Published online May 16, 2025. doi: 10.12998/wjcc.v13.i14.101981
Revised: December 14, 2024
Accepted: December 27, 2024
Published online: May 16, 2025
Processing time: 103 Days and 13.2 Hours
This article discusses a case involving a 63-year-old man with non-small cell lung cancer, who was treated with a combination of chemotherapy and immunothe
After the fifth cycle of treatment, the patient developed skin itching and a vitiligo-like rash, which are known side effects of immunotherapy. Despite dermatologi
The case highlights the use of immunotherapy in patients with non-small cell lung cancer and the potential side effect of vitiligo-like rash. The patient’s tumor res
Core Tip: The article presents a case study of a 63-year-old male with non-small cell lung cancer treated effectively with chemotherapy and immunotherapy, despite developing immune-related skin adverse events manifesting as a vitiligo-like rash. Treatment with sintilimab, a programmed death 1 inhibitor, resulted in tumor regression and highlights the need for further research into the prognostic significance and mechanisms of vitiligo-like rash in patients with lung cancer undergoing immunotherapy.
- Citation: Mao XM, Wang WH. Vitiligo-like rash in a patient with lung cancer caused by sintilimab: A case report. World J Clin Cases 2025; 13(14): 101981
- URL: https://www.wjgnet.com/2307-8960/full/v13/i14/101981.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i14.101981
Lung cancer remains a leading cause of cancer-related mortality worldwide, with non-small cell lung cancer (NSCLC) accounting for approximately 85% of all cases[1]. Advances in understanding the molecular landscape of NSCLC have revolutionized treatment approaches, particularly with the introduction of targeted therapies and immunotherapies. Among the latter, immune checkpoint inhibitors (ICIs) have emerged as a cornerstone in the management of various solid tumors, including NSCLC. These agents work by releasing the brakes on the immune system, allowing it to recognize and attack cancer cells[2]. However, the use of ICIs is not without its challenges, as they can also lead to immune-related adverse events (irAEs) that affect multiple organs, including the skin[3].
Right chest pain for 3 months and shortness of breath after activities for 1 month.
The patient was admitted to the hospital for chest pain and shortness of breath after activity and was diagnosed with lung adenocarcinoma. After combined chemotherapy and immunotherapy, the clinical evaluation demonstrated that the treatment was effective.
The patient was previously in good health.
Parents died of unknown causes; siblings are healthy.
The patient was conscious, with thick breathing sounds in both lungs, no rales, soft abdomen, and no edema in both lower limbs.
Medical thoracoscopy showed multiple pleural nodules in the right parietal layer, and moderate pleural effusion. Right pleural biopsy showed infiltration of lung adenocarcinoma. Immunohistochemical results showed cytokeratin 7 (CK7) (+), thyroid transcription factor 1 (+), napsin A (+), caudal type homeobox 2 (-), anaplastic lymphoma kinase (ALK) Ventana (-), CK5/6 (+), calretinin (-), and p40 (-). Gene mutation detection was negative, which included epidermal growth factor receptor, KRAS, NRAS, BRAF, human epidermal growth factor receptor 2, MET, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha mutation and ALK, ROS1, RET fusion. Programmed death ligand 1 (PD-L1) expression by tumor proportion score was 60%. T lymphocyte classification showed CD4+/CD8+ was 4.51 (Table 1).
| T lymphocytes | February 2021 (before the appearance of the rash) | March 2021 (after the appearance of the rash) | April 2021 (after the development of the rash) |
| Total absolute number of T lymphocytes (per microliter) | 1860 | 1085 | 1406 |
| Absolute number of helper/induced T lymphocytes CD4+ (per microliter) | 1506 | 841 | 1012 |
| Absolute number of suppressor/cytotoxic T lymphocytes CD8+ (per microliter) | 333 | 242 | 364 |
| Percentage of helper/induced T lymphocytes | 60.93 | 56.07 | 47.36 |
| Suppressor/cytotoxic T lymphocyte percentage | 13.50 | 16.13 | 17.02 |
| Helper/suppressor T lymphocyte ratio CD4+/CD8+ | 4.51 | 3.48 | 2.78 |
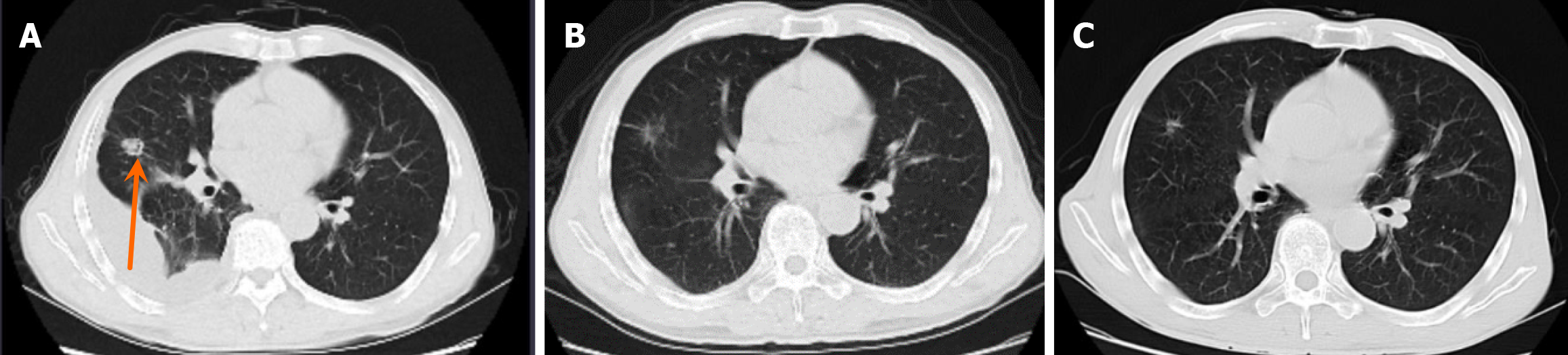
Chest computed tomography showed a 15 mm × 14 mm nodule in the upper lobe of the right lung and right pleural effusion (Figure 1).
The patient was diagnosed with NSCLC.
Five cycles of chemotherapy (pemetrexed and carboplatin, every 3 weeks) combined with sintilimab injection 200 mg im
After the fifth cycle of treatment, the patient developed skin itching and a vitiligo-like rash, which are known side effects of immunotherapy. Despite dermatological consultation and treatment with topical corticosteroids, the rash worsened while the itching subsided. The patient continued with the treatment, and after 15 cycles, the tumor showed a response with a reduction in size. The vitiligo-like rash increased, but the antitumor treatment remained effective.
ICIs have been shown to be effective against a wide range of solid organ malignant tumors. These monoclonal antibodies lead to activation of cytotoxic T cells and subsequent elimination of cancer cells[4]. Implementation of ICIs have shown improved and sustained responses in patients with cancer[5]. With the support of a large number of clinical trials, these drugs have been approved for the treatment of melanoma, NSCLC, renal cell cancer, bladder cancer, and head and neck cancer, among others. Sintilimab injection is a monoclonal antibody against programmed death 1 (PD-1). National Medical Products Administration approves first-line treatment of sintilimab combined with pemetrexed/platinum che
Vitiligo, an acquired pigmentary disorder of unknown origin, is the most frequent cause of depigmentation worldwide[9]. Treatment with ICIs in patients with melanoma may lead to vitiligo. The overall cumulative incidence of vitiligo was 3.4% in patients with melanoma treated with ICIs, which is higher than the normal prevalence rate of 1%[9-13]. Immuno
Vitiligo is a complex disease whose pathogenesis results from the interaction of genetic components, cellular oxidative stress, and immune factors. Research has indicated that regulatory T cells (Tregs) are significantly reduced in vitiligo skin. The PD-1/PD-L1 pathway may be involved in active generalized vitiligo and may have a role in Treg exhaustion[17,18]. CD8+ T cells are considered the main effector cells that recognize these common melanocyte antigens[19-22]. The CD4 (+)/CD8 (+) ratio is lower in active generalized patients with vitiligo compared with stable generalized patients with vitiligo[23]. It is generally believed that vitiligo-like rashes do not subside after stopping immunotherapy[24]. There is a case report that a patient with NSCLC who had a history of vitiligo for 10 years was treated with the anti-PD-1 antibody camrelizumab. The symptoms of vitiligo worsened significantly within half a year, resulting in systemic skin depigmen
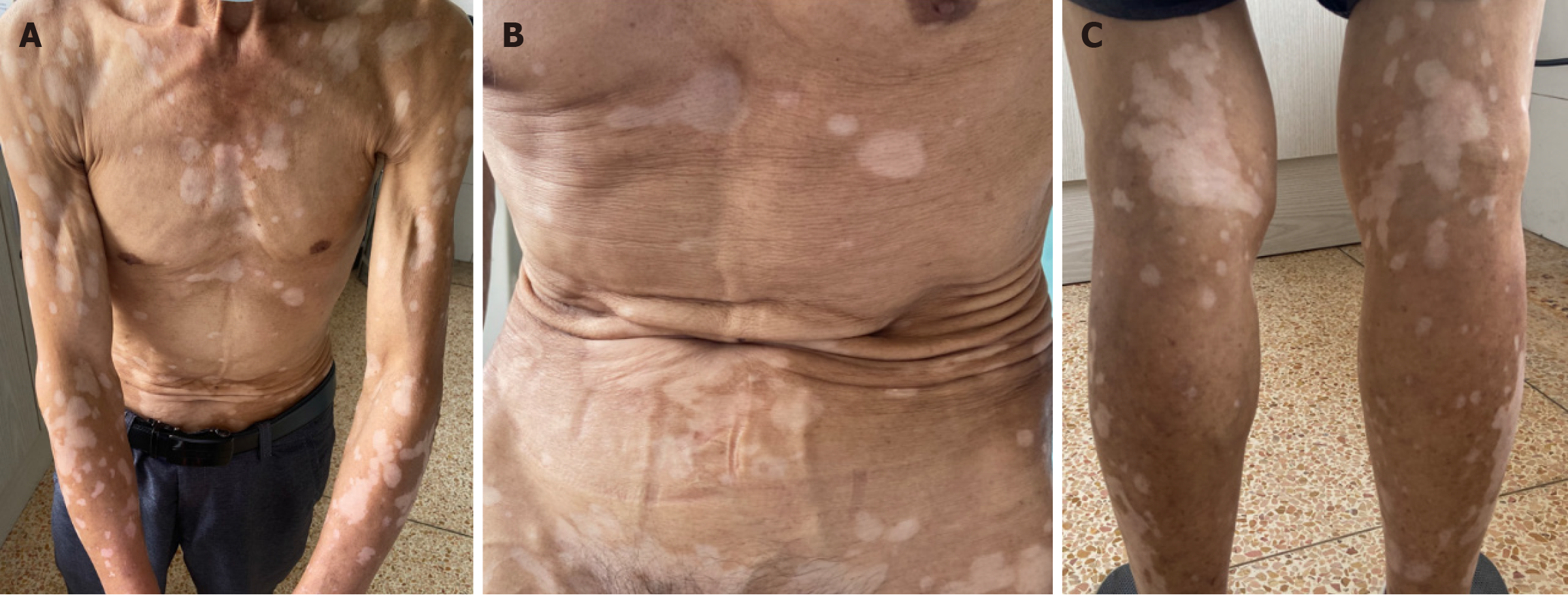
In our case, this patient with lung cancer has no previous history of vitiligo. The patient was treated with anti-PD-1 antibody (sintilimab) for almost 2 years and the tumor response evaluation was “stable disease” during the treatment period. Vitiligo-like rash appeared after 5 months of lung cancer treatment. Over the next 1.5 years, the vitiligo-like rash spread over the patient’s chest, back, abdomen, and both upper and lower extremities and face (Figure 2). In our case, the ratio of CD4+/CD8+ decreased with the progression of vitiligo over time (Table 1). The patient did not receive specific treatment such as glucocorticoids for vitiligo in the follow-up, as his quality of life was not affected. We will continue to monitor the vitiligo-like rashes in the following discontinuation period.
Vitiligo development in patients with melanoma treated with ICIs can be seen as a good prognostic sign[27]. Favorable prognosis in a 63-year-old female with NSCLC developed vitiligo after the use of pembrolizumab was reported. This patient demonstrated “complete remission” at 16 months of follow-up. The vitiligo might be associated with increased efficacy of pembrolizumab in metastatic lung adenocarcinoma[28]. In our case, this patient with lung cancer has now survived for more than 2 years and is currently in stable primary tumor disease. It is unclear whether the presence of vitiligo is a favorable prognostic factor in patients with lung cancer. Therefore, we report a case of vitiligo like rash in a patient with lung cancer after receiving ICI treatment. Systematic analysis of vitiligo-like rash in patients with lung cancer received ICI therapy is still lacking. The mechanism of vitiligo in patients with lung cancer receiving ICI therapy remains to be explored. Whether patients with lung cancer developed vitiligo-like rash after ICI therapy have an improved prognosis needs to be studied further.
The case highlights the use of immunotherapy in patients with NSCLC and the potential side effect of vitiligo-like rash. The patient’s tumor responded well to the treatment, and despite the skin reaction, the treatment was not discontinued due to its effectiveness. The article suggests that additional studies are needed to understand the mechanism behind vitiligo in patients with lung cancer receiving ICIs and whether the development of vitiligo-like rash after ICI therapy is associated with improved prognosis. The case also underscores the importance of managing irAEs in the context of effective antitumor treatment.
| 1. | Economopoulou P, Mountzios G. The emerging treatment landscape of advanced non-small cell lung cancer. Ann Transl Med. 2018;6:138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Remon J, Vilariño N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): Approaches on special subgroups and unresolved burning questions. Cancer Treat Rev. 2018;64:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Blum SM, Rouhani SJ, Sullivan RJ. Effects of immune-related adverse events (irAEs) and their treatment on antitumor immune responses. Immunol Rev. 2023;318:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Malviya N, Tattersall IW, Leventhal J, Alloo A. Cutaneous immune-related adverse events to checkpoint inhibitors. Clin Dermatol. 2020;38:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features. J Am Acad Dermatol. 2020;83:1130-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Zhou C, Wang J, Wang B, Cheng Y, Wang Z, Han B, Lu Y, Wu G, Zhang L, Song Y, Zhu B, Hu Y, Wang Z, Song Q, Ren S, He Y, Hu X, Zhang J, Yao Y, Zhao H, Wang Z, Chu Q, Duan J, Liu J, Qin S. [Chinese Experts Consensus on Immune Checkpoint Inhibitors for Non-small Cell Lung Cancer (2020 Version)]. Zhongguo Fei Ai Za Zhi. 2021;24:217-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3123] [Article Influence: 446.1] [Reference Citation Analysis (0)] |
| 8. | Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, Kern JA, Lacouture ME. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 9. | Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 10. | Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 589] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 11. | Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, Tomasic G, Soria JC, Champiat S, Texier M, Lanoy E, Robert C. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016;152:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 494] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 12. | Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, Maruyama H, Fujisawa Y, Matsuya T, Fujimoto M, Yamamoto A. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol. 2017;44:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, Carlino MS, Kefford R, Fernandez-Penas P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol. 2016;74:455-61.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Quach HT, Dewan AK, Davis EJ, Ancell KK, Fan R, Ye F, Johnson DB. Association of Anti-Programmed Cell Death 1 Cutaneous Toxic Effects With Outcomes in Patients With Advanced Melanoma. JAMA Oncol. 2019;5:906-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Ramondetta A, Ribero S, Conti L, Fava P, Marra E, Broganelli P, Caliendo V, Picciotto F, Guida M, Fierro MT, Quaglino P. Clinical and Pathological Relevance of Drug-induced Vitiligo in Patients Treated for Metastatic Melanoma with Anti-PD1 or BRAF/MEK Inhibitors. Acta Derm Venereol. 2020;100:adv00001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Liu RC, Consuegra G, Chou S, Fernandez Peñas P. Vitiligo-like depigmentation in oncology patients treated with immunotherapies for nonmelanoma metastatic cancers. Clin Exp Dermatol. 2019;44:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Mukhatayev Z, Dellacecca ER, Cosgrove C, Shivde R, Jaishankar D, Pontarolo-Maag K, Eby JM, Henning SW, Ostapchuk YO, Cedercreutz K, Issanov A, Mehrotra S, Overbeck A, Junghans RP, Leventhal JR, Le Poole IC. Antigen Specificity Enhances Disease Control by Tregs in Vitiligo. Front Immunol. 2020;11:581433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Tembhre MK, Parihar AS, Sharma VK, Sharma A, Chattopadhyay P, Gupta S. Alteration in regulatory T cells and programmed cell death 1-expressing regulatory T cells in active generalized vitiligo and their clinical correlation. Br J Dermatol. 2015;172:940-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Le Gal FA, Avril MF, Bosq J, Lefebvre P, Deschemin JC, Andrieu M, Dore MX, Guillet JG. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Marchioro HZ, Silva de Castro CC, Fava VM, Sakiyama PH, Dellatorre G, Miot HA. Update on the pathogenesis of vitiligo. An Bras Dermatol. 2022;97:478-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Pehlivan S, Ozkinay F, Alper S, Onay H, Yuksel E, Pehlivan M, Ozkinay C. Association between IL4 (-590), ACE (I)/(D), CCR5 (Delta32), CTLA4 (+49) and IL1-RN (VNTR in intron 2) gene polymorphisms and vitiligo. Eur J Dermatol. 2009;19:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Birlea SA, Laberge GS, Procopciuc LM, Fain PR, Spritz RA. CTLA4 and generalized vitiligo: two genetic association studies and a meta-analysis of published data. Pigment Cell Melanoma Res. 2009;22:230-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R. Decreased regulatory T-cells and CD4(+) /CD8(+) ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res. 2013;26:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | de Golian E, Kwong BY, Swetter SM, Pugliese SB. Cutaneous Complications of Targeted Melanoma Therapy. Curr Treat Options Oncol. 2016;17:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Xu Y, Cai Y, Zu J, Wang X, Wang Y, Sun C, Guo Y, Shao G, Yang Z, Qiu S, Ma K. Aggravation of depigmentation for a non-small-cell lung cancer patient with pre-existing vitiligo using anti-programmed cell death-1 therapy: case report. Immunotherapy. 2020;12:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Gao Z, Xu Y, Zu J, Wang X, Sun C, Qiu S, Guo Y, Ma K. The time window for the reversal of depigmentation from aggravation to recovery in a non-small-cell lung cancer patient with pre-existing vitiligo using anti-programmed cell death-1 therapy: A case report. Front Immunol. 2022;13:946829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Wu CE, Yang CK, Peng MT, Huang PW, Chang CF, Yeh KY, Chen CB, Wang CL, Hsu CW, Chen IW, Lin CT, Ueng SH, Lin G, Lin YF, Cheng CY, Chang JW. The association between immune-related adverse events and survival outcomes in Asian patients with advanced melanoma receiving anti-PD-1 antibodies. BMC Cancer. 2020;20:1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Hasan Ali O, Diem S, Markert E, Jochum W, Kerl K, French LE, Speiser DE, Früh M, Flatz L. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology. 2016;5:e1231292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |