Published online May 16, 2025. doi: 10.12998/wjcc.v13.i14.103501
Revised: December 19, 2024
Accepted: January 2, 2025
Published online: May 16, 2025
Processing time: 55 Days and 1.5 Hours
Diffuse panbronchiolitis (DPB) is a rare, chronic inflammatory lung disease mar
A 12-year-old girl, diagnosed with asthma at age five and managed with inhaled corticosteroids and long-acting beta-2 agonists, developed a history of chronic productive cough and chronic sinusitis for a year. On examination, she exhibited wheezing and coarse crackles. Despite receiving treatment for an asthma exacerbation, her symptoms did not improve. A chest X-ray revealed reticulonodular infiltration in both lower lungs, prompting further evaluation with high-resolu
This case highlights the importance of early diagnosis and prompt treatment in achieving favorable outcomes for DPB.
Core Tip: Diffuse panbronchiolitis presents progressively worsening symptoms such as chronic cough, shortness of breath during exertion, and persistent sinus infections. Often misdiagnosed as asthma, the condition can be identified through purulent sputum history or squawks detected during auscultation. Prompt and accurate diagnosis ensures effective treatment and improved clinical outcomes.
- Citation: Klubdaeng A, Tovichien P. Diffuse panbronchiolitis in children misdiagnosed as asthma: A case report. World J Clin Cases 2025; 13(14): 103501
- URL: https://www.wjgnet.com/2307-8960/full/v13/i14/103501.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i14.103501
Diffuse panbronchiolitis (DPB) is a rare chronic lung disease of unknown origin, characterized by persistent cough, exertional dyspnea, and chronic sinusitis[1]. DPB is most prevalent in Asian populations, particularly in Japan and Korea, likely due to genetic or environmental factors[2]. While the cause remains unclear, it may involve genetic predisposition or immune system imbalances[3]. DPB primarily affects individuals aged 20 to 40, but pediatric cases, which account for 13%, are often misdiagnosed as asthma[2]. Early diagnosis is critical to prevent mismanagement and ensure effective treatment. Long-term macrolide therapy, including erythromycin, clarithromycin, and azithromycin, has proven effec
Productive cough and chronic sinusitis persisting for a year.
A 12-year-old girl experienced a persistent productive cough and chronic sinusitis for a year. Despite her symptoms, she did not report fever or blood-tinged sputum. She was initially treated at a provincial hospital for an asthma flare-up with inhaled bronchodilators and corticosteroids. However, her symptoms persisted, resulting in her referral to our hospital.
She was diagnosed with asthma at age five and managed her symptoms using inhaled corticosteroids and long-acting beta-2 agonists.
She had no history of exposure to the offending antigens and denied vaping electronic cigarettes. There was no family history of cystic fibrosis, primary ciliary dyskinesia, immunodeficiency, or autoimmune disorders.
The physical examination showed normal vital signs, including a temperature of 36.5°C, blood pressure of 105/63 mmHg, pulse and respiratory rates of 100 beats per minute and 20 breaths per minute, respectively, and an oxygen saturation of 96% on room air. Examination of the nasal cavity revealed swelling, reddened mucosa, and a whitish discharge. Lung auscultation revealed bilateral wheezing and coarse crackles at the lung bases, while other findings were unremarkable.
Spirometry indicated an FEV1/FVC ratio of 81%. Pulmonary tuberculosis was ruled out with negative results for the tuberculin skin test, sputum acid-fast bacillus smear, and culture. Blood chemistry and immunological profiles, including IgG, IgM, IgA, and IgE, were normal, ruling out primary immune deficiency. However, a 128-fold increase in cold agglutinin levels was observed in her serum. Flexible bronchoscopy and bronchoalveolar lavage (BAL) showed a normal airway structure. Cytology revealed 1720 nucleated cells per cubic millimeter, comprising 80% neutrophils, 2% lympho
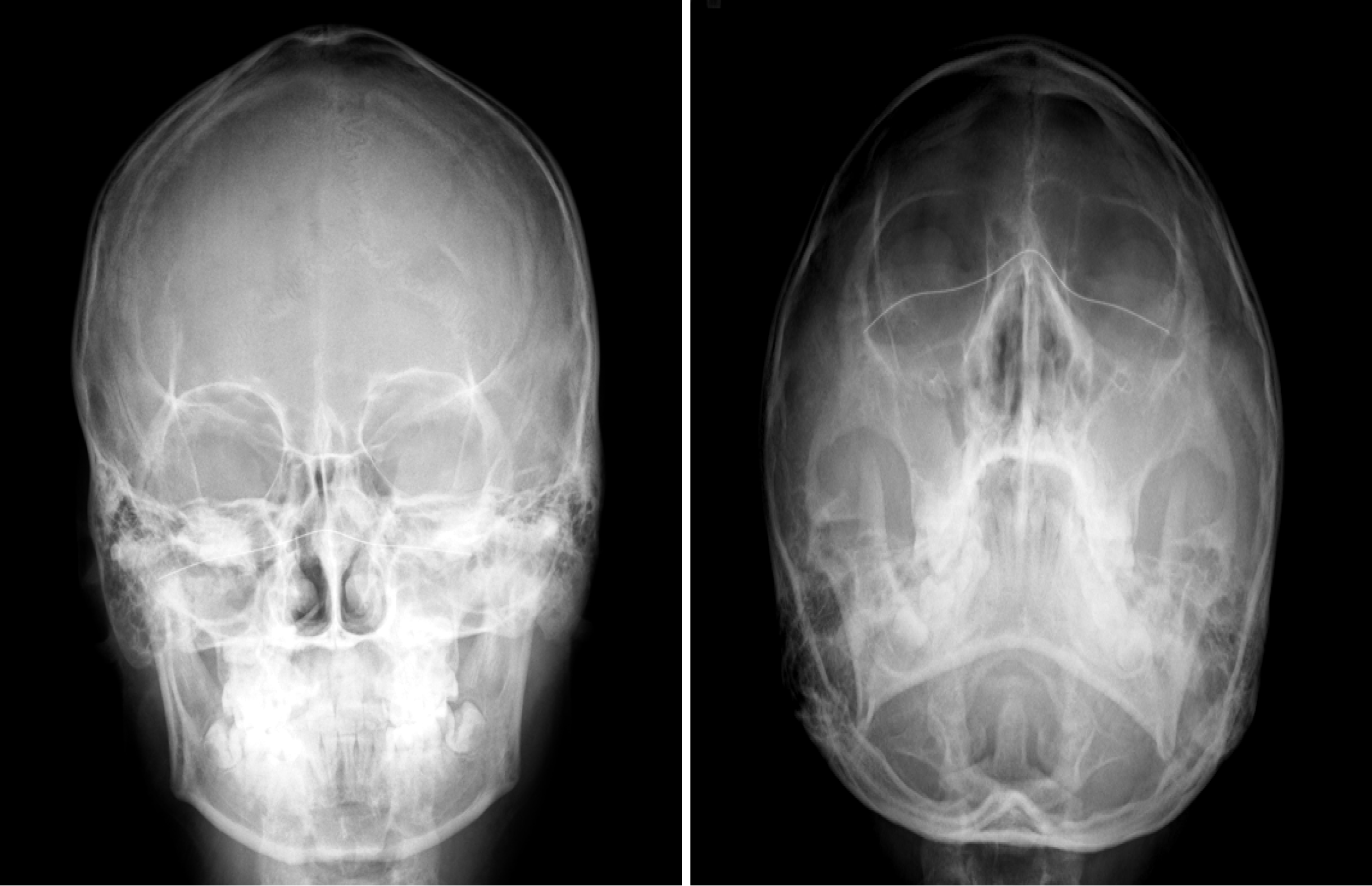
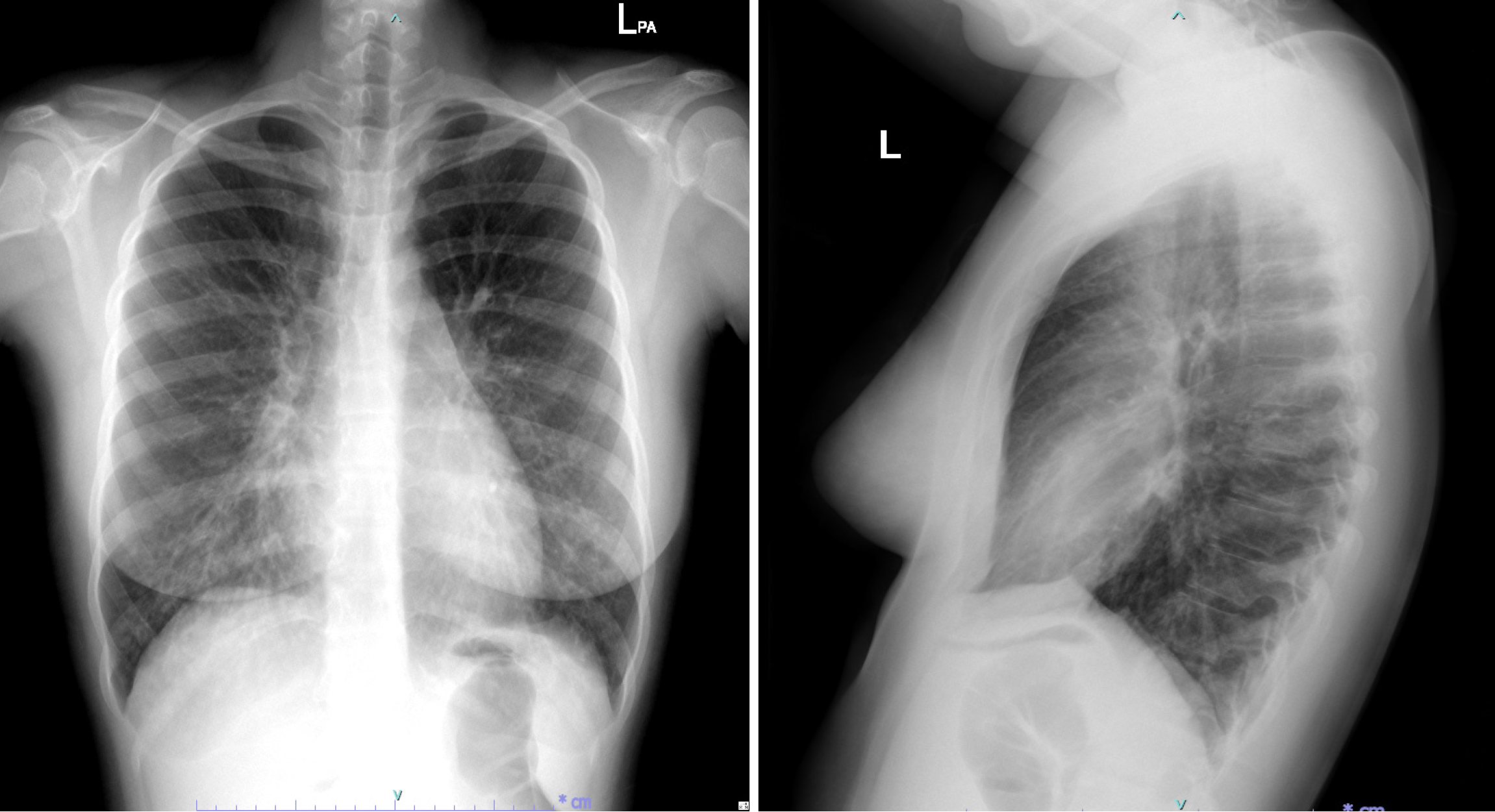
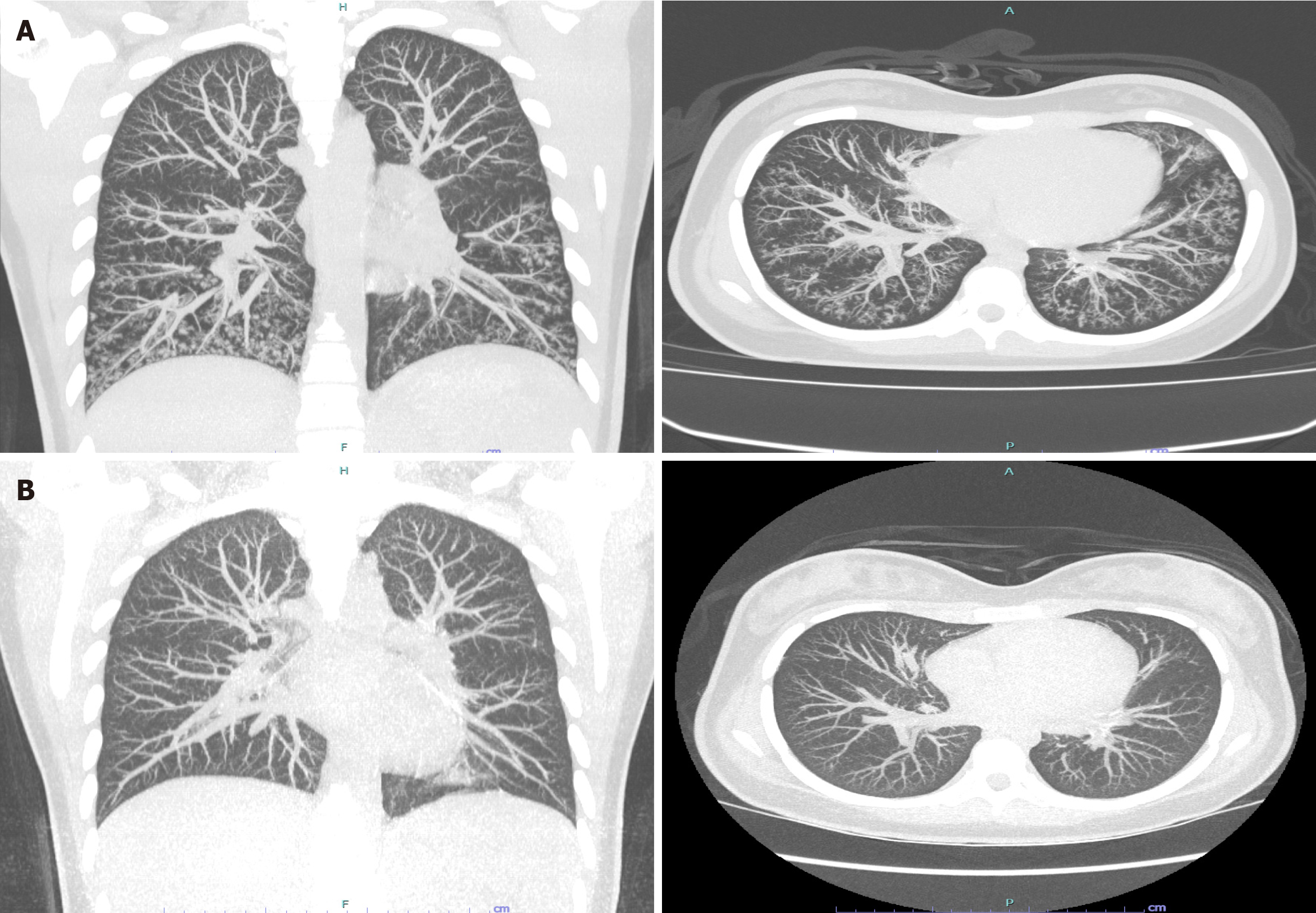
Paranasal sinus X-rays (Figure 1) showed opacification in the maxillary sinuses with bilateral air-fluid levels. A chest radiograph (Figure 2) revealed reticulonodular infiltrates in both lower lungs. High-resolution computed tomography (HRCT) (Figure 3A) demonstrated centrilobular nodules, a "tree-in-bud" pattern, and non-tapering bronchi indicative of inflammatory bronchiolitis.
The diagnosis of DPB was confirmed using clinical features and the authoritative diagnostic criteria established by the Ministry of Health and Welfare of Japan. This patient met the following major criteria: (1) Persistent cough, sputum production, and exertional dyspnea; (2) A history of recurrent chronic sinusitis; and (3) Bilateral diffuse nodular shadows observed on imaging. The diagnosis was further supported by two minor criteria: (1) Coarse crackles, occasional whee
Treatment began with a 2-week course of levofloxacin, followed by long-term azithromycin therapy at a dosage of 500 mg administered orally once daily. One month into treatment, productive cough and sinusitis symptoms were fully resolved. After symptom resolution, azithromycin was continued, while the doses of inhaled fluticasone-salmeterol and intranasal fluticasone furoate were gradually reduced.
After 1 year of treatment, chest HRCT (Figure 3B) revealed normal findings. Azithromycin was continued for 2 years without any adverse effects, and no symptoms recurred during the 2 years following its discontinuation.
DPB, a rare condition first described in Japan in the 1960s[7], is characterized by cough, shortness of breath, and chronic sinusitis[8]. Diagnosis requires three major criteria: (1) Persistent cough, sputum production, and exertional dyspnea; (2) History of recurrent chronic sinusitis; and (3) Bilateral diffuse nodular shadows on imaging. Additionally, at least two minor criteria must be present to confirm diagnosis. Minor criteria include: (1) Coarse crackles, occasional wheezing, rhonchi, or squawks; (2) Abnormal FEV1/FVC and partial oxygen pressure; and (3) Elevated cold hemagglutinin titers. It is essential to rule out differential diagnoses, including tuberculosis, primary ciliary dyskinesia, cystic fibrosis, and immu
The exact cause of DPB remains unknown; however, genetic, environmental, and systemic factors are thought to contribute to its development. Genetic predisposition, particularly in Japan, has been linked to specific markers like HLA-B54 haplotypes[9]. Additionally, Pseudomonas aeruginosa has been identified as a major factor contributing to persistent airway inflammation and structural lung damage[10]. In this case, Pseudomonas aeruginosa persisted until long-term macrolide therapy and targeted antibiotics were administered, effectively controlling the disease.
DPB is mainly diagnosed in middle-aged adults but can also affect children, often misdiagnosed as asthma[2,6]. Males account for 64% of cases, with diagnoses ranging from ages 10 years to over 70 years[2]. Pediatric cases are frequently identified after persistent asthma symptoms are reevaluated, with diagnostic delays of 6 months to 12 years. In adole
Diagnosing DPB is challenging in clinical practice due to the lack of a definitive diagnostic test. However, clinical symptoms like chronic productive cough and exertional dyspnea can suggest DPB. These symptoms, while suggestive, are also seen in more common conditions like bronchiectasis and asthma. Symptoms often persist for years before diagnosis. In many cases, chronic sinusitis precedes pulmonary symptoms by several years[2]. On physical examination, findings such as wheezing and crackles further support the suspicion of DPB. Wheezing is primarily expiratory, while inspiratory crackles are typically heard at the lung bases.
When asthma is initially suspected in patients with these symptoms, a lack of improvement following a course of systemic corticosteroids should prompt further evaluation. This evaluation should include consideration of DPB. The presence of purulent sputum or squawks, uncommon asthma findings, can guide clinicians toward diagnosing DPB correctly. Squawks are abnormal lung sounds, described as short inspiratory wheezes, and are associated with conditions like hypersensitivity pneumonitis, pneumonia, and interstitial lung disease[12]. While rare in children, these findings provide a useful distinction between DPB and asthma, aiding in accurate diagnosis.
Although sputum cultures were inconsistently reported, Pseudomonas aeruginosa was frequently identified. It had also been detected in a previously documented pediatric case[5]. Pulmonary function tests often showed a mixed obstructive pattern with occasional restrictive features. Mild hypoxemia was commonly observed alongside a reduced diffusing capacity (DLCO) in measured cases. Radiologic findings complemented these results, often revealing diffuse small nodules on chest X-rays. HRCT scans of the chest consistently showed multiple centrilobular nodules and a characteristic tree-in-bud appearance. Dilated bronchioles and bronchiectasis were also frequently observed[2].
Lung biopsies in patients with DPB revealed fine nodules primarily located in the centrilobular regions. These nodules comprised thickened respiratory bronchiole walls with infiltration of lymphocytes, plasma cells, and histiocytes. A key histopathological feature was the accumulation of foamy histiocytes in the walls of the respiratory bronchioles and alveolar ducts[13]. If characteristic findings of DPB are observed on HRCT scans, a trial of azithromycin may be initiated without requiring biopsy confirmation.
Early diagnosis of DPB is crucial to prevent severe complications, including bronchiectasis and respiratory failure. Because DPB symptoms often persist for long periods before diagnosis, patients were initially treated with asthma medications. However, antibiotics targeting bacteria like Pseudomonas aeruginosa had little to no impact on disease progression. The standard treatment involves long-term macrolide therapy, with doses and durations customized for each patient[1,2]. Macrolides are thought to treat DPB through several mechanisms. These include reducing mucus hypersecretion, inhibiting neutrophil accumulation in the mucosal epithelium, and suppressing lymphocyte activity[14].
Erythromycin is a primary treatment for DPB, significantly enhancing lung function, radiographic outcomes, and survival rates. However, its use is limited by side effects such as gastrointestinal discomfort, liver dysfunction, and frequent dosing[15-18]. The first pediatric DPB case involved a 13-year-old girl with progressive disease over 12 years who showed significant improvement during a 6-month erythromycin regimen (5-10 mg/kg/day)[19]. Similarly, a 13-year-old boy experienced marked recovery within 4 weeks of erythromycin (500 mg/day), allowing discontinuation of other therapies, with maintenance treatment (500 mg every other day) sustaining his recovery for another year[20]. In contrast, a 16-year-old boy experienced persistent symptoms and lifestyle disruptions despite erythromycin treatment (200-400 mg/day), suggesting the need for alternative macrolides like clarithromycin[21]. Pediatric case series further indicate improved outcomes with switching to clarithromycin in some patients[6]. However, for those with severe lung impairment or advanced disease, such as a 12-year-old girl who deteriorated despite clarithromycin (250 mg/day), treatment responses were minimal, reflecting irreversible damage[4].
Azithromycin, a macrolide with fewer side effects and infrequent dosing, is an effective treatment for DPB[22-25]. Studies in China show it improves symptoms, lung function, and chest HRCT findings[24,25]. In Li et al's study[25], 27.5% of patients fully recovered, 70.6% improved, and the 5-year survival rate was 94.1%. This was achieved with a regimen starting with intravenous azithromycin (500 mg daily for 1-2 weeks), followed by oral doses (500 mg daily for 3 months) and tapering to three times weekly for up to 12 months. Hui et al[24] confirmed significant lung function improvements and HRCT findings, including reductions in nodular shadows and bronchial wall abnormalities after 6 months of azithromycin treatment.
The effectiveness of azithromycin in treating childhood DPB remains unclear, requiring further study. A previous case of a 10-year-old boy showed that early azithromycin treatment, an initial dose of 500 mg, 250 mg daily for 2 weeks, and 500 mg 3 times/week for 3 years, resolved symptoms and normalized lung function for a year after stopping therapy[5]. However, he experienced a relapse 3 years later, marked by severe rhinosinusitis and pulmonary symptoms, both reso
Conducting a high-quality randomized controlled trial (RCT) for DPB is challenging due to specific limitations inhe
We emphasize the importance of early diagnosis and treatment of DPB, particularly in cases of difficult-to-manage asthma or recurrent/chronic sinopulmonary symptoms, especially when purulent sputum is present, as early inter
The authors would like to express gratitude to the patient and the staff of the Radiology Department, Faculty of Medicine Siriraj Hospital, Mahidol University, for chest imaging and to Siriraj Medical Research Center (SiMR) for publication support.
| 1. | Poletti V, Casoni G, Chilosi M, Zompatori M. Diffuse panbronchiolitis. Eur Respir J. 2006;28:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Weinberger M, Lesser D. Diffuse panbronchiolitis: A progressive fatal lung disease that is curable with azithromycin, but only if diagnosed! Pediatr Pulmonol. 2019;54:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Keicho N, Hijikata M. Genetic predisposition to diffuse panbronchiolitis. Respirology. 2011;16:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Aslan AT, Ozcelik U, Talim B, Haliloglu M, Dogru D, Dalgic F, Kiper N. Childhood diffuse panbronchiolitis: a case report. Pediatr Pulmonol. 2005;40:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Weinberger M, Fischer A, Kao S. Diffuse panbronchiolitis in a 10-year-old boy. Pediatr Pulmonol. 2015;50:E32-E34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Fujita S, Suzuki R, Sagara N, Aota A, Akashi K, Katsunuma T. Three cases of diffuse panbronchiolitis in children with a past history of difficult-to-treat bronchial asthma: A case report from a single medical facility. Allergol Int. 2020;69:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Yamanaka A, Saiki S, Tamura S, Saito K. [Problems in chronic obstructive bronchial diseases, with special reference to diffuse panbronchiolitis]. Naika. 1969;23:442-451. [PubMed] |
| 8. | Swaminathan AC, Carney JM, Tailor TD, Palmer SM. Overview and Challenges of Bronchiolar Disorders. Ann Am Thorac Soc. 2020;17:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Sugiyama Y, Kudoh S, Maeda H, Suzaki H, Takaku F. Analysis of HLA antigens in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1990;141:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 888] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 11. | Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168:1277-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Paciej R, Vyshedskiy A, Bana D, Murphy R. Squawks in pneumonia. Thorax. 2004;59:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tsang KW, Ooi CG, Ip MS, Lam WK, Ngan H, Chan EY, Hawkins B, Ho CS, Amitani R, Tanaka E, Itoh H. Clinical profiles of Chinese patients with diffuse panbronchiolitis. Thorax. 1998;53:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 167] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157:1829-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 392] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Lin X, Lu J, Yang M, Dong BR, Wu HM. Macrolides for diffuse panbronchiolitis. Cochrane Database Syst Rev. 2015;1:CD007716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Chuang MC, Chou YT, Lin YC, Hsieh MJ, Tsai YH. Diffuse panbronchiolitis-The response and recurrence after erythromycin therapy. J Formos Med Assoc. 2016;115:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Zhao SY, Peng Y, Zhou CJ, Jiao AX, Jiang ZF. [Diffuse panbronchiolitis in a child: case report and literature review]. Zhonghua Er Ke Za Zhi. 2007;45:504-507. [PubMed] |
| 20. | Zhao NN, Cao H, Zhang SS, Cao GQ. Successful treatment of diffuse panbronchiolitis in a child from Western China: A case report. Exp Ther Med. 2017;13:2094-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Okumura H, Kawashima A, Hanamoto A. Squawks as an important physical finding for differentiation of diffuse panbronchiolitis from asthma in children: A case report. J Gen Fam Med. 2023;24:261-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Healy DP. Macrolide immunomodulation of chronic respiratory diseases. Curr Infect Dis Rep. 2007;9:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Bush A. Azithromycin is the answer in paediatric respiratory medicine, but what was the question? Paediatr Respir Rev. 2020;34:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis. 2013;5:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Li H, Zhou Y, Fan F, Zhang Y, Li X, Yu H, Zhao L, Yi X, He G, Fujita J, Jiang D. Effect of azithromycin on patients with diffuse panbronchiolitis: retrospective study of 51 cases. Intern Med. 2011;50:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |