Published online Apr 16, 2025. doi: 10.12998/wjcc.v13.i11.98979
Revised: November 27, 2024
Accepted: December 13, 2024
Published online: April 16, 2025
Processing time: 162 Days and 23.1 Hours
Thymic epithelial neoplasms are rare malignant neoplasms originating in the thymus gland. There have been case reports of patients with advanced thymomas treated with a methylprednisolone pulse or with glucocorticoid (GCs) shock be
We report a case of a patient with thymoma who had a significant response to preoperative low-dose GC therapy. A mediastinal tumor was detected in the patient via computerized tomography upon admission. The tumor was initially suspected to be a thymic tumor, but lymphoma could not be ruled out. The tumor shrank significantly after low-dose (5 mg/day) GC therapy. Thoracoscopic thy
This case highlights that low-dose GCs are effective in the treatment of thymomas, and we believe that GCs should be applied more frequently and studied more thoroughly in the treatment of thymomas.
Core Tip: This study reports a case of a thymoma patient whose tumor significantly shrank following preoperative treatment with low-dose glucocorticoids (GCs). The low-dose GC therapy demonstrated promising efficacy, promoting tumor reduction, minimizing intraoperative adhesions, and facilitating smooth postoperative recovery without recurrence. This study highlights the potential of GCs in the management of thymoma and calls for further investigation into their application in therapeutic strategies.
- Citation: Yao JK, He ZY, Zhu Z, Huang HT. Treatment of thymoma with low-dose glucocorticoids before surgery for significant tumor shrinkage: A case report. World J Clin Cases 2025; 13(11): 98979
- URL: https://www.wjgnet.com/2307-8960/full/v13/i11/98979.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i11.98979
Thymic epithelial tumors are rare malignant tumors originating in the thymus[1]. The overall incidence of thymomas in the United States is 0.13 per 100000 person-years[2]. Histologically, thymic epithelial tumors are classified into various subtypes, including type A, AB, B1, B2, B3, and C thymic carcinomas[3]. Thymomas can present with various manifestations, such as myasthenia gravis and local symptoms, or are asymptomatic and detected as mediastinal masses on chest X-ray[4-6]. For resectable thymomas, especially for invasive thymomas, postoperative radiotherapy is recommended[7]. Complete thymectomy is typically performed in specific cases[8]. Platinum chemotherapy is still the standard treatment for advanced thymomas that cannot be completely resected by surgery[9]. Initiation of chemotherapy to optimize surgical excision and consolidation to control residual lesions may improve the prognosis of patients with advanced thymomas[10-12].
At present, there are case reports that advanced thymomas can be controlled by methylprednisolone pulse therapy with good efficacy[13]. Moreover, preoperative glucocorticoid (GC) impact therapy has a significant effect on stage B1 thymoma, and the preoperative tumor burden in patients is significantly reduced[14]. However, there are currently almost no clinical studies on the application of low-dose GCs in the treatment of thymoma. We hope that this report on the preoperative use of low-dose GCs in the treatment of a thymoma will provide new ideas for the application of GCs in the treatment of thymomas.
A 44-year-old young patient with chest tightness examined chest computed tomography (CT) and found anterior mediastinal space.
In August 2023, a 44-year-old patient with chest tightness was examined via chest CT and an anterior mediastinal space tumor was detected. The patient was admitted with a temperature of 38.2 °C. The patient was healthy in the past and had no bad habits, such as smoking or drinking. The patient had occasional chest tightness and pain but no related clinical symptoms of myasthenia gravis and sought treatment at our hospital because of the aggravation of chest tightness symptoms.
The patient had no specific past medical history.
The patient had no personal and family history.
He had a physical examination but found no obvious positive signs.
Routine blood tests revealed that the white blood cell count was 15.97 × 109/L, the lymphocyte count was 3.11 × 109/L, and the monocyte count was 2.25 × 109/L. Other blood tests showed no obvious abnormality.
Chest CT revealed a soft-tissue shadow that was visible on the right side of the anterior mediastinum.
The patient was eventually diagnosed with thymoma.
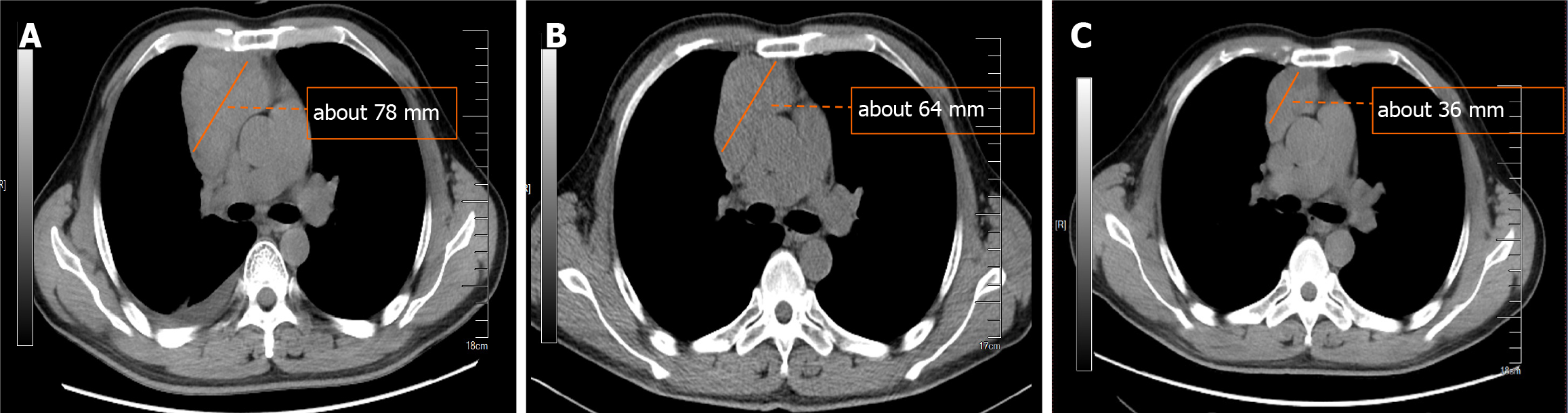
The cooling effect of daily intravenous injection of 5 mg of dexamethasone sodium phosphate was significant, as shown in Figure 1, and chest CT reexamination revealed a significant reduction in the thymic mass.
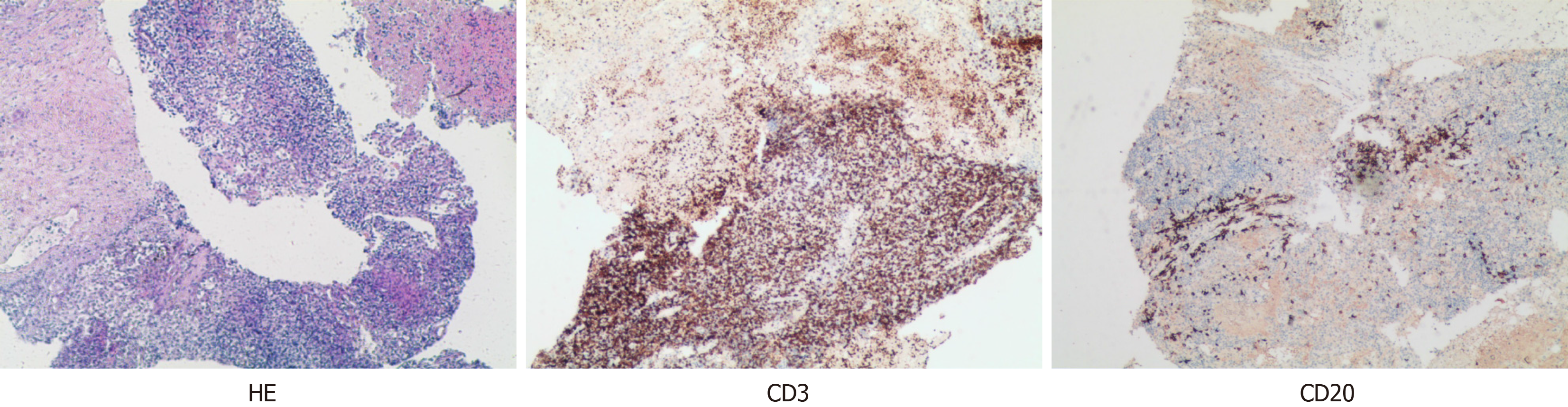
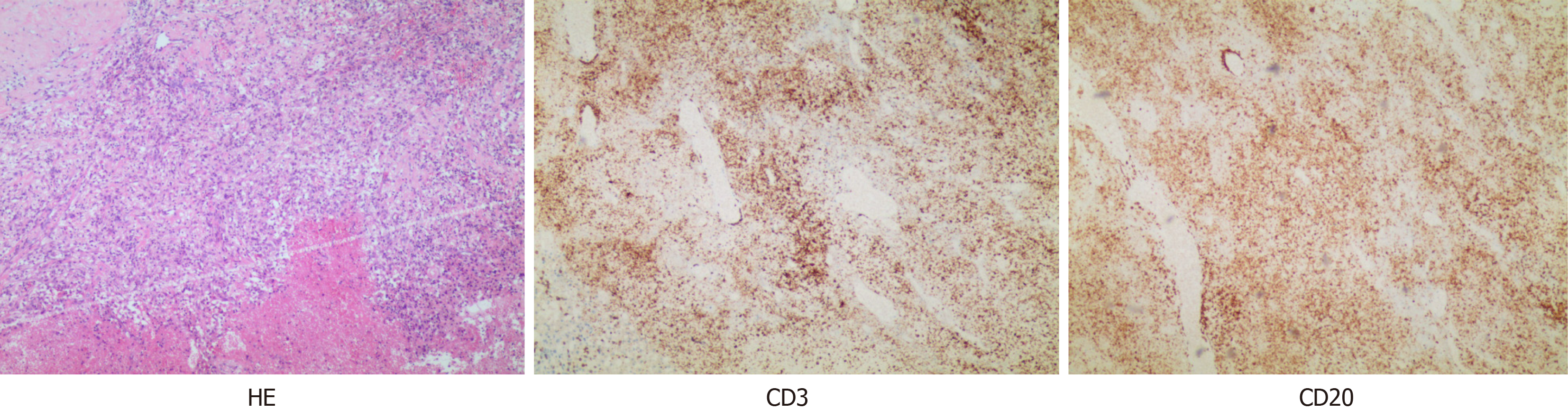
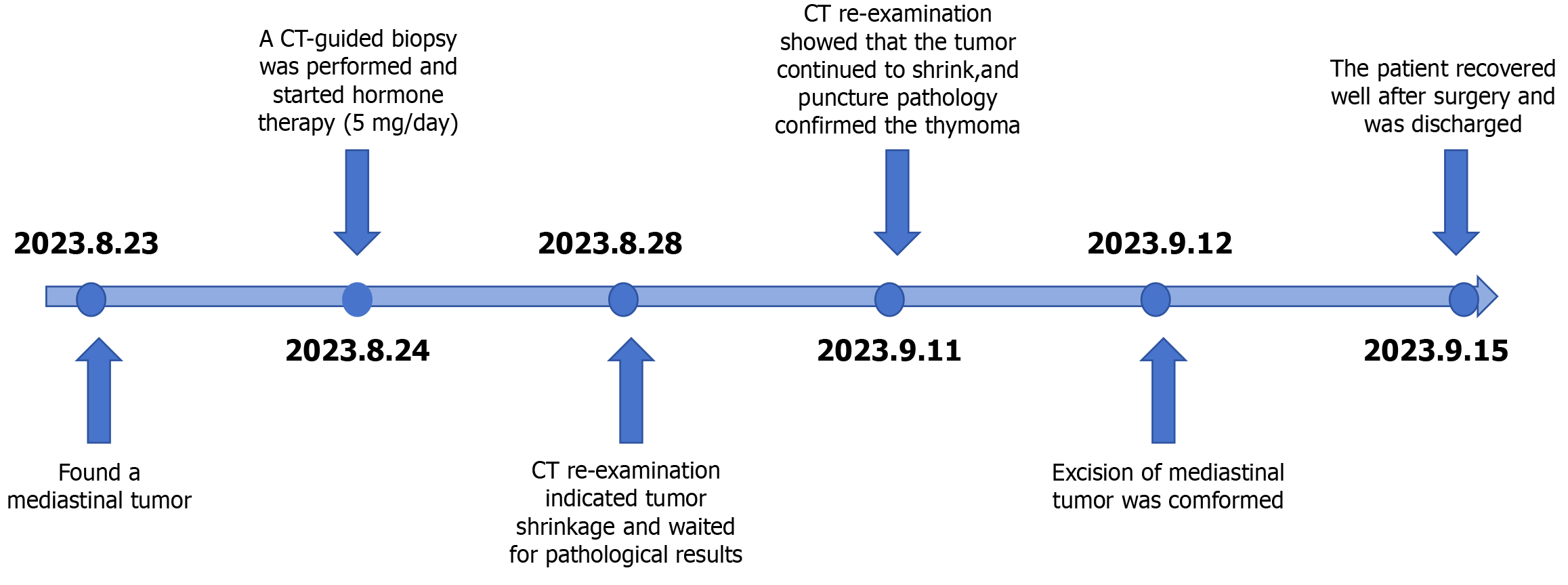
When professor Huang’s expert team discussed the condition, they consulted the relevant literature and found that in other countries, the preoperative application of GCs could effectively reduce the thymus tumor volume and improve the surgical effect; thus, dexamethasone sodium phosphate was used for 2 weeks. Considering that the patient's disease could not exclude the possibility of lymphoma, B-ultrasound-guided transesophageal mediastinal mass puncture was performed after admission. Thymoma was pathologically confirmed (Figure 2), and surgery was scheduled. Extubation was performed 3 days after surgery, and routine pathology revealed a type B2 anterior mediastinal thymoma (Figure 3). To date, the patient has recovered well, and the tumor has not returned. The entire process of patient treatment, from admission to discharge, is shown in Figure 4.
In this case, the patient experienced excellent GC therapy outcomes before surgery. The mass was significantly reduced, there was less intraoperative adhesion, and the separation of the pericardium and thymus tissue was smooth. These findings suggest that the GC promoted a significant shrinkage of the thymoma in this patient. Indeed, there have been previous reports of thymoma regression after GC therapy[14,15], which is consistent with the findings reported in this case. These findings suggest that GCs can promote the obvious shrinkage of thymomas in patients. After the patient was admitted to the hospital, we consulted the relevant literature and found many case reports of GC shock therapy for thymomas. Some scholars have conducted prospective studies with the administration of GC shock therapy for 2 weeks before surgery and reported that the effects on B1, B2 and B3 thymomas were significant, and most patients experienced partial remission before surgery and no recurrence after surgery[14]. In Nakamura et al’s report[13], a patient with a metastatic thymoma that was well controlled by methylprednisolone pulse therapy plus immunosuppressants was described. In patients with metastatic thymomas, especially in those with myasthenia gravis, the use of GCs has been affirmed by many studies for their positive effects[16]. However, no studies have discussed in detail the influence of the duration of GC use and its benefits before and after surgery. In addition, these studies were based on high-dose corticosteroid shock therapy, while our patient achieved significant tumor shrinkage with 5 mg of a corticosteroid per day. In previous studies, only one patient with thymoma-induced pleural effusion was reported to have been treated with a low dose of GCs, but the effect was significant[17]. These findings suggest that it may be possible to achieve good results with small amounts of hormones in patients with preoperative thymoma.
GCs, a class of steroid hormones, are important regulatory molecules that control inflammation, cell growth and differentiation through the activity of specific intracellular GC receptors (GRs)[18,19]. In the normal thymus, GRs are expressed not only in immature thymus cells but also in epithelial cells, and high GR expression can also be detected in thymoma cells. Therefore, the effect of GCs on thymomas is related mainly to the effects of GRs on thymoma cells and epithelial cells. A study by Mimae et al[20] revealed high GR expression rates in thymomas, and multivariate analysis revealed that GR expression was associated with a better prognosis in patients with thymomas, including those with surgically removed thymomas.
In our case, there were positive pathological changes before and after hormone therapy, which provided effective case support for our study of the effects of GCs on thymoma. We report the case of a patient who was hospitalized for a large thymoma, with a pre-steroid biopsy showing immature TdT (+) and CD5 (+), CD20 (+), and CD3 (+) T lymphocytes, and post-steroid resection pathology revealing TdT (-), CD5 (-), CD20 (-), and CD3 (-) cells. These findings suggest that GCs cause the loss of immature lymphocytes in thymus tumors. This finding is consistent with the findings of Tateyama et al[21], who reported that corticosteroids might cause degenerative changes in epithelial cells and immature T lymphocytes. Kobayashi et al[14] noted that the reduction in the size of thymomas rich in these immature lymphocytes with steroid treatment could be explained by the reduction in the number of most lymphocytes. This could explain why the tumors shrunk so much after GC therapy. Kobayashi et al[14] also reported that preoperative GC pulse treatment resulted in a much greater reduction in B1 thymomas than in other types of thymomas because B1 thymomas have large numbers of immature double-positive [CD4 (+) and CD8 (+)] lymphocytes, which are very sensitive to GC-induced apoptosis.
Notably, studies have shown that GCs can also directly inhibit the proliferation of tumor cells. This may be because GCs affect tumor cells by inducing G1 phase cell cycle arrest in human thymus tumor epithelial cells[22]. In this study, there were a small number of cases in which tumors still grew slowly after steroid therapy, which the authors believe may be related to the short duration of steroid hormone use and the type of pathology. We hope that further studies will confirm the appropriate duration of preoperative steroid use and analyze its benefits depending on the pathological types in patients. In summary, we believe that GCs can effectively control the progression of thymomas. We also believe that for thymoma patients with large tumor volumes or chest tightness, the use of low-dose GC therapy can be considered before surgical treatment, which may be conducive to curing patients. We also look forward to more similar cases or studies to provide clinical evidence for preoperative GC therapy. At present, there are no authoritative guidelines for the preoperative use of hormone therapy for thymomas. This case provides a better idea for the treatment of thymoma and has certain reference value for clinicians. The explanation of the mechanism of action of hormones in the treatment of thymoma could be improved in the future, and the application of hormone therapy in the treatment of thymoma could be supported by a systematic theory. In the future, we may conduct more in-depth research on the timing of hormone therapy before thymoma surgery and whether to use hormone therapy after surgery so that patients with thymomas can receive better treatment.
This report reports a case of thymoma sensitive to low dose GCs. It highlights the need to consider preoperative trials of low-dose GC therapy in patients with significant discomfort or large, suspected thymomas.
| 1. | Siesling S, van der Zwan JM, Izarzugaza I, Jaal J, Treasure T, Foschi R, Ricardi U, Groen H, Tavilla A, Ardanaz E; RARECARE Working Group. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer. 2012;48:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 2. | Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5:S260-S265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 3. | Scorsetti M, Leo F, Trama A, D'Angelillo R, Serpico D, Macerelli M, Zucali P, Gatta G, Garassino MC. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 4. | Thomas CR, Wright CD, Loehrer PJ. Thymoma: state of the art. J Clin Oncol. 1999;17:2280-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 5. | Couture MM, Mountain CF. Thymoma. Semin Surg Oncol. 1990;6:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 6. | Salyer WR, Eggleston JC. Thymoma: a clinical and pathological study of 65 cases. Cancer. 1976;37:229-249. [PubMed] [DOI] [Full Text] |
| 7. | Gomez D, Komaki R, Yu J, Ikushima H, Bezjak A. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol. 2011;6:S1743-S1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 8. | Imbimbo M, Ottaviano M, Vitali M, Fabbri A, Leuzzi G, Fiore M, Franceschini D, Pasello G, Perrino M, Schiavon M, Pruneri G, Dei Tos AP, Sangalli C, Garassino MC, Berardi R, Alessi A, Calareso G, Petrini I, Scorsetti M, Scotti V, Rosso L, Rea F, Pastorino U, Casali PG, Ramella S, Ricardi U, Abate-Daga L, Torri V, Trama A, Palmieri G, Marino M, Zucali PA; TYME network collaborators. Best practices for the management of thymic epithelial tumors: A position paper by the Italian collaborative group for ThYmic MalignanciEs (TYME). Cancer Treat Rev. 2018;71:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 9. | Fornasiero A, Daniele O, Ghiotto C, Piazza M, Fiore-Donati L, Calabró F, Rea F, Fiorentino MV. Chemotherapy for invasive thymoma. A 13-year experience. Cancer. 1991;68:30-33. [PubMed] [DOI] [Full Text] |
| 10. | Loehrer PJ Sr, Kim K, Aisner SC, Livingston R, Einhorn LH, Johnson D, Blum R. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol. 1994;12:1164-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 197] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 11. | Lucchi M, Ambrogi MC, Duranti L, Basolo F, Fontanini G, Angeletti CA, Mussi A. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg. 2005;79:1840-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 12. | Allen AM, Czerminska M, Jänne PA, Sugarbaker DJ, Bueno R, Harris JR, Court L, Baldini EH. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 13. | Nakamura S, Kawaguchi K, Fukui T, Hakiri S, Ozeki N, Mori S, Goto M, Hashimoto K, Ito T, Yokoi K. Multimodality therapy for thymoma patients with pleural dissemination. Gen Thorac Cardiovasc Surg. 2019;67:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 14. | Kobayashi Y, Fujii Y, Yano M, Sasaki H, Yukiue H, Haneda H, Suzuki E, Endo K, Kawano O. Preoperative steroid pulse therapy for invasive thymoma: clinical experience and mechanism of action. Cancer. 2006;106:1901-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 15. | Berg J, Tonev GT, Grimnes JO, Suhrke P, Hammarström C, Vu HPN. Thymoma with pleural metastases. Tidsskr Nor Laegeforen. 2023;143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 16. | Goldman AJ, Herrmann C Jr, Keesey JC, Mulder DG, Brown WJ. Myasthenia gravis and invasive thymoma: a 20-year experience. Neurology. 1975;25:1021-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 17. | Hanibuchi M, Saijo A, Mitsuhashi A, Kajimoto T, Kitagawa T. A rare case of invasive thymoma presented a dramatic response to low-dose prednisolone as a single-drug therapy. J Med Invest. 2021;68:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 18. | Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2099] [Cited by in RCA: 2101] [Article Influence: 105.1] [Reference Citation Analysis (1)] |
| 19. | Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1379] [Article Influence: 34.5] [Reference Citation Analysis (1)] |
| 20. | Mimae T, Tsuta K, Takahashi F, Yoshida A, Kondo T, Murakami Y, Okada M, Takeuchi M, Asamura H, Tsuda H. Steroid receptor expression in thymomas and thymic carcinomas. Cancer. 2011;117:4396-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 21. | Tateyama H, Takahashi E, Saito Y, Fukai I, Fujii Y, Niwa H, Eimoto T. Histopathologic changes of thymoma preoperatively treated with corticosteroids. Virchows Arch. 2001;438:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 22. | Funakoshi Y, Shiono H, Inoue M, Kadota Y, Ohta M, Matsuda H, Okumura M, Eimoto T. Glucocorticoids induce G1 cell cycle arrest in human neoplastic thymic epithelial cells. J Cancer Res Clin Oncol. 2005;131:314-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (1)] |