Published online Apr 16, 2025. doi: 10.12998/wjcc.v13.i11.100161
Revised: November 19, 2024
Accepted: December 16, 2024
Published online: April 16, 2025
Processing time: 134 Days and 23.7 Hours
Lung cancer is one of the most common and deadly cancers worldwide. As the disease progresses and due to the side effects of treatment, patients’ physical activity significantly decreases.
To systematically review and conduct a meta-analysis on the effects of exercise rehabilitation on the physical activity of lung cancer patients and determine the best implementation methods to provide clinical guidance.
Literature was searched through multiple electronic databases. A random effects model was used to combine effect sizes through standardized mean difference (SMD). The Cochrane risk of bias tool was used to assess the quality of the lite
A total of 11 studies involving 541 patients were included in this study. The phy
Exercise rehabilitation effectively improved muscle function in NSCLC patients, especially strength training, respiratory training, and cross-training. Cardiopulmonary function also showed improvement, particularly when exercise duration exceeded 1 hour, age was ≥ 65 years, and the intervention period was more than 3 months. A single exercise duration of more than 0.5 hours can enhance patients’ physical endurance. Appropriately increasing exercise duration and selecting suitable exercise forms can effectively improve the physical activity of NSCLC patients.
Core Tip: This study conducted a meta-analysis on the effects of exercise rehabilitation for lung cancer patients. It found that exercise, especially strength, respiratory, and cross-training, significantly improved muscle function. Cardiopulmonary function also improved, particularly with exercise exceeding 1 hour. A single exercise duration over 0.5 hours enhanced physical endurance. Increasing exercise duration and choosing suitable forms can improve non-small cell lung cancer patients’ activity.
- Citation: Xu SH, Xu H, Xiao KW, Mao SJ. Exercise rehabilitation on patients with non-small cell lung cancer: A meta-analysis of randomized controlled trials. World J Clin Cases 2025; 13(11): 100161
- URL: https://www.wjgnet.com/2307-8960/full/v13/i11/100161.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i11.100161
Lung cancer is a malignant tumor occurring in the lungs. It is one of the most common cancers globally and a leading cause of cancer-related deaths[1]. Lung cancer typically forms due to genetic mutations in lung cells, causing uncontro
The current main treatments for NSCLC include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. These treatments significantly impact patients’ lung function decline and respiratory muscle weakness, leading to postoperative cardiopulmonary function impairment, respiratory system disorders, decreased exercise tolerance, and limited physical activity, severely affecting patients’ quality of life and postoperative recovery. Maintaining and impro
Numerous studies have shown that implementing pulmonary rehabilitation training can effectively enhance the exercise endurance and quality of life for lung cancer patients[7]. Preoperative exercise training helps improve patients’ surgical tolerance and reduces postoperative complications, while systematic postoperative exercise rehabilitation acce
This study has been registered in the International Prospective Register of Systematic Reviews and Meta-Analyses Database[11] with the registration number CRD42024546506. To ensure the integrity and reproducibility of the research, the writing of this study strictly follows the PRISMA 2020 checklist.
Inclusion criteria: (1) Participants: Patients who have been diagnosed with lung cancer, including symptomatic and asymptomatic patients, and all included patients must have undergone surgical treatment; (2) Study type: Only ran
Exclusion criteria: (1) Non-randomized or uncontrolled studies are excluded; (2) Case studies or special case reports are excluded; (3) Studies with incomplete outcome data or data that cannot be extracted are excluded; (4) Review articles and animal studies are excluded; and (5) Non-English literature is excluded.
Multiple electronic databases were used for literature retrieval, including PubMed, EMBASE, Cochrane Library, EBScohost, and Web of Science. Based on the PICOS principle, both Medical Subject Headings and free terms were used to determine the search terms (Table 1), with P: Pulmonary neoplasms, and I: Exercise rehabilitation. To ensure comprehensiveness and relevance, no restrictions were placed on the control group, outcome indicators, or type of literature during the search, and logical operators were used to combine search terms. Additionally, citations from relevant reviews and meta-analyses were traced to expand the search depth. The search timeframe was set from the establishment of each database to October 2023, and the search language was limited to English.
| Search strategy | Subject terms | Mesh terms |
| T1 | Lung neoplasms | Pulmonary neoplasms OR neoplasms, lung OR lung neoplasm OR neoplasm, lung OR neoplasms, pulmonary OR neoplasm, pulmonary OR pulmonary neoplasm OR lung cancer OR cancer, lung OR cancers, lung OR lung cancers OR pulmonary cancer OR cancer, pulmonary OR cancers, pulmonary OR pulmonary cancers OR cancer of the lung OR cancer of lung |
| T2 | Exercise | Exercises OR physical activity OR activities, physical OR activity, physical OR physical activities OR exercise, physical OR exercises, physical OR physical exercise OR physical exercises OR acute exercise OR acute exercises OR exercise, acute OR exercises, acute OR exercise, isometric OR exercises, isometric OR isometric exercises OR isometric exercise OR exercise, aerobic OR aerobic exercise OR aerobic exercises OR exercises, aerobic OR exercise training OR exercise trainings OR training, exercise OR trainings, exercise |
| T3 | T1 and T2 | |
| Search strategy | Subject terms | Medical Subject Headings terms |
We implemented a detailed literature screening process to ensure the accuracy and systematic nature of the research. In the initial screening stage, we managed the search results using the EndnoteX20 reference management software. After deduplication, studies were excluded based on titles and abstracts according to the inclusion and exclusion criteria, eliminating studies that did not align with the research objectives. Subsequently, the studies that met the preliminary screening criteria underwent full-text review to further assess their eligibility. Exclusion criteria included non-RCTs, case studies, and studies with incomplete data. To ensure transparency and reproducibility, all information and results from the screening process were recorded and managed via EndnoteX20. The study selection process was independently conducted by two researchers, Xiao KW and Xu SH. Any discrepancies or disputes were resolved by a third party, Xu H, aiming to enhance the credibility and scientific rigor of the research results through stringent standards and transparent processes.
In this study, the data collection and extraction process was strictly conducted according to the Cochrane data extraction protocol[12]. Data extraction was independently performed by two researchers, Xiao KW and Xu SH, using customized data extraction forms, which helped standardize information collection and reduce bias. The extraction forms included several key areas: Baseline characteristics of lung cancer patients, surgical status, details of the intervention measures, outcome indicators, and information on whether the control group participants were taking specific medications. Researchers first independently extracted data from the literature and then compared their results to identify any inconsistencies. If discrepancies or disputes arose during the data extraction process, they were reviewed and decided upon by a third researcher, Xu H. Extracted baseline characteristics included patients’ age, gender, disease stage, and other relevant health indicators. Surgical status was recorded as whether surgery had been performed. Details of the interventions included the type (AE, strength training, cross-training, breathing exercises), frequency, and duration.
In this meta-analysis, we included indicators related to exercise capacity to evaluate the impact of exercise rehabilitation on lung cancer patients. Functional strength, muscle strength, endurance, and cardiopulmonary function collectively reflect the overall exercise capacity of lung cancer patients. The six-minute walk test (6MWT) demonstrated an indi
Specific indicators included in this study were the 6MWT, predicted percentage, and walking distance to comprehensively evaluate the patients’ functional endurance. Muscle strength test indicators included thigh muscle strength, grip strength test, Timed Up and Go test, 30-second sit-to-stand test, and one-leg stand test to explore patients’ muscle strength and endurance[14]. By observing maximal oxygen consumption (VO2max) peak and maximum values, exercise thresholds, and their predicted percentages, we aimed to clarify the effects of exercise rehabilitation on lung cancer patients’ exercise capacity and further explore its role and potential benefits in the rehabilitation of lung cancer patients.
In this study, to ensure the quality of the included literature and reduce the risk of bias, we employed quality assessment and bias risk evaluation methods. All included RCTs were assessed for quality using the Cochrane risk of bias[15] Tool via Revman 5.4. This tool specifically evaluates potential bias risks in RCTs, including random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other biases. “Other biases” refer to potential sources of bias beyond the six domains mentioned above. According to the Cochrane Handbook guidelines, other biases may include baseline imbalance bias, early termination bias, learning effect bias, duplicate publication bias, and funding source bias, among others, which could impact study outcomes. Each study was classified as “low risk”, “uncertain”, or “high risk” based on these dimensions.
To assess potential publication bias, we performed Egger’s test to detect small study effects and asymmetry risk, commonly used in meta-analyses to evaluate bias. To further verify the robustness of our results, we also conducted sensitivity analyses using the leave-one-out approach. This involved re-performing the meta-analysis by sequentially excluding each study to examine the impact of any single study on the overall effect estimate and to identify studies that might excessively influence the overall analysis results.
In this meta-analysis, we used Stata16.1 statistical software, employing both fixed-effect and random-effect models to combine effect sizes. Preliminary statistical tests (I2 statistics) were used to assess heterogeneity among studies. If heterogeneity was not significant (I2 < 40%), a fixed-effect model was used; otherwise, if heterogeneity was significant (I2 ≥ 40%), a random-effect model was applied. Due to differences in the units of measurement for the included study indicators, we calculated effect sizes and heterogeneity using the standardized mean difference (SMD), and generated forest plots, publication bias graphs, and sensitivity analysis charts.
Subgroup analyses were performed based on the classification of participants’ age, intervention duration, exercise type, and single intervention duration to explore the potential impact of these factors on the overall effect estimate. This aimed to more accurately understand the effects of exercise rehabilitation on lung cancer patients under different conditions. To verify the robustness of our research results, sensitivity analyses were conducted on the included studies, which helped identify and confirm key factors affecting the study conclusions and ensure the reliability of the results. Throughout the meta-analysis process, we strictly adhered to the Cochrane Handbook’s data analysis standards to ensure the transparency and scientific integrity of the analysis.
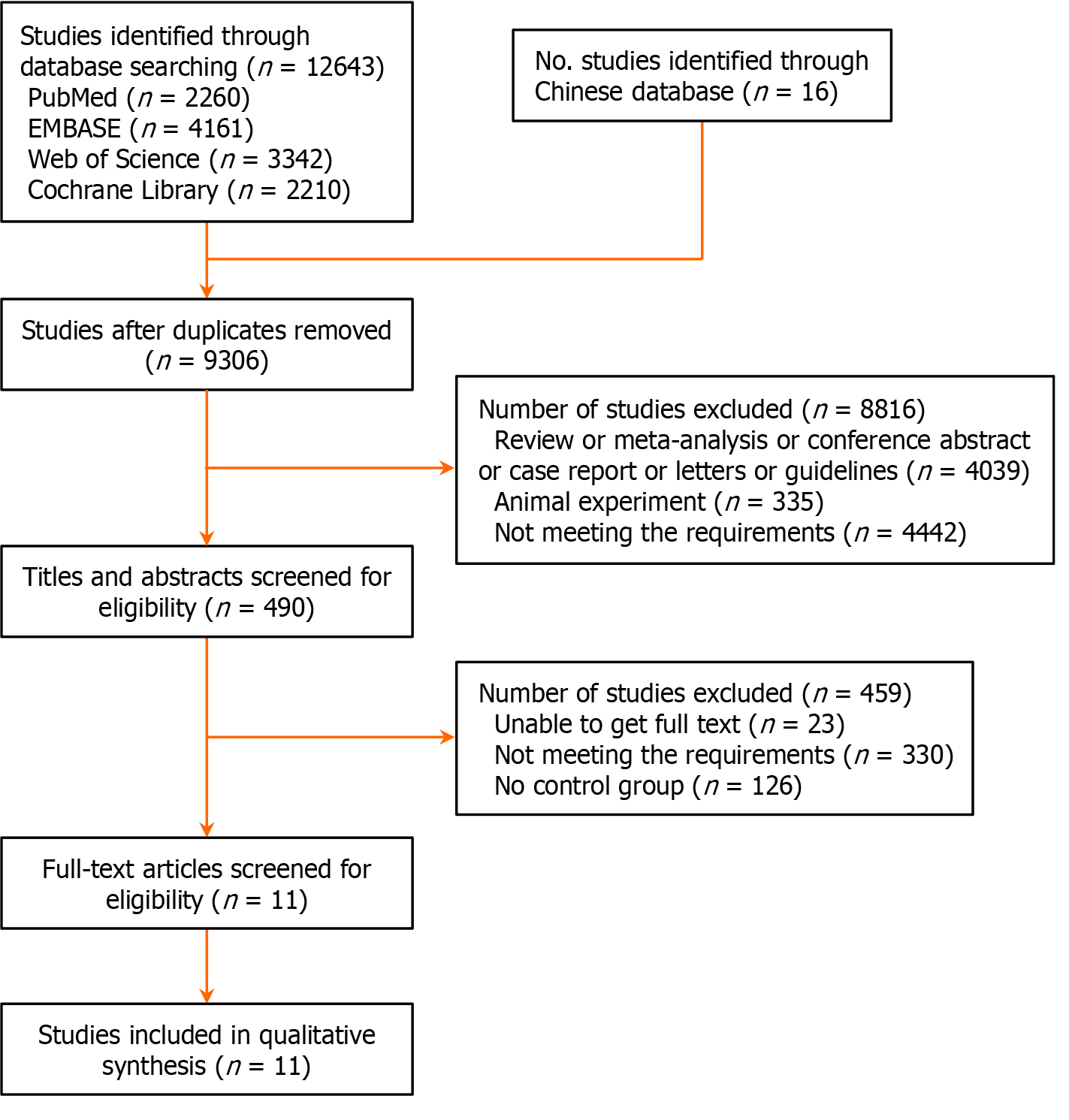
This study searched databases such as PubMed, EMBASE, Web of Science, and the Cochrane Library. A total of 12643 articles were retrieved, along with 16 additional articles from other sources. After removing duplicates, 9306 articles remained. Initial screening based on titles and abstracts, following inclusion and exclusion criteria, resulted in 490 articles for further review. After full-text screening, 479 articles were excluded, and ultimately, 11 RCTs on exercise rehabilitation for improving exercise capacity in NSCLC patients were included in this meta-analysis (Figure 1).
This meta-analysis included 11 RCTs on exercise rehabilitation improving exercise capacity in lung cancer patients (Table 2), involving 541 patients, all of whom had NSCLC. The exercise rehabilitation interventions included AE, RT, high-intensity interval training (HIIT), and XT. The experimental groups’ ages ranged from 56 to 72 years, with exercise intervention durations varying from less than 0.5 hours to over 1 hour per session, and frequencies ranging from twice weekly to six times weekly. Most intervention periods lasted 12 weeks, with control groups typically receiving standard care or no specific intervention.
| Authors | Year | Preoperative/ postoperative | Type | Medication | Experimental group age, mean ± SD | Experimental group gender (male/female) | Intervention method | Duratio (hour/time) | Frequency (time/week) | Period (week) | Control group age, mean ± SD | Control group gender | Control method |
| Gill Arbane | 2010 | After surgery | NSCLC | Analgesia | 65.4 ± 8.75 | N/A | ST | < 0.5 | 2 | 12 | 62.6 ± 3.75 | N/A | UC |
| Denise Shuk Ting Cheung | 2021 | Preoperative | NSCLC | N/A | 61.00 ± 12.12 | 5/5 | AE | 0.5-1 | 2 | 12 | 58.36 ± 9.32 | 5/6 | SM |
| Denise Shuk Ting Cheung | 2021 | Preoperative | NSCLC | N/A | 61.11 ± 7.01 | 6/3 | RT | 0.5-1 | 2 | 12 | 58.36 ± 9.32 | 5/6 | SM |
| Raquel Sebio García | 2017 | Preoperative | NSCLC | N/A | 70.9 ± 6.1 | 9/1 | ST | 0.5-1 | 3-5 | According to actual condition | 69.4 ± 9.4 | 11/1 | UC |
| Chueh-Lung Hwang | 2012 | Preoperative + after surgery | NSCLC | N/A | 61.0 ± 6.3 | 5/8 | HIIT | 0.5-1 | 3 | < 12 | 58.5 ± 8.2 | 7/4 | UC |
| Marcus Jonsson | 2019 | Preoperative + after surgery | NSCLC | Analgesia | 68.7 ± 7.4 | 29/25 | HIIT | < 0.5 | 6 | 12 | 68.4 ± 8.3 | 18/35 | N/A |
| Marta K. Mikkelsen | 2022 | Preoperative + after surgery | NSCLC | N/A | 72.1 ± 1.8 | 19/22 | XT | 0.5-1 | 2 | 12 | 71.5 ± 1.625 | 17/26 | UC |
| Maria T. Morano | 2013 | Preoperative | NSCLC | N/A | 68.8 ± 7.3 | 5/7 | RT | < 0.5 | 4 | < 12 | 64.8 ± 8 | 4/8 | UC |
| Morten Quist | 2020 | Preoperative | NSCLC | N/A | 65.2 ± 8.2 | 55/55 | XT | > 1 | 2 | 12 | 63.5 ± 8.7 | 52/56 | UC |
| Anna Rutkowska | 2019 | Preoperative | NSCLC | N/A | 59.1 ± 6.8 | 18/2 | XT | 0.5-1 | 5 | < 12 | 61.3 ± 8.8 | 9/1 | UC |
| Francesco Stefanelli | 2013 | Preoperative | NSCLC | Improve lung function | 65.5 ± 7.4 | N/A | RT | > 1 | 5 | < 12 | 64.8 ± 7.3 | N/A | UC |
| Rui-Chen Ma | 2021 | Preoperative | NSCLC | N/A | 56.97 ± 7.09 | 13/21 | AE | 0.5-1 | 2 | According to actual condition | 54.91 ± 10.09 | 8/27 | UC |
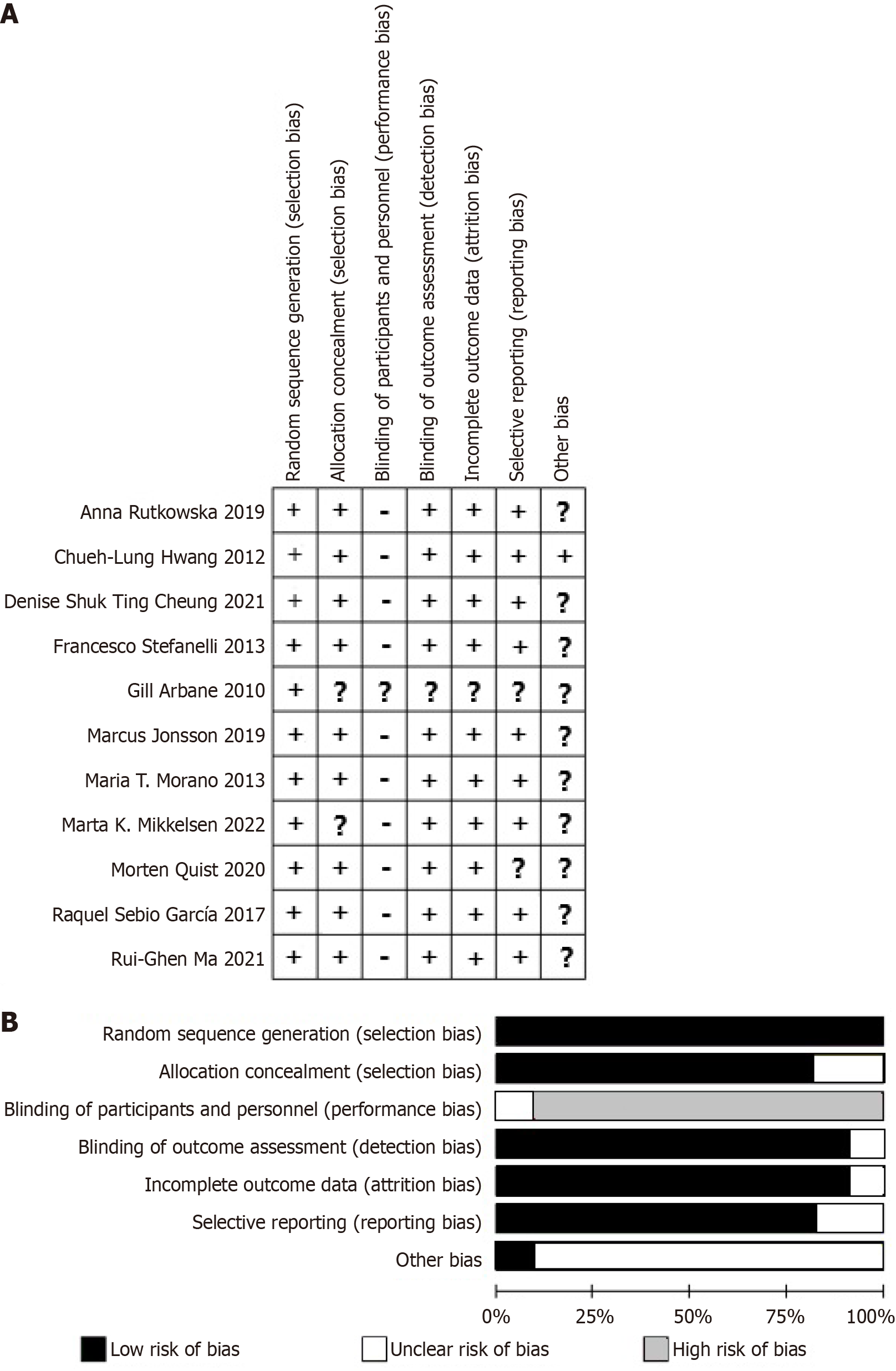
This study evaluated the quality of the included studies using the Cochrane risk of bias tool. The quality assessment chart displays the scores of each study across seven risks of bias items. As shown in Figure 2A, “+” represent low risk; “?” represent unclear risk, and “-” represent high risk. Overall, Gill (2010) showed low risk only in random allocation, with other items not clearly described. Most studies had a high risk in blinding of participants and personnel due to the nature of lung cancer patients and the interventions, making blinding impractical. Most studies showed low risk in random sequence generation and allocation concealment, while other biases were not specified (Figure 2B). From the summary of publication bias results (Figure 3), most studies showed low risk in random sequence generation, allocation concealment, incomplete outcome data, and selective reporting. However, there was some uncertainty and high risk in blinding of participants and personnel, as well as other biases. Other biases identified include incomplete reporting of baseline characteristics and inconsistent standardization of intervention implementation. These bias risks might affect the reliability and interpretability of the overall analysis results.
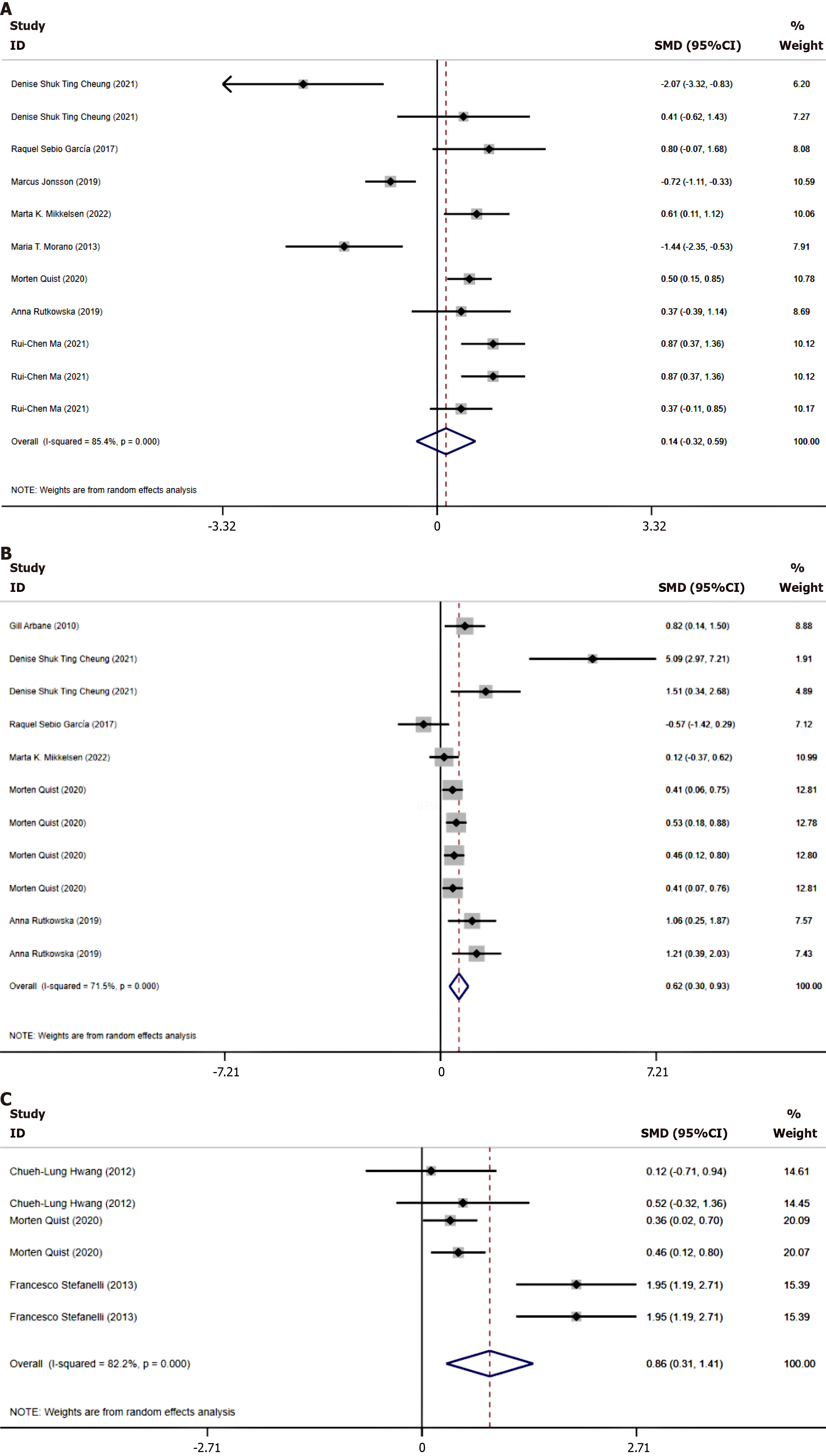
Effect of exercise rehabilitation on physical endurance in lung cancer patients: The 6MWT is a recognized standard test for measuring patients’ physical endurance, effectively reflecting the maximum distance a patient can walk within a specified time, thereby assessing their cardiopulmonary endurance and functional status. In this study, we evaluated the physical endurance of lung cancer patients by combining the 6MWT indicators, finding high heterogeneity (I2 = 85.4%), thus choosing to use a random effects model. Due to the inclusion of both time and distance units, the SMD was used for effect size consolidation (Figure 4A). The results of this meta-analysis showed that the combined effect size SMD = 0.14, P = 0.558, with no statistical significance. Further subgroup analysis revealed that when the exercise duration per session was between 0.5 and 1 hour, the SMD = 0.44, P ≤ 0.05; and when it was more than 1 hour, the SMD = 0.5, P ≤ 0.05. This indicates that when the exercise duration exceeds 0.5 hours, there is a significant improvement in the physical endurance of lung cancer patients. This result supports that longer exercise durations in rehabilitation can enhance physical endu
| Included indicators | Analysis indicators | Group | SMD | 95%CI | P value | I2 |
| Physical endurance | Age subgroup analysis | Age < 65 | 0.31 | -0.25 to 0.86 | 0.28 | 76.20% |
| Age ≥ 65 | 0.14 | -0.32 to 0.59 | 0.95 | 89.90% | ||
| Intervention, method, subgroup | AE | -2.07 | -3.32 to -0.83 | 0.01 | - | |
| RT | -2 | -1.34 to 0.94 | 0.733 | - | ||
| ST | 0.8 | -0.07 to 1.68 | 0.07 | - | ||
| HIIT | -0.72 | -1.11 to -0.33 | 0.0001 | - | ||
| XT | 0.65 | 0.43 to 0.86 | 0.0001 | 0% | ||
| Single intervention duration | < 0.5 hours | -0.96 | -1.62 to -0.30 | 0.034 | 50.60% | |
| 0.5-1 hour | 0.44 | 0.03 to 0.85 | 0.004 | 67.30% | ||
| > 1 hour | 0.5 | 0.15 to 0.85 | 0.005 | - | ||
| Intervention period | < 3 months | 0.27 | -0.39 to 0.93 | 0.421 | 80.80% | |
| ≥ 3 months | 0.04 | -0.60 to 0.68 | 0.906 | 87.50% | ||
| Overall | 0.14 | -0.32 to 0.59 | 0.558 | 85.40% | ||
| Muscle function | Age subgroup analysis | Age ≥ 65 | 0.394 | 0.205 to 0.582 | 0.0001 | 26.80% |
| Age < 65 | 1.842 | 0.725 to 2.960 | 0.001 | 76% | ||
| Intervention, method, subgroup | AE | 5.09 | 2.965 to 7.215 | 0.825 | - | |
| RT | 1.514 | 0.345 to 2.684 | 0.011 | - | ||
| ST | 0.152 | -1.203 to 1.508 | 0.001 | 83.80% | ||
| XT | 0.477 | 0.298 to 0.656 | 0.0001 | 71.50% | ||
| Single intervention duration | < 0.5 hours | 0.818 | 0.140 to 1.497 | 0.018 | - | |
| 0.5-1 hour | 1.087 | 0.154 to 2.021 | 0.022 | 85.10% | ||
| > 1 hour | 0.451 | 0.279 to 0.624 | 0.0001 | 0 | ||
| Intervention period | < 3 months | 2.197 | 0.330 to 4.064 | 0.021 | 0.86 | |
| ≥ 3 months | 0.437 | 0.213 to 0.661 | 3.83 | 0.453 | ||
| Overall | 0.619 | 0.304 to 0.933 | 0.0001 | 0.715 | ||
| Cardiorespiratory function | Age subgroup analysis | Age < 65 | 0.313 | -0.275 to 0.902 | 0.297 | 0 |
| Age ≥ 65 | 1.097 | 0.370 to 1.824 | 0.003 | 88.70% | ||
| Intervention, method, subgroup | HIIT | 0.313 | -0.275 to 0.902 | 0.297 | 0 | |
| RT | 1.950 | 1.413 to 2.487 | 0.001 | 0 | ||
| XT | 0.409 | 0.166 to 0.652 | 0.0001 | 0 | ||
| Single intervention duration | 0.5-1 hour | 0.313 | -0.275 to 0.902 | 0.297 | 0 | |
| > 1 hour | 1.097 | 0.370 to 1.824 | 0.297 | 0 | ||
| Intervention period | 3 months | 0.313 | -0.275 to 0.902 | 0.003 | 88.70% | |
| ≥ 3 months | 1.097 | 0.370 to 1.824 | 0.002 | 82.7% | ||
| Overall | 0.856 | 0.307 to 1.406 | - | - | ||
Effect of exercise rehabilitation on muscle function in lung cancer patients: In this study, we conducted a combined effect size analysis of multiple muscle function indicators in lung cancer patients. Considering the high heterogeneity among the included studies (I2 = 71.5%), a random effects model was chosen. The main muscle function tests involved in the analysis included thigh muscle strength tests, grip strength tests, Timed Up and Go tests, 30-second sit-to-stand tests, and single-leg stand tests, thus using SMD as the combined effect size (Figure 4B). The results of the muscle function evaluation showed an overall combined effect size SMD = 0.619 (P = 0.001), which was statistically significant, indicating that exercise intervention effectively improved muscle function in lung cancer patients overall. Subgroup analysis further revealed differences in the effects of various intervention forms, with strength training, RT, and XT all showing significant improvements in muscle function (P < 0.05), while AE did not reach statistical significance (P > 0.05). Additionally, the effect was significant when exercise duration exceeded 1 hour or was less than 0.5 hours (P ≤ 0.05), reflecting that exercise within these time ranges is particularly effective in enhancing physical endurance in lung cancer patients. When exercise duration was between 0.5 and 1 hour, the effect was not significant (P > 0.05) (Table 3). These findings emphasize the need to consider the form and duration of exercise when designing rehabilitation programs to optimize therapeutic effects.
Impact of exercise rehabilitation on cardiopulmonary function in lung cancer patients: This study assessed the cardiopulmonary function of lung cancer patients using peak VO2, maximum VO2, exercise threshold, and their predicted percentages. Due to the different measurement units for each indicator, the SMD was used to combine effect sizes. High heterogeneity among the studies (I2 = 82.7%) necessitated the use of a random effects model for the meta-analysis (Figure 4C). The results show that exercise rehabilitation significantly improves the cardiopulmonary function of lung cancer patients, with an SMD of 0.856, 95% confidence interval (CI) = 0.307-1.406, P = 0.002, indicating statistical significance. This demonstrates the effectiveness of exercise rehabilitation in enhancing the cardiopulmonary endurance of lung cancer patients. Further subgroup analysis found that when the single session exercise duration was between 0.5 to 1 hour using HIIT, and the patients were under 65 years of age, the P values were all > 0.05, suggesting that under these specific conditions, the exercise intervention does not significantly improve cardiopulmonary function. Conversely, when the exercise duration exceeded 1 hour, the age was over 65, and the interventions included RT and XT with an intervention period ≥ 3 months, the P values were ≤ 0.05, indicating significant effects of exercise on cardiopulmonary function under these conditions (Table 3). Thus, it can be seen that appropriately increasing exercise duration and selecting suitable forms of exercise can more effectively enhance cardiopulmonary endurance, and exercise rehabilitation is particularly effective in improving outcomes for older adults.
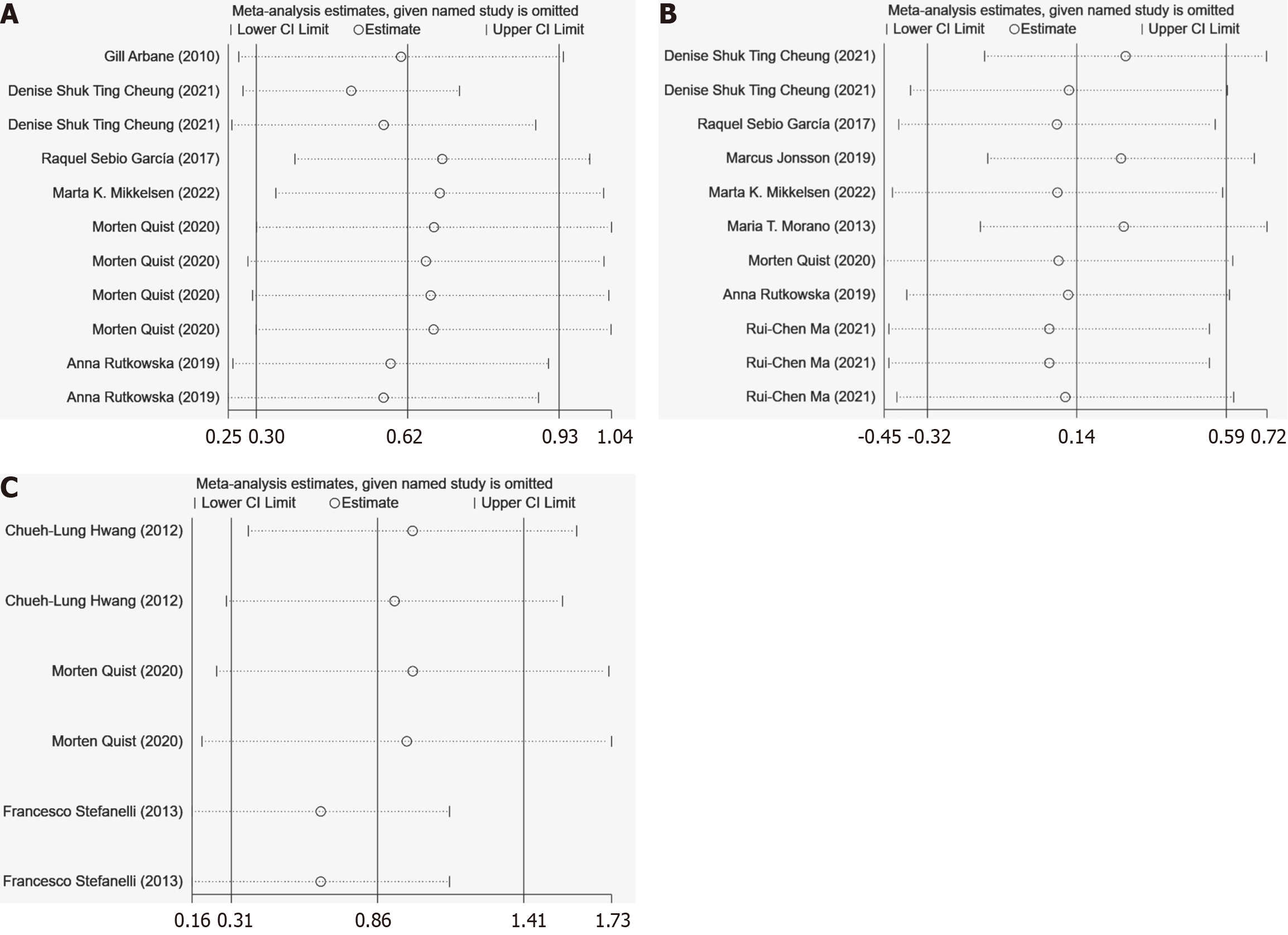
This study employed influence analysis and the leave-one-out method for sensitivity analysis. Influence analysis confirmed that the results were consistent with the combined effect sizes (Figure 5), indicating no potential outliers had a significant impact on the meta-analysis results, thus demonstrating the stability of the study outcomes. The leave-one-out method was used, and recalculating the meta-analysis statistics showed that the results remained stable after this process, with no single study significantly influencing the overall conclusions, affirming the reliability of the study results.
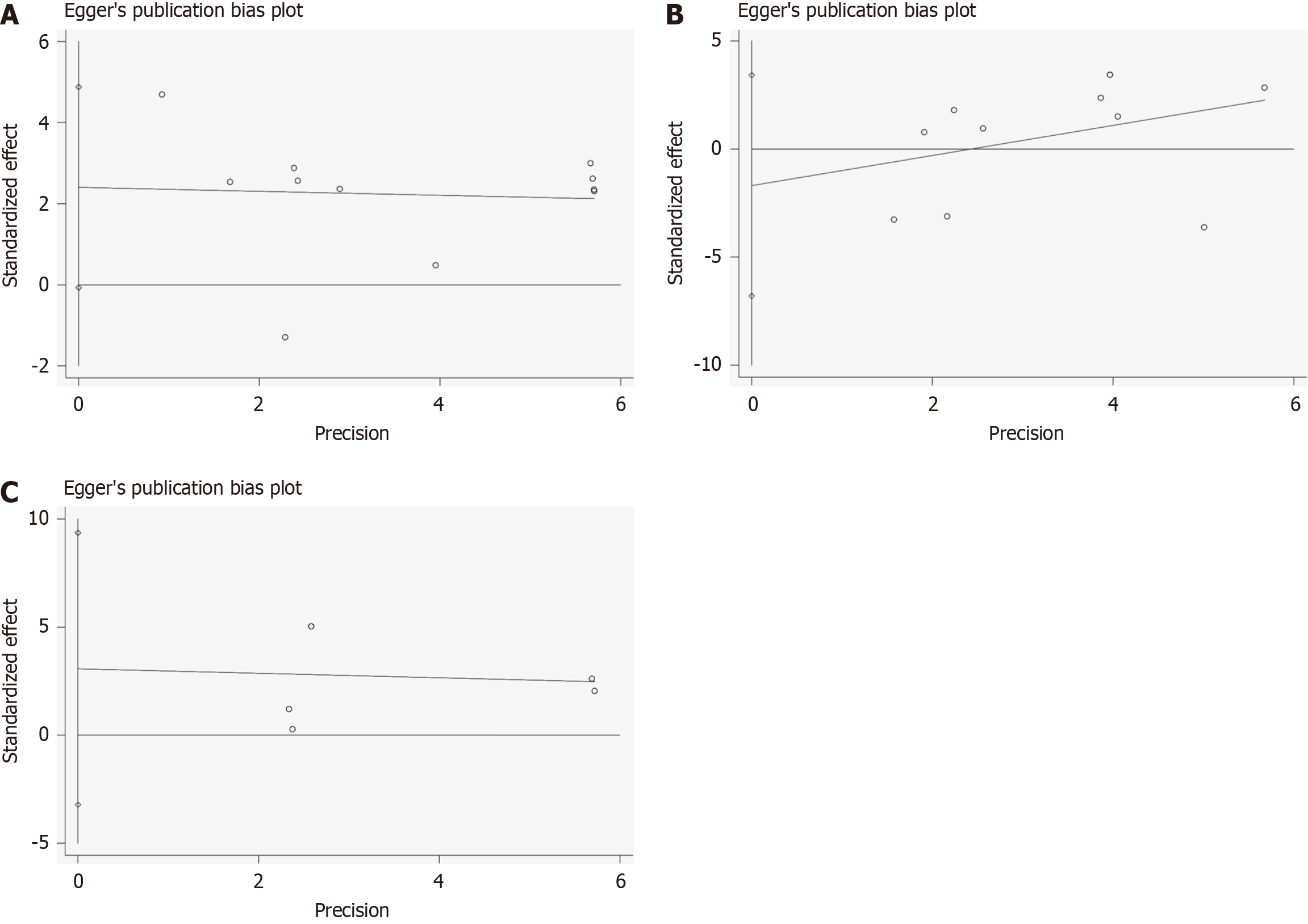
In the Egger’s test analysis, no significant publication bias was observed in the data related to physical endurance, muscle function, or cardiopulmonary function. The publication bias analysis for physical endurance showed that both the slope and bias were not statistically significant (slope P = 0.30, bias P = 0.47), indicating no obvious publication bias. The 95%CI for the slope ranged from -0.72 to 2.11, and for the bias from -6.79 to 3.41, both encompassing zero, further supporting the conclusion of no significant bias. The muscle function Egger’s test results showed an estimated slope of -0.05, standard error of 0.28, t value of -0.18, P value of 0.86, indicating no statistical significance for the slope, with a 95%CI ranging from -0.67 to 0.57, indicating no significant publication bias. The analysis of cardiopulmonary function data also showed no significant publication bias. The P values for the slope and bias analyses were 0.87 and 0.25, respectively, indicating no statistical significance, showcasing the stability and reliability of the data. The confidence intervals for both slope and bias included zero, further confirming the consistency of the research results and the absence of systematic bias.
The exercise capacity of patients with NSCLC plays a crucial role in their overall health and quality of life. Maintaining and enhancing exercise capacity not only helps them cope better with the side effects of treatment but also improves their daily activity levels and mental health. However, there is still considerable debate regarding the applicability and programs of exercise rehabilitation. Our meta-analysis found that exercise rehabilitation effectively enhances muscle function and cardiopulmonary function; moreover, a single session of exercise rehabilitation lasting more than 0.5 hours significantly improves patients’ physical endurance. This meta-analysis demonstrates that exercise rehabilitation significantly enhances the muscle function of NSCLC patients, aligning with previous research findings. Appropriate exercise rehabilitation not only strengthens muscle power but also improves overall physical function. Research by Peddle-McIntyre et al[16] has shown that cancer patients can significantly increase muscle strength and physical endurance through strength and endurance training. Lung cancer patients participating in systematic exercise rehabilitation programs have shown significant improvements in muscle strength and function. Exercise rehabilitation also significantly enhances the cardiopulmonary function of NSCLC patients. Numerous studies indicate that cardiopulmonary function is a critical factor affecting the quality of life and prognosis of lung cancer patients[17,18]. Research by Lindenmann et al[19] in 2020 has found that lung cancer patients significantly improve their peak VO2 and maximum heart rate through postoperative aerobic training, consistent with the results of this study.
Our meta-analysis found that when the duration of a single exercise rehabilitation session exceeds 0.5 hours, there is an improvement in the 6MWT scores of NSCLC patients. Research by Rodríguez-Cañamero et al[20] in 2022 has shown that prolonged aerobic training significantly enhances the physical endurance of lung cancer patients. A large-scale epidemiological survey found that more than 150 minutes of moderate-intensity exercise per week can significantly improve the physical endurance and survival rates of lung cancer patients[21]. However, some studies have noted that short-duration, HIIT can also effectively improve physical endurance. This inconsistency may relate to factors such as research design, patient characteristics, and the intensity and frequency of exercise.
Exercise rehabilitation can improve and enhance muscle function; exercise promotes the balance of muscle protein synthesis and degradation, making muscle fibers stronger and more enduring. The mechanical load induced by exercise stimulates the growth of muscle fibers, enhancing muscle protein synthesis rates through signaling pathways such as mammalian target of rapamycin and adenosine 5’-monophosphate-activated protein kinase[22]. Exercise also enhances mitochondrial function in muscles, improving energy metabolism efficiency, and overall muscle endurance and function[23]. Due to the prolonged disease and treatment process, NSCLC patients often experience muscle atrophy and strength decline. Exercise rehabilitation can reduce inflammatory cytokines’ damage to muscles through its anti-inflammatory effects and decrease oxidative stress to muscle tissues through its antioxidant actions, thereby protecting and restoring muscle function. Personalized exercise rehabilitation programs can effectively enhance the muscle function of NSCLC patients, with gradually increasing loads stimulating the adaptive enhancement and strength improvement of muscles. Large-scale epidemiological surveys have found that cancer patients participating in exercise rehabilitation significantly outperform non-participants in physical, psychological, and social functions. Compared to traditional drug treatments, it not only improves muscle function but also enhances their quality of life, alleviates adverse reactions from treatment, and improves patient survival quality.
This study, through subgroup analysis, found that exercise durations exceeding 1 hour or less than 0.5 hours sig
The mechanism by which exercise enhances the cardiopulmonary function of NSCLC patients primarily involves adaptive changes in the cardiovascular and respiratory systems. Physical activity increases cardiac output and blood oxygen transport capacity, enhancing alveolar gas exchange efficiency and thereby improving oxygenation capabilities[24]. Long-term physical activity can strengthen myocardial contractility, lower heart rates, and enhance cardiac efficiency. These physiological changes not only improve patients’ exercise endurance but also reduce respiratory distress during physical activities. NSCLC patients often face a decline in cardiopulmonary function due to cancer and its treat
This study found that when exercise interventions exceed 0.5 hours, patients’ physical endurance improves. This may be due to the continuous exercise stimulus that promotes adaptive changes in the cardiovascular and muscular systems[31]. Extended periods of exercise increase cardiac output, enhance blood oxygen transport efficiency, and promote alveolar gas exchange, improving oxygenation capabilities. NSCLC patients often suffer from systemic inflammation and oxidative stress, which can affect their physical endurance and rehabilitation outcomes[32]. Long-term exercise rehabilitation can improve the cellular microenvironment by reducing inflammation markers and oxidative stress levels, promoting tissue repair and functional recovery. Past studies have indicated that sustained AE significantly reduces levels of C-reactive protein and interleukin-6 in NSCLC patients, decreasing systemic inflammation[33]. Past research has shown that regular and prolonged exercise rehabilitation significantly reduces mortality and recurrence rates in NSCLC patients[34], enhancing quality of life and functional independence. However, short-term exercise interventions often fail to provide sufficient stimulus and may not significantly reduce inflammation markers and oxidative stress levels, thus limiting their effectiveness in improving overall patient health[35].
In this meta-analysis, the results of the subgroup analysis should be interpreted with caution due to methodological limitations. The subgroup analysis in this study was conducted at the study level, based on 11 RCTs, rather than at the individual level of the 541 patients. Although the original studies were designed as RCTs, key baseline characteristics (age, gender ratio, disease stage, comorbidities) were not fully balanced across subgroups after stratification. Uneven sample sizes across subgroups may have further reduced statistical power. Additionally, the distribution of potential confounding factors (chemotherapy regimens, nutritional status, and complications) was not effectively controlled among the subgroups. Therefore, the results of this subgroup analysis should be regarded as hypothesis-generating rather than definitive conclusions.
The research limitations of this article are as follows: (1) This study included only 11 RCTs, which is a small sample size that may limit the generalizability and external validity of the results. Future research should expand the sample size and conduct more RCTs for different types and stages of lung cancer patients; (2) Due to the limitations of the included studies, this research did not perform subgroup analyses for preoperative and postoperative interventions. This may lead to an incomplete understanding of the effects at different intervention time points. Future studies should explore in detail the specific impacts of preoperative and postoperative interventions on the rehabilitation of lung cancer patients; and (3) Due to the specificity of lung cancer patients and the nature of exercise rehabilitation interventions, it is difficult to implement blinding for participants and personnel administering the interventions, posing a high risk of bias. Although bias is reduced through subgroup analysis and sensitivity analysis, it cannot be completely eliminated.
This meta-analysis assessed physical endurance, muscular function, and cardiopulmonary function to explore the impact of exercise rehabilitation on the exercise capacity of NSCLC patients. The results indicate that exercise rehabilitation significantly enhances muscular function, with an overall effect size of SMD = 0.619, P = 0.001. Subgroup analyses further reveal that strength training, RT, and XT significantly improve muscular function (P < 0.05), while the effect of AE did not reach statistical significance (P > 0.05). Moreover, interventions lasting more than one hour per session showed more significant effects. In terms of cardiopulmonary function, exercise rehabilitation also demonstrated significant improvements (SMD = 0.856, 95%CI = 0.307-1.406, P = 0.002). Subgroup analyses showed particularly significant im
| 1. | Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, Zhang Z, Gao T, Zhang Y, Li L. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). 2023;136:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 113] [Reference Citation Analysis (32)] |
| 2. | Wadowska K, Bil-Lula I, Trembecki Ł, Śliwińska-Mossoń M. Genetic Markers in Lung Cancer Diagnosis: A Review. Int J Mol Sci. 2020;21:4569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (32)] |
| 3. | Rodak O, Peris-Díaz MD, Olbromski M, Podhorska-Okołów M, Dzięgiel P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers (Basel). 2021;13:4705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (31)] |
| 4. | Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, Bivona T, Diehn M, Dive C, Dziadziuszko R, Leighl N, Malapelle U, Mok T, Peled N, Raez LE, Sequist L, Sholl L, Swanton C, Abbosh C, Tan D, Wakelee H, Wistuba I, Bunn R, Freeman-Daily J, Wynes M, Belani C, Mitsudomi T, Gandara D. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J Thorac Oncol. 2021;16:1647-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 369] [Article Influence: 92.3] [Reference Citation Analysis (32)] |
| 5. | Deo SVS, Sharma J, Kumar S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann Surg Oncol. 2022;29:6497-6500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 205] [Article Influence: 68.3] [Reference Citation Analysis (33)] |
| 6. | Huang J, Deng Y, Tin MS, Lok V, Ngai CH, Zhang L, Lucero-Prisno DE 3rd, Xu W, Zheng ZJ, Elcarte E, Withers M, Wong MCS. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest. 2022;161:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (33)] |
| 7. | Codima A, das Neves Silva W, de Souza Borges AP, de Castro G Jr. Exercise prescription for symptoms and quality of life improvements in lung cancer patients: a systematic review. Support Care Cancer. 2021;29:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (33)] |
| 8. | Yang M, Liu L, Gan CE, Qiu LH, Jiang XJ, He XT, Zhang JE. Effects of home-based exercise on exercise capacity, symptoms, and quality of life in patients with lung cancer: A meta-analysis. Eur J Oncol Nurs. 2020;49:101836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (31)] |
| 9. | Zhou C, Qin Y, Zhao W, Liang Z, Li M, Liu D, Bai L, Chen Y, Chen Y, Cheng Y, Chu T, Chu Q, Deng H, Dong Y, Fang W, Fu X, Gao B, Han Y, He Y, Hong Q, Hu J, Hu Y, Jiang L, Jin Y, Lan F, Li Q, Li S, Li W, Li Y, Liang W, Lin G, Lin X, Liu M, Liu X, Liu X, Liu Z, Lv T, Mu C, Ouyang M, Qin J, Ren S, Shi H, Shi M, Su C, Su J, Sun D, Sun Y, Tang H, Wang H, Wang K, Wang K, Wang M, Wang Q, Wang W, Wang X, Wang Y, Wang Z, Wang Z, Wu L, Wu D, Xie B, Xie M, Xie X, Xie Z, Xu S, Xu X, Yang X, Yin Y, Yu Z, Zhang J, Zhang J, Zhang J, Zhang X, Zhang Y, Zhong D, Zhou Q, Zhou X, Zhou Y, Zhu B, Zhu Z, Zou C, Zhong N, He J, Bai C, Hu C, Li W, Song Y, Zhou J, Han B, Varga J, Barreiro E, Park HY, Petrella F, Saito Y, Goto T, Igai H, Bravaccini S, Zanoni M, Solli P, Watanabe S, Fiorelli A, Nakada T, Ichiki Y, Berardi R, Tsoukalas N, Girard N, Rossi A, Passaro A, Hida T, Li S, Chen L, Chen R. International expert consensus on diagnosis and treatment of lung cancer complicated by chronic obstructive pulmonary disease. Transl Lung Cancer Res. 2023;12:1661-1701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (32)] |
| 10. | Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnago D, Bria E, Jones LW, Milella M, Lanza M, Pilotto S. Physical Activity and Exercise in Lung Cancer Care: Will Promises Be Fulfilled? Oncologist. 2020;25:e555-e569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (33)] |
| 11. | Bernardo WM. PRISMA statement and PROSPERO. Int Braz J Urol. 2017;43:383-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 12. | Noyes J, Booth A, Flemming K, Garside R, Harden A, Lewin S, Pantoja T, Hannes K, Cargo M, Thomas J. Cochrane Qualitative and Implementation Methods Group guidance series-paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018;97:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (1)] |
| 13. | Pires IM, Denysyuk HV, Villasana MV, Sá J, Marques DL, Morgado JF, Albuquerque C, Zdravevski E. Development Technologies for the Monitoring of Six-Minute Walk Test: A Systematic Review. Sensors (Basel). 2022;22:581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 14. | Pinckard K, Baskin KK, Stanford KI. Effects of Exercise to Improve Cardiovascular Health. Front Cardiovasc Med. 2019;6:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 15. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24648] [Article Influence: 1760.6] [Reference Citation Analysis (3)] |
| 16. | Peddle-McIntyre CJ, Singh F, Thomas R, Newton RU, Galvão DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev. 2019;2:CD012685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 17. | Wang Q, Zhou W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J Sport Health Sci. 2021;10:201-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 18. | Sejbuk M, Mirończuk-Chodakowska I, Witkowska AM. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients. 2022;14:1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 19. | Lindenmann J, Fink-Neuboeck N, Fediuk M, Maier A, Kovacs G, Balic M, Smolle J, Smolle-Juettner FM. Preoperative Peak Oxygen Consumption: A Predictor of Survival in Resected Lung Cancer. Cancers (Basel). 2020;12:836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 20. | Rodríguez-Cañamero S, Cobo-Cuenca AI, Carmona-Torres JM, Pozuelo-Carrascosa DP, Santacruz-Salas E, Rabanales-Sotos JA, Cuesta-Mateos T, Laredo-Aguilera JA. Impact of physical exercise in advanced-stage cancer patients: Systematic review and meta-analysis. Cancer Med. 2022;11:3714-3727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (1)] |
| 21. | Yang JJ, Yu D, White E, Lee DH, Blot W, Robien K, Sinha R, Park Y, Takata Y, Gao YT, Smith-Byrne K, Monninkhof EM, Kaaks R, Langhammer A, Borch KB, Al-Shaar L, Lan Q, Sørgjerd EP, Zhang X, Zhu C, Chirlaque MD, Severi G, Overvad K, Sacerdote C, Aune D, Johansson M, Smith-Warner SA, Zheng W, Shu XO. Prediagnosis Leisure-Time Physical Activity and Lung Cancer Survival: A Pooled Analysis of 11 Cohorts. JNCI Cancer Spectr. 2022;6:pkac009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 22. | Vainshtein A, Sandri M. Signaling Pathways That Control Muscle Mass. Int J Mol Sci. 2020;21:4759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 23. | Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med Sci Sports Exerc. 2015;47:1922-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 24. | Fresiello L, Meyns B, Di Molfetta A, Ferrari G. A Model of the Cardiorespiratory Response to Aerobic Exercise in Healthy and Heart Failure Conditions. Front Physiol. 2016;7:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 26. | Maeda K, Higashimoto Y, Honda N, Shiraishi M, Hirohata T, Minami K, Iwasaki T, Chiba Y, Yamagata T, Terada K, Matsuo Y, Shuntoh H, Tohda Y, Fukuda K. Effect of a postoperative outpatient pulmonary rehabilitation program on physical activity in patients who underwent pulmonary resection for lung cancer. Geriatr Gerontol Int. 2016;16:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 27. | Pouliopoulou DV, Macdermid JC, Saunders E, Peters S, Brunton L, Miller E, Quinn KL, Pereira TV, Bobos P. Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2023;6:e2333838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (1)] |
| 28. | Su XE, Hong WP, He HF, Lin S, Wu SH, Liu F, Lin CL. Recent advances in postoperative pulmonary rehabilitation of patients with nonsmall cell lung cancer (Review). Int J Oncol. 2022;61:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 29. | Morishita S, Hamaue Y, Fukushima T, Tanaka T, Fu JB, Nakano J. Effect of Exercise on Mortality and Recurrence in Patients With Cancer: A Systematic Review and Meta-Analysis. Integr Cancer Ther. 2020;19:1534735420917462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 30. | Helminen O, Valo J, Andersen H, Lautamäki A, Vuohelainen V, Sihvo E. Real-world guideline-based treatment of lung cancer improves short- and long-term outcomes and resection rate: A population-based study. Lung Cancer. 2020;140:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Hughes DC, Ellefsen S, Baar K. Adaptations to Endurance and Strength Training. Cold Spring Harb Perspect Med. 2018;8:a029769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 32. | Matei B, Winters-Stone KM, Raber J. Examining the Mechanisms behind Exercise's Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation. Antioxidants (Basel). 2023;12:1423. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Ni HJ, Pudasaini B, Yuan XT, Li HF, Shi L, Yuan P. Exercise Training for Patients Pre- and Postsurgically Treated for Non-Small Cell Lung Cancer: A Systematic Review and Meta-analysis. Integr Cancer Ther. 2017;16:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Alhebshi A, Alsharif N, Thorley J, James LJ, Clifford T. The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials. Antioxidants (Basel). 2021;11:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |