INTRODUCTION
Hereditary hemorrhagic telangiectasia (HHT), also known as Rendu-Osler-Weber disease, is a dominantly-inherited disorder with an estimated prevalence of 1 in 5000-8000 individuals[1]. The underlying pathology of HHT involves a lack of capillary beds, resulting in arteriovenous malformations (AVMs), which can lead to spontaneous epistaxis, multiple mucocutaneous telangiectasias, and arteriovenous shunts in various organs[2]. HHT can be diagnosed through genetic testing or following the Curaçao Criteria, which includes the presence of epistaxis, telangiectasias, visceral lesions, or a family history. A diagnosis of HHT is considered “definite” if at least three criteria are present and “possible” if two are met[3]. The main genetic mutations responsible for HHT are endoglin and activin receptor-like kinase 1 (ACVRL1), resulting in HHT type 1 (HHT1) and HHT2, respectively. Mutations in the growth differentiation factor 2 gene, which belongs to HHT5, and in the decapentaplegic homolog 4 have also been reported. All these genes play a role in the transforming growth factor beta (TGF-β) signaling pathway involved in angiogenesis[4].
Pulmonary arterial hypertension (PAH) is a condition characterized by elevated mean PAH resulting from various underlying pathologies[5]. PAH is a rare but severe complication of HHT. The pathophysiological mechanisms linking HHT to PAH include interference with the TGF-β pathway and systemic vasculopathy[4]. Heritable PAH (HPAH) is a subtype of PAH, with less than 1% of HHT patients developing HPAH due to mutations in the ACVRL1 gene. Because of the association between PAH and genetic mutations in HHT, heritable diseases are now classified as group 1 PAH[6]. Patients with HHT complicated by PAH are thought to have worse outcomes than those with PAH alone. Notably, PAH patients with an ACVRL1 mutation are diagnosed at a younger age and have a worse prognosis, despite receiving similar therapy and demonstrating better hemodynamics at the time of diagnosis[7]. The management of HHT involves medical or interventional therapy, which can be challenging in HHT-PAH patients. Postpartum PAH complicated with HHT is a rare condition. Diagnosing and treating PAH in pregnant or postpartum HHT patients can be particularly challenging. There are few reports investigating the efficacy of pulmonary vasodilators in improving hemodynamics in postpartum patients with this condition. In this case report, we present the effects of different pulmonary vasodilators on a postpartum woman with an ACVRL1 mutation who was diagnosed with both HHT and PAH.
CASE PRESENTATION
Chief complaints
A 28-year-old woman, who had recently given birth, was admitted to our hospital with complaints of dyspnea on exertion for the past five months, as well as chest tightness and shortness of breath for the past ten days.
History of present illness
At 24-week gestation, the patient experienced a cold and fever, followed by a dry cough that persisted for about a month. Her dyspnea on exertion worsened over time. Ten days prior to admission, she delivered a healthy baby boy vaginally, after which her dyspnea became markedly more severe.
History of past illness
The patient reported having giving birth to a healthy girl three years ago. Her first pregnancy was uneventful, except for recurrent episodes of epistaxis, for which she did not see medical treatment. During her second pregnancy, she experienced recurrent epistaxis, as well as episodes of dizziness and ocular blurring.
Personal and family history
The patient disclosed that her mother had also experienced recurrent epistaxis during both of her pregnancies.
Physical examination
Upon admission, the physical examination showed a pulse rate of 111 beats per minute, blood pressure of 134/80 mmHg, body temperature of 36.5 °C, and a respiration rate of 30 breaths per minute. Despite receiving high-flow oxygen therapy (FiO2: 80%, flow rate: 50 L/minute), the pulse oxygen saturation remained at 88%. Cyanosis of the lips and facial erythema were observed.
Laboratory examinations
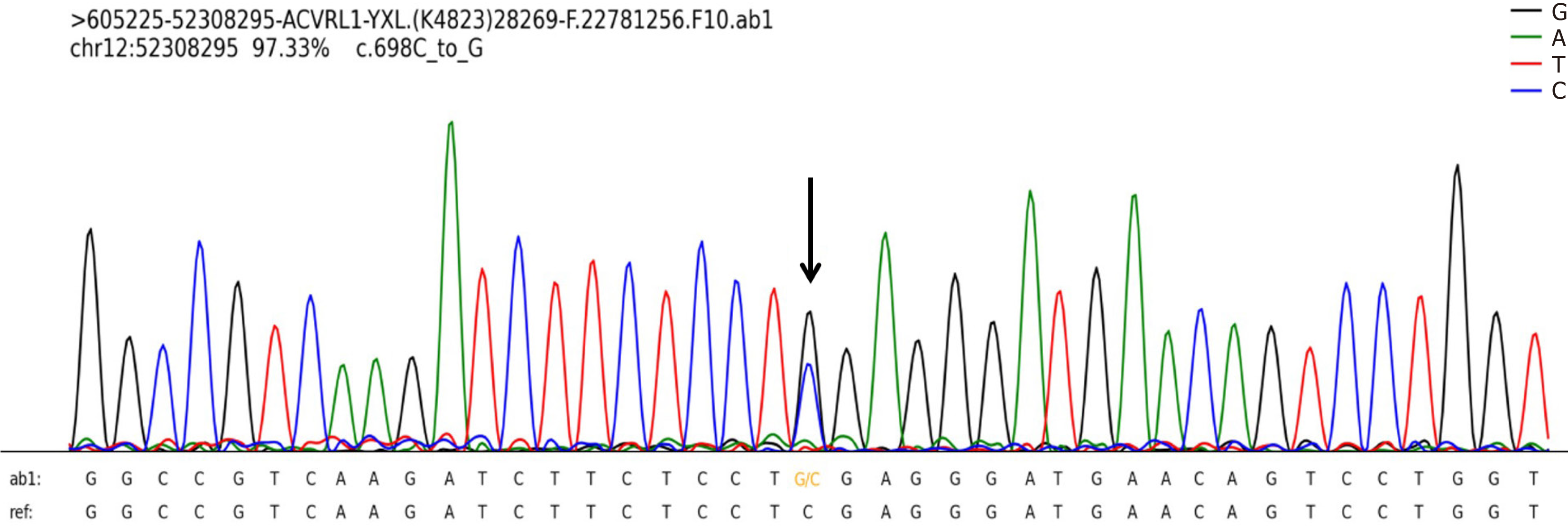
Upon arrival at the hospital, the patient’s arterial blood gas (ABG) test revealed type I respiratory failure (PaO2/FiO2 ratio: 134.75 mmHg, PaO2: 53.9 mmHg, PaCO2: 26.8 mmHg, PH: 7.42). She was subsequently admitted to the intensive care unit. Her complete blood count was within normal limits, but her hemoglobin level was elevated (151 g/L). Liver, kidney and cardiac indicators showed slightly decreased albumin (37 g/L), increased lactate dehydrogenase (276 U/L), alanine aminotransferase (106 U/L), uric acid (458 μmol/L), and N-terminus pro-brain natriuretic peptide (NT-proBNP) (140.8 pg/mL). Immunological markers and immunoglobulin levels were normal. Coagulation function tests revealed elevated D-dimer (1.59 mg/L) and fibrinogen (4.33 g/L). Genetic testing identified a mutation in ACVRL1 [chr12: 52308295, M_000020.3, c.698C>G, (p.S233W)] (Figure 1).
Figure 1 Identification of a heterozygous variant of ACVRL1 in the patient.
Imaging examinations
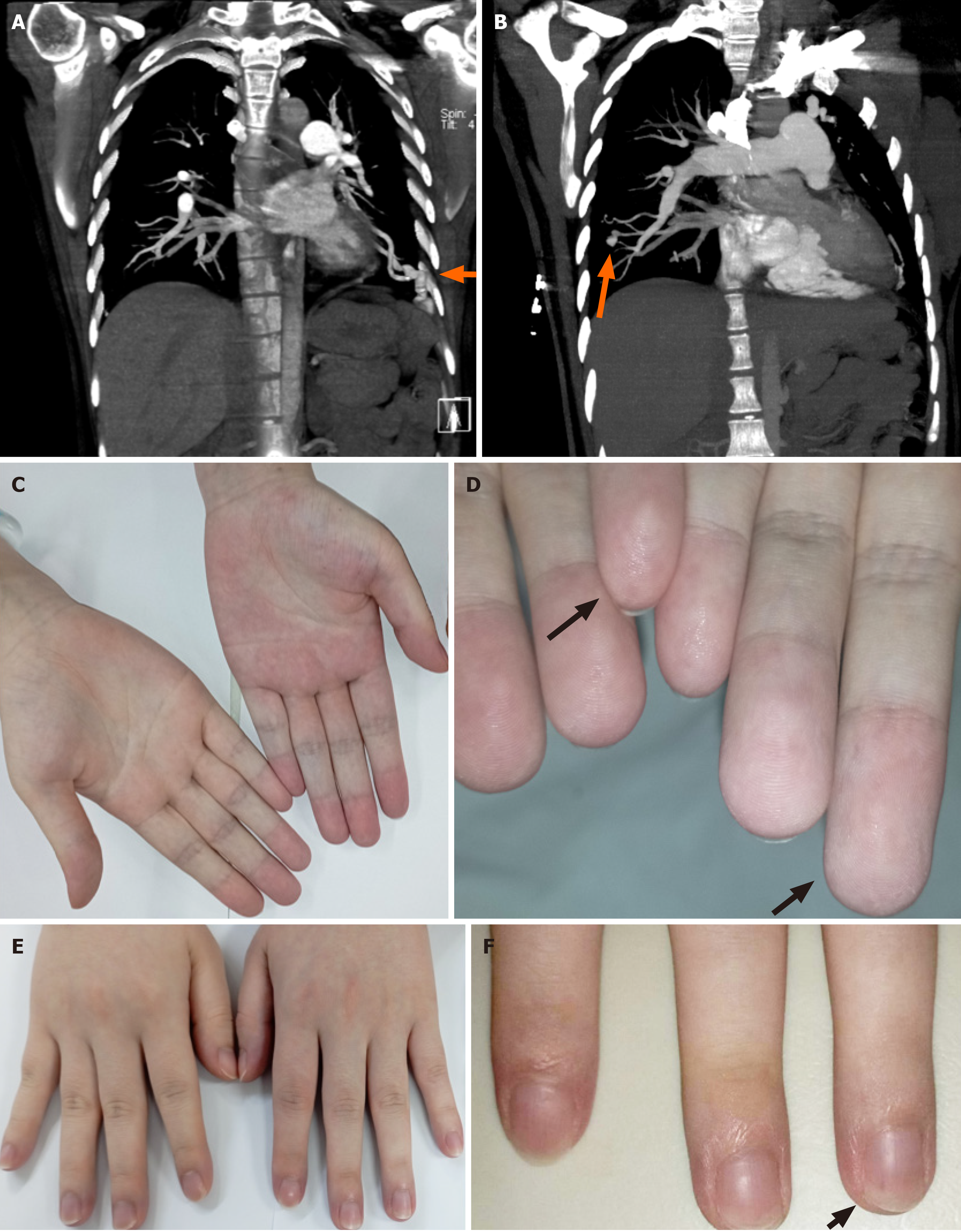
Initial imaging raised concerns about pulmonary embolism. However, chest computed tomography angiography performed on the day of arrival revealed multiple bilateral pulmonary AVMs (PAVMs) (Figure 2A and B). Cyanosis and telangiectasias were observed on the patient’s fingertips (Figure 2C-F) and lips.
Figure 2 The patient’s clinical manifestations at admission.
A and B: The patient’s imaging presentation on admission, bilateral pulmonary arteriovenous malformations shown by the orange arrows; C and D: Telangiectases (black arrows) are observed in both distant and closer views of the palm of the patient’s hands; E and F: Telangiectases (black arrows) are observed in both distant and closer views of back of the patient’s hands.
TREATMENT
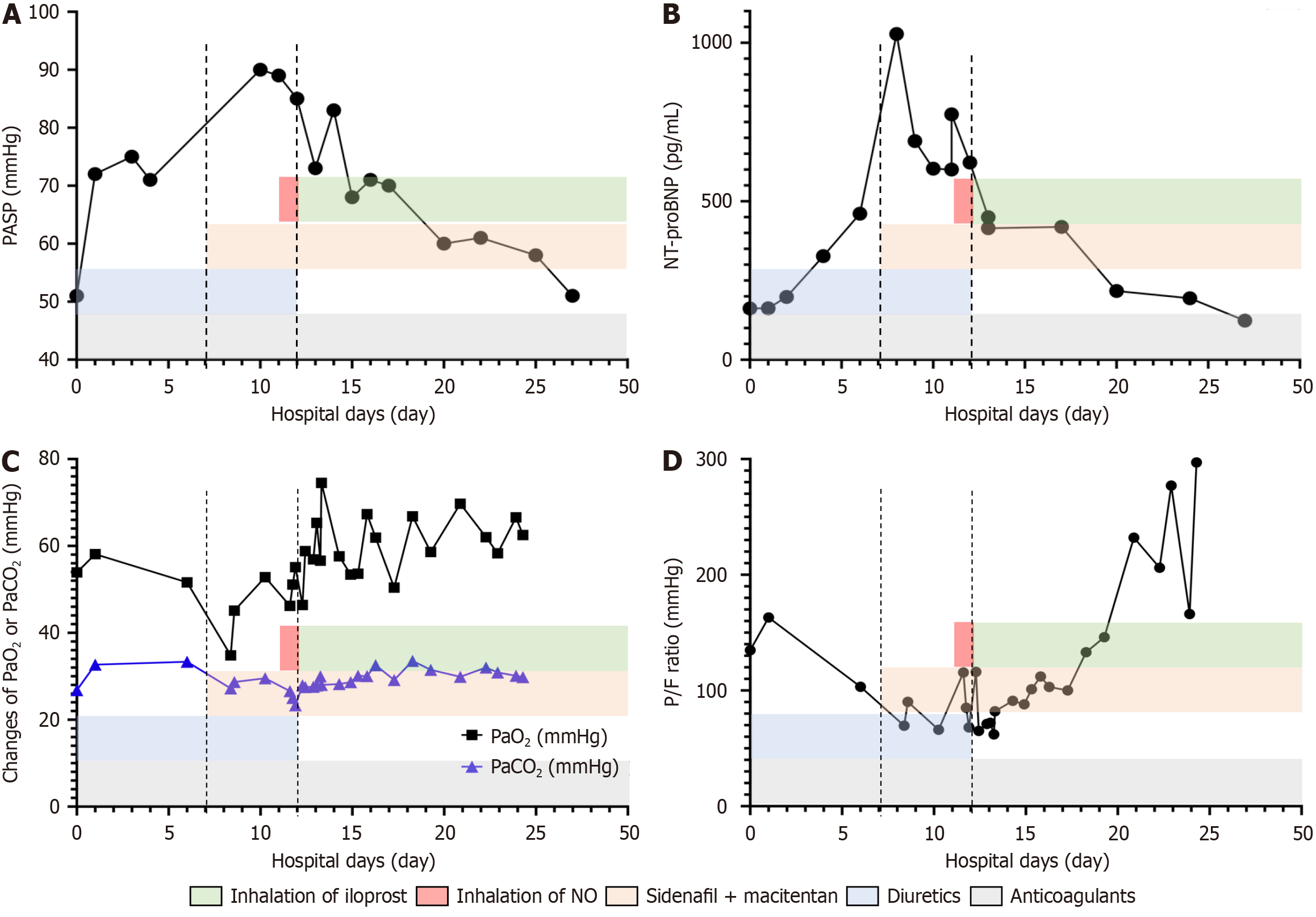
The alterations in PASP, NT-proBNP, and ABG levels under different treatments are depicted in Figure 3. Specifically, within the first seven days of admission, the patient was treated with diuretics (hydrochlorothiazide, triamterene) and anticoagulant (low molecular weight heparin calcium). However, her dyspnea continued to deteriorate, accompanied by a decrease in PaO2/FiO2 ratio (from 134.75 mmHg to 82 mmHg), an increase in PASP (from 51 mmHg to 80 mmHg), and a rise in NT-proBNP (from 162 pg/mL to 461 pg/mL). Considering her PAH diagnosis, treatment was initiated with sildenafil 18.75 mg three times a day, a phosphodiesterase type 5 inhibitor, and macitentan 5 mg once daily, a dual endothelin receptor antagonist for four days. This was followed by 24-hour nitric oxide (NO) inhalation administered via high flow nasal cannula. The oxygen supplement of high flow nasal cannula (FiO2 increased from 0.8 to 0.9, flow from 30 L/minute to 50 L/minute) was provided for her instead of mask (flow: 10 L/minute). Despite these interventions, there was no significant improvement in her dyspnea or clinical indicators. As a result, Ventavis® (iloprost) inhalation therapy was introduced (1.25 μg in 3 mL of 0.9% NaCl solution per administration every three hours) for eight days. During this period, the patient’s PaO2/FiO2 ratio improved significantly (from 68 mmHg to 133 mmHg), and her PASP decreased (from 85 mmHg to 70 mmHg). Due to limited availability of Ventavis®, the dosage was reduced (1.25 μg in 3 mL of 0.9% NaCl solution every six hours) and continued for five days. The patient was discharged on the 27th day with improved symptoms and indicators, as shown in Figure 3 (PaO2/FiO2 ratio: 297 mmHg, NT-proBNP: 123 pg/mL, and PASP: 50 mmHg). By discharge, she was able to walk 100 m with assistance without experiencing dyspnea or requiring oxygen supplementation. However, the patient declined to continue taking medication post-discharge.
Figure 3 Changes in this case at the time of treatment.
A: Changes in pulmonary artery systolic pressure ratio of this case under treatments; B: Changes in N-terminus pro-brain natriuretic peptide ratio of this case under treatments; C: Changes in PaO2 or PaCO2 ratio of this case under treatments; D: Changes in PaO2/FiO2 ratio of this case under treatments. PASP: Pulmonary artery systolic pressure; NT-proBNP: N-terminus pro-brain natriuretic peptide; P/F: PaO2/FiO2; NO: Nitric oxide.
DISCUSSION
We hereby present a case report on a postpartum woman with concurrent pulmonary hypertension and HHT, complicated by an ACVRL1 mutation. Notably, inhalation of iloprost led to a prompt amelioration of symptoms and improvement in examination findings, ultimately resulting in a successful hospital discharge. Several intriguing clinical manifestations were observed in this case, which may provide valuable insights into the management of similarly afflicted patients. HHT is a systemic disease that affects multiple organ systems, including the brain, liver, and lungs, leading to diverse clinical presentations[8]. Its management depends on the organs involved and the specific clinical manifestations, with treatment options including blood transfusion, surgery, embolization, drainage, and diuretics[9]. During pregnancy or delivery, HHT patients are at increased risk of developing life-threatening complications, including bleeding (0.8%-4.3%), stroke (1.3%), and myocardial infarction (0.5%)[10]. In the case presented, the patient experienced progressively worsening dyspnea during her pregnancy, which became more severe after delivery. Echocardiography revealed significantly elevated PAP, along with typical HHT features including epistaxis and PAVMs, presenting considerable challenges in both diagnosis and management.
PAH, as a complication of HHT, is gaining increasing recognition. Mutations in ACVRL1 genes have been associated with PAH and HHT[11]. A study evaluating ACVRL1 genotype and PAP in adult HHT patients found that, among 68 patients, 27 had a mutation in ACVRL1, and 9 had elevated PASP. Further investigation confirmed that 7 of the 9 patients with elevated PASP also had a mutation in ACVRL1[12]. While these patients did not exhibit severe pulmonary hypertension, many had coexisting vascular anomalies detected by contrast echocardiography, which could complicate the estimation of pulmonary vascular resistance[12]. Further investigation is necessary to determine whether HHT is a consequence of PAH or whether the two conditions coexist. Although research on postpartum changes in pulmonary blood flow is limited, hormonal and hemodynamic alterations during pregnancy are known to exacerbate PAVMs[13]. Pulmonary arteriovenous fistulas, frequently associated with HHT, may exhibit increased pulmonary shunts during pregnancy[14]. When PAVMs expand beyond the capacity of normal pulmonary capillary beds, the proportion of pulmonary shunts may increase due to the elevate blood volume in late pregnancy[15]. Pulmonary shunting and resultant hypoxemia may lead to pulmonary vascular constriction and an increased PAP, creating a vicious cycle of pulmonary arteriovenous shunting.
In this case, the patient’s dyspnea worsened gradually at 24-week pregnancy and continued to deteriorate after delivery. Our assessment suggests that the patient’s PAH and hypoxia may be linked to postpartum fluid volume expansion and HHT-related PAVMs. Diuretics were administered to address potential fluid overload, but the patient’s symptoms persisted, indicating that additional factors may have been contributing. One possibility is that the patient had HPAH, which was aggravated during pregnancy. The presence of HHT may induce arteriovenous shunting in the patient’s pulmonary circulation via AVM, thereby reducing pressure on the right heart, similar to the effect of balloon atrial septostomy. Although PAVM embolization is a common treatment method for pregnant patients with HHT, in this case, we believe that occluding these open pulmonary AVMs could potentially worsen the patient’s PAH, or leading to the opening or worsening of other arteriovenous fistulas post-occlusion.
A recent literature review identified 24 cases of severe complications in HHT pregnant women between 1986 and 2022[16]. Among them, seven women had hypoxemia but were not monitored with echocardiography or PAP assessments. Three patients had received PAVM treatment for HHT prior to pregnancy, but the PAVMs reoccurred during pregnancy, resulting in hemoptysis. In addition, three patients with both PAH and HHT were reported, all of whom had ACVRL1 mutations. Two of them developed symptoms of PAH before being diagnosed with HHT. None of these three patients responded to acute vasodilator testing, and did not initially respond to treatment with oxygen and/or inhaled NO[17]. Managing pregnant women with severe hypoxia due to PAH and HHT is particularly challenging. Following consultations with the radiology, cardiology, and obstetrics and gynecology departments, and considering the patient’s lack of obvious bleeding symptoms and the ineffectiveness of diuretics, we decided to proceed with PAP reduction treatment.
The pathophysiology of PAH is complex. Current therapeutic strategies focus on targeting the three primary molecular pathways: Prostacyclin, endothelin-1, and NO. All of these pathways primarily function by modulating the tone of the pulmonary vasculature, leading to vasodilation[18]. Phosphodiesterase 5 inhibitors block the degradation of cyclic guanosine monophosphate, enhancing NO signaling to produce significant vasodilation and antiproliferative effects, particularly in the pulmonary vasculature[19]. Ambrisentan, tadalafil, and sildenafil are phosphodiesterase 5 inhibitors that have been used in the treatment of cases with HHT-PAH associated with ACVRL1 mutations[20,21]. Endothelin receptor antagonists, such as bosentan, inhibit endothelin-1 activity, leading to vasodilation, and have been reported in case studies for treating PAH in HHT patients with ACVRL1 mutations[22-24]. Case reports also describe the use of intravenous or oral epoprostenol in treating PAH in these patients[25]. Prostacyclin, a potent vasodilator produced by endothelial cells, and its synthetic derivatives (epoprostenol, treprostinil, and iloprost) also induce pulmonary vasodilation. ACVRL1 is a type I receptor of the TGF-β family. Its mutation can disrupt TGF-β signaling, leading to dysfunction of pulmonary endothelial and/or smooth muscle cells and the proliferative features of PAH[22,26]. Jerkic et al[27] reported that endothelial cell dysfunction and reduced NO production were observed in ACVRL1 gene knockout mice but not in wild-type mice. There are few reports on the treatment of HHT-PAH with ACVRL1 mutations, and even fewer cases in the postpartum context. While the drugs mentioned above are theoretically effective based on their mechanisms, this may not always be the case in practice. For example, inhaled NO has been reported as ineffective in all cases of HHT-PAH with ACVRL1 mutations. Wu et al[28] reported a 45-year-old female with HHT-PAH and ACVRL1 mutations who, despite treatment with ambrisentan, died of heart failure within ten months. Jamindar et al[25] reported that a female patient, after three months of sildenafil treatment, still exhibited signs of right heart failure, and her symptoms only stabilized after switching to bosentan and intravenous epoprostenol. Some reports also suggest that personalized treatment, and combination therapy might be effective in improving patient conditions in HHT-PAH. However, these reports do not focus on the postpartum state, and there are no detailed reports on the immediate improvement in oxygenation or symptom relief. Bleeding and anemia have been reported in these case studies, but it remains unclear whether they are adverse effects of systemic treatments (e.g., endothelin receptor antagonists, which may compromise endothelial integrity and increase bleeding risk) or a consequence of the underlying disease. Inhaled nebulized medications offer several advantages, such as the ability to deliver the drug directly to the target organ, resulting in higher local concentrations and reduced systemic toxicity. Additionally, inhalation has minimal impact on the ventilation-perfusion ratio in the lungs, thereby exerting only a minimal effect on gas exchange. Lastly, for the same drug, the inhaled dosage is typically lower, which helps reduce costs and alleviate the patient’s financial burden[29].
In this case, sildenafil and macitentan were used to target the endothelin receptors and inhibit phosphodiesterase-5, but no significant improvement was seen in either PAP or ABG levels. Inhaled NO also failed to alleviate the patient’s condition, consistent with previous reports. In Bonderman et al’s report[30], two female patients with HHT-related PAH showed improved PAH after one year of bosentan treatment. However, these patients were not genetically tested, and they were not pregnant at the time. Regarding our case, the limited duration of treatment with sildenafil and macitentan may help explain the lack of significant improvement observed. In contrast, inhaled iloprost has shown promising efficacy in the treatment of PAH. According to the guidelines[31], it is recommended as a class I monotherapy for patients with PAH in World Health Organization functional class III (with level B evidence) and as a class IIb monotherapy for those in World Health Organization functional class IV (with level C evidence). Our case reports that inhalation of iloprost led to significant improvements in symptoms and indicators in a patient with HHT-PAH associated with an ACVRL1 mutation. Furthermore, no bleeding symptoms, such as epistaxis, gastrointestinal bleeding, or hemoptysis, were observed during the treatment.