto T, Watanabe T, Akasaka T, Doi Y, Kimura T, Kitakaze M, Kosuge M, Takayama M, Tomoike H; Japanese Circulation Society Joint Working Group. JCS/JHFS 2018 Guideline on the Diagnosis and Treatment of Cardiomyopathies. Circ J. 2021;85:1590-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (33)] |
| 8. | Gu M, Jin H, Hua W, Niu HX, Wang J, Ding LG, Xue C, Zhang S. [Clinical features and outcomes of cardiac resynchronization therapy in 16 patients with dilated-phase hypertrophic cardiomyopathy]. Zhongguo Xunhuan Zazhi. 2017;32:461-464. [DOI] [Full Text] |
| 9. | Morimoto R, Ito R, Araki T, Mizutani T, Kimura Y, Kazama S, Oishi H, Kuwayama T, Sugiura Y, Hiraiwa H, Kondo T, Okumura T, Kobayashi K, Mutsuga M, Murohara T. Contractile pericarditis-like hemodynamics in dilated-phase hypertrophic cardiomyopathy with giant atrium. J Cardiol Cases. 2023;27:199-202. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (34)] |
| 10. | Takemura G, Onoue K, Arimoto T, Watanabe T, Tsujimoto A, Takada C, Okada H, Nakano T, Sakaguchi Y, Miyazaki N, Watanabe T, Kanamori H, Ogura S, Saito Y, Fujiwara T, Fujiwara H, Hotta Y. Vacuolated cardiomyocytes in human endomyocardial biopsy specimens. J Cardiol Cases. 2020;21:54-58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (31)] |
| 11. | Kuno T, Imaeda S, Asakawa Y, Nakamura H, Takemura G, Asahara D, Kanamori A, Kabutoya T, Numasawa Y. Mitochondrial Cardiomyopathy Presenting as Dilated Phase of Hypertrophic Cardiomyopathy Diagnosed with Histological and Genetic Analyses. Case Rep Cardiol. 2017;2017:9473917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Kitahara H, Nawata K, Kinoshita O, Itoda Y, Shintani Y, Fukayama M, Ono M. Implantation of a Left Ventricular Assist Device for Danon Cardiomyopathy. Ann Thorac Surg. 2017;103:e39-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Higashi H, Nishimura K, Iio C, Watanabe Y, Kono T, Uetani T, Aono J, Nagai T, Inoue K, Suzuki J, Okura T, Higaki J, Ikeda S. Coronary spasm as an exaggerating factor of mitral regurgitation in a patient with dilated-phase hypertrophic cardiomyopathy. Int J Cardiol. 2016;223:410-411. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Miyoshi T, Okayama H, Hiasa G, Kawata Y, Yamada T, Kazatani Y. Contrast-enhanced ultrasound for the evaluation of acute renal infarction. J Med Ultrason (2001). 2016;43:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Kolekar S, Munjewar C, Sharma S. Dabigatran for left ventricular thrombus. Indian Heart J. 2015;67:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Kitamura T, Otsuki M, Yamaoka M, Saitoh Y, Shimomura I. The temporary drop of serum octreotide concentration deteriorated ventricular tachycardia in an acromegalic patient. Endocr J. 2013;60:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Sato T, Seguchi O, Morikawa N, Hieda M, Watanabe T, Sunami H, Murata Y, Yanase M, Hata H, Fujita T, Nakatani T. A heart transplant candidate with severe pulmonary hypertension and extremely high pulmonary vascular resistance. J Artif Organs. 2013;16:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka H, Tatsumi K, Matsumoto K, Kawai H, Hirata K. Emerging role of three-dimensional speckle tracking strain for accurate quantification of left ventricular dyssynchrony. Echocardiography. 2013;30:E292-E295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Sato A, Sakamoto N, Ando K, Kaneshiro T, Uekita H, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Sato M, Tamagawa K, Arimura T, Kimura A, Takeishi Y. Dilated phase of hypertrophic cardiomyopathy caused by two different sarcomere mutations, treated with surgical left ventricular reconstruction and cardiac resynchronization therapy with a defibrillator. Intern Med. 2012;51:2559-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Canpolat U, Şahiner L, Kaya EB, Aytemir K. Melting heart: dilated phase of hypertrophic cardiomyopathy. Anadolu Kardiyol Derg. 2012;12:E36-E37. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ueda A, Fukamizu S, Soejima K, Tejima T, Nishizaki M, Nitta T, Kobayashi Y, Hiraoka M, Sakurada H. Clinical and electrophysiological characteristics in patients with sustained monomorphic reentrant ventricular tachycardia associated with dilated-phase hypertrophic cardiomyopathy. Europace. 2012;14:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Fukuzawa K, Yoshida A, Onishi T, Suzuki A, Kanda G, Takami K, Kumagai H, Torii S, Takami M, Fukuda Y, Kawai H, Hirata K. Dilated phase of hypertrophic cardiomyopathy caused by Fabry disease with atrial flutter and ventricular tachycardia. J Cardiol. 2009;54:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Matsushita T, Kawase T, Tsuda E, Kawazoe K. Apicoaortic conduit for the dilated phase of hypertrophic obstructive cardiomyopathy as an alternative to heart transplantation. Interact Cardiovasc Thorac Surg. 2009;8:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Ueno T, Sakata R, Iguro Y, Yamamoto H, Ueno M, Ueno T. Subvalvular procedure for functional mitral regurgitation in a patient with dilated-phase hypertrophic cardiomyopathy. Gen Thorac Cardiovasc Surg. 2007;55:170-173. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Matsuo S, Sato Y, Nakae I, Masuda D, Matsumoto N, Horie M. Evaluation of cardiac resynchronization therapy in drug-resistant dilated-phase hypertrophic cardiomyopathy by means of Tc-99m sestamibi ECG-gated SPECT. Ann Nucl Med. 2006;20:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Benezet-Mazuecos J, Ibanez B, Farre J. Atypical left bundle branch block in dilative "burned-out" phase of hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2005;28:1357-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Kawai M, Kihara Y, Hasegawa K, Matsumori A, Sasayama S. Dilated phase of hypertrophic cardiomyopathy with mid-ventricular obstruction after 20-year follow-up. Jpn Circ J. 2000;64:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Suzuki K, Hasegawa K, Nohara R, Matsumori A, Sasayama S. A patient with hypertrophic cardiomyopathy accompanied by right ventricular dilation of unknown cause. Jpn Circ J. 1999;63:137-140. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Matsuda H, Fukushima N, Sawa Y, Nishimura M, Matsumiya G, Shirakura R. First brain dead donor heart transplantation under new legislation in Japan. Jpn J Thorac Cardiovasc Surg. 1999;47:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Akazawa H, Kuroda T, Kim S, Mito H, Kojo T, Shimada K. Specific heart muscle disease associated with glycogen storage disease type III: clinical similarity to the dilated phase of hypertrophic cardiomyopathy. Eur Heart J. 1997;18:532-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ding CL. [Characteristics of patients with hypertrophic cardiomyopathy expansion phase of research and living conditions]. Linchuang Xinxueguanbing Zazhi. 2017;33:133-136. [DOI] [Full Text] |
| 32. | Ishihara K, Kubota Y, Matsuda J, Imori Y, Tokita Y, Asai K, Takano H. Predictive Factors for Decreasing Left Ventricular Ejection Fraction and Progression to the Dilated Phase of Hypertrophic Cardiomyopathy. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (33)] |
| 33. | Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 861] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 34. | Fu D, Wang S, Luo Y, Wu S, Peng D. Identification of a novel splicing-altering LAMP2 variant in a Chinese family with Danon disease. ESC Heart Fail. 2023;10:2479-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (33)] |
| 35. | Lopes LR, Zekavati A, Syrris P, Hubank M, Giambartolomei C, Dalageorgou C, Jenkins S, McKenna W; Uk10k Consortium, Plagnol V, Elliott PM. Genetic complexity in hypertrophic cardiomyopathy revealed by high-throughput sequencing. J Med Genet. 2013;50:228-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 36. | Wang S, Zou Y, Fu C, Xu X, Wang J, Song L, Wang H, Chen J, Wang J, Huan T, Hui R. Worse prognosis with gene mutations of beta-myosin heavy chain than myosin-binding protein C in Chinese patients with hypertrophic cardiomyopathy. Clin Cardiol. 2008;31:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 38. | Watkins H, Rosenzweig A, Hwang DS, Levi T, McKenna W, Seidman CE, Seidman JG. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992;326:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 485] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 39. | Greber-Platzer S, Marx M, Fleischmann C, Suppan C, Dobner M, Wimmer M. Beta-myosin heavy chain gene mutations and hypertrophic cardiomyopathy in Austrian children. J Mol Cell Cardiol. 2001;33:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Posen BM, Moolman JC, Corfield VA, Brink PA. Clinical and prognostic evaluation of familial hypertrophic cardiomyopathy in two South African families with different cardiac beta myosin heavy chain gene mutations. Br Heart J. 1995;74:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Woo A, Rakowski H, Liew JC, Zhao MS, Liew CC, Parker TG, Zeller M, Wigle ED, Sole MJ. Mutations of the beta myosin heavy chain gene in hypertrophic cardiomyopathy: critical functional sites determine prognosis. Heart. 2003;89:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Aizawa Y, Tanimoto Y, Hirata Y, Fujisawa T, Fukuoka R, Nakajima K, Katsumata Y, Nishiyama T, Kimura T, Yuasa S, Kohno T, Kohsaka S, Murata M, Maekawa Y, Furukawa Y, Takatsuki S, Fukuda K. Incidence, Clinical Characteristics, and Long-term Outcome of the Dilated Phase of Hypertrophic Cardiomyopathy. Keio J Med. 2019;68:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (31)] |
| 43. | Goto D, Kinugawa S, Hamaguchi S, Sakakibara M, Tsuchihashi-Makaya M, Yokota T, Yamada S, Yokoshiki H, Tsutsui H; JCARE-CARD Investigators. Clinical characteristics and outcomes of dilated phase of hypertrophic cardiomyopathy: report from the registry data in Japan. J Cardiol. 2013;61:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Hamada T, Kubo T, Kitaoka H, Hirota T, Hoshikawa E, Hayato K, Shimizu Y, Okawa M, Yamasaki N, Matsumura Y, Yabe T, Takata J, Doi YL. Clinical features of the dilated phase of hypertrophic cardiomyopathy in comparison with those of dilated cardiomyopathy. Clin Cardiol. 2010;33:E24-E28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |