Published online Apr 6, 2025. doi: 10.12998/wjcc.v13.i10.101796
Revised: October 31, 2024
Accepted: December 3, 2024
Published online: April 6, 2025
Processing time: 82 Days and 23.8 Hours
Cauda equina syndrome (CES) is characterized by a group of symptoms that may be caused by inflammation, spinal cord compression, venous congestion, or ischemia. This syndrome is commonly an indication for surgical intervention but has not been determined as a postoperative complication following surgery for lumbar spine disease.
To report the case of a 54-year-old male patient who had CES following spinal surgery, with no obvious compression lesions found during re-exploration, suggesting that vascular insufficiency may have contributed to the condition. Furthermore, a series of urodynamic studies on bladder recovery patterns in such complications have also been investigated.
Postoperative CES requires urgent imaging and exploration to rule out com
Core Tip: This report discusses postoperative Cauda equina syndrome (CES) with neurogenic bladder as a potential complication of spinal surgery. Emergency imaging is crucial to exclude spinal cord compression. If stenosis remains, further exploration is needed. Compressive lesions might not explain the patient's symptoms, suggesting vascular insufficiency. In noncompressive CES, conservative management may be appropriate. Postoperative cystometrogram findings might initially show no bladder contraction and delayed first sensation, with contractility improving later.
- Citation: Yang KW, Lai WH, Huang DW. Cauda equina syndrome with urinary retention as a postoperative complication of lumbar spine surgery: A case report. World J Clin Cases 2025; 13(10): 101796
- URL: https://www.wjgnet.com/2307-8960/full/v13/i10/101796.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i10.101796
Cauda equina syndrome (CES) is characterized by a group of symptoms that result from nerve compression or damage to the cauda equina. It includes low back pain, sciatica, lower-extremity weakness, saddle-type anesthesia, and bowel and bladder dysfunction. Prompt treatment of CES is crucial as it is usually considered a surgical emergency to prevent further neurological impairments. However, the indications and timing of repeat surgery in postoperative CES need to be investigated further. Due to the absence of organic compression, some experts recommend re-exploration for unexplained neurological deficits[1], whereas others prefer conservative management with close monitoring[2]. This study aims to report a case of CES following spinal surgery, including urodynamic findings and relevant literature.
A 54-year-old Chinese male had bilateral leg numbness with neurogenic claudication for 3 months. Patient received spinal surgery but urine retention was noted after operation.
Patient underwent spinal surgery. On the second postoperative day, the patient was unable to urinate after the removal of the Foley catheter and required reinsertion. He complained of constipation and mild weakness in both legs while standing.
Patient had lumbago for a decade and progressed recently. The magnetic resonance imaging (MRI) findings indicated severe stenosis and disk bulging at L4-L5. To treat this condition, on October 22, 2020, the patient underwent L4 bilateral laminotomy, L4-L5 microdiscectomy, and interbody fusion with cage and bone graft, along with posterior lateral fixation using pedicle screws and rods. The implants were confirmed using C-arm X-ray fluoroscopy. No difficulties were encountered in hemostasis or closure. After surgery, he experienced severe numbness in the bilateral shank, radiating to the planta and perineum, except in the L5 area.
Patient had hypertension and chronic kidney disease, stage 3. The patient denied any family history of neurological malignancy.
On physical examination, severe numbness and weakness in the bilateral shank, radiating to the planta and perineum in S1,2 dermatome but except in the L5 area. Urine retention and constipation were also noted.
No abnormality was found in routine blood and urine analyses, including electrolyte imbalance or urinary tract infection.
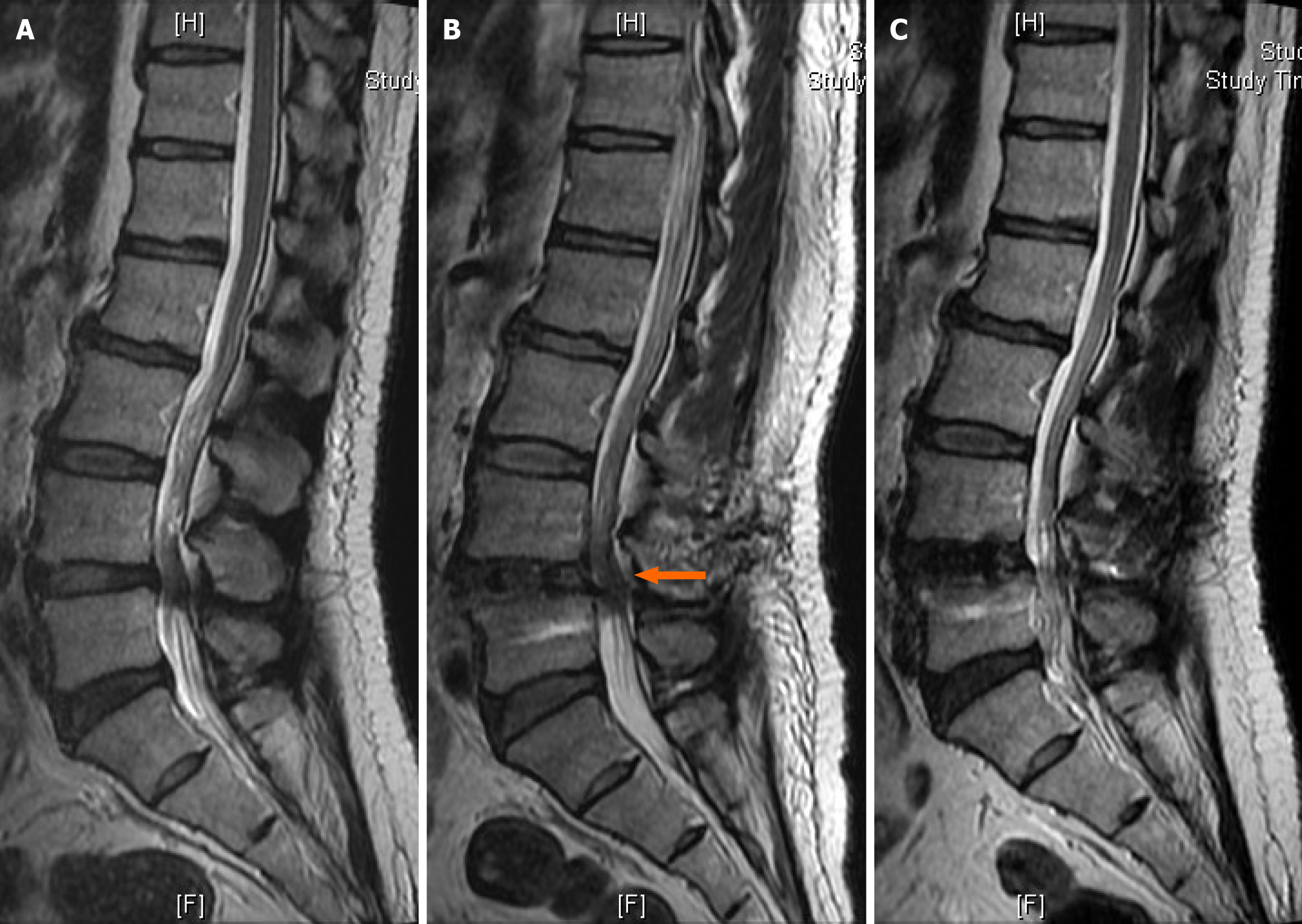
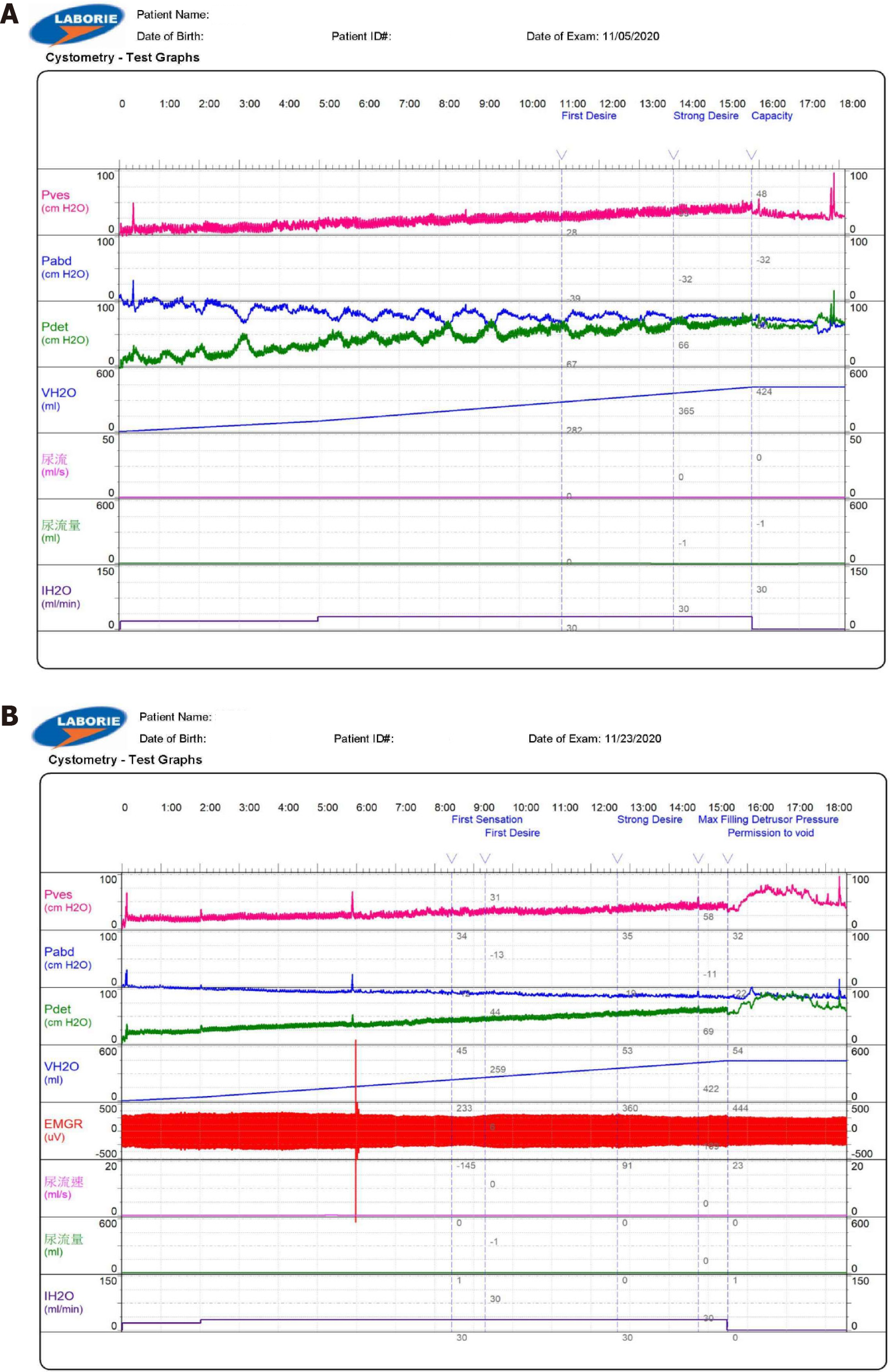
Postoperative MRI findings indicated signs of epidural hematoma and compressive stenosis at L4-L5 (Figure 1). On the seventh postoperative day, the patient underwent re-exploration, and only minimal hematoma, Gelfoam, and DuraGem were observed at the epidural space. The dura was loosened after removal of the hematoma and Gelfoam. Although slight improvement was observed in the perineum and shank numbness, urinary retention persisted. The cystometrogram results indicated no bladder contraction or a delayed first sensation (Figure 2A). Transrectal ultrasound indicated a prostate size of approximately 23 mL and prostate enlargement was not associated with his symptoms.
The final diagnosis was CES with urinary retention, vascular insufficiency related.
The patient underwent rehabilitation and acupuncture, as part of the treatment plan and kept Foley catheter for urine retention. The results of the repeat cystometrogram three weeks later indicated some bladder contraction, but with high compliance (Figure 2B).
After one month of follow-up, the Foley catheter was successfully removed and the patient was able to walk and voided smoothly. However, only partial perineum sensation returned.
Intermittent claudication frequently affects older adults, manifested as leg pain during walking. In cases of neurogenic claudication, an extended lumbar posture compresses a degenerative, stenotic spinal canal, leading to either direct mechanical pressure or indirect vascular compression of nerve roots and/or the cauda equina[3]. Patients with CES may experience lower back pain, unilateral or bilateral sciatica, saddle anesthesia, sexual dysfunction, fecal incontinence, bladder dysfunction, leg weakness, and sensory loss in the perineum, buttocks, and upper posterior thighs. However, symptom severity varies depending on the condition’s location and extent.
CES as a postoperative complication is rare with an incidence of 0.08%-2.8%. Lincoln reported 23 of 28395 (0.08%) patients undergoing no more than two-level lumbar discectomies without fusion or instrumentation[4]. Duncan also reported an incidence of 2.8% (5 of 175) for CES as a sequela of the decompressive laminectomy procedure for spinal stenosis, although the patient numbers were relatively low[5]. However, the incidence figure has to be interpreted cautiously, as the denominator is uncertain.
Postoperative CES can be classified into two groups based on compressive and noncompressive causes, which include anesthetic agents or vascular insufficiency. The cauda equina is particularly vulnerable to vascular distress, as its arterial supply lacks anastomotic branches, leading to a hypovascular area. Olmarker[6] revealed that the arteriolar and venular blood supply of the cauda equina was less developed compared to that of the peripheral nerves, as evidenced by ink-injection experiments. Moreover, venous congestion can occur in the presence of relative spinal stenosis at the operated and adjacent levels. Research has shown that double-level compression has a more significant impact on nerve impulse than single-level compression, potentially due to venous congestion caused by stasis[7].
Researchers have suggested intraoperative techniques to reduce the formation of shear force and avoid complications, such as not pulling the nerve root across the central line of the spinal process and ensuring the nerve retractor’s plane is parallel to the dural sac at the pulling point[8]. However, bladder and rectal sphincter paralysis typically involve the S3-S5 nerve roots, which are relatively close to the middle of the cauda equina. Our patient underwent L4 bilateral laminotomy and L4-L5 microdiscectomy, and L5 nerve function was maintained. Thus, direct nerve injury during surgery was unlikely.
If postoperative CES occurs, emergency imaging should be considered. However, interpreting postoperative MRI or computed tomography (CT) myelography findings to determine the cause of the condition can be difficult. Annertz et al[9] found no correlation between the symptoms and the size or nature of the abnormal tissue in the spinal canal on MRI, and concluded that edema and scar formation contributed to the limitations of the interpretation. Similar issues exist in other studies of CT scans[8]. Despite these changes, MRI, with its multiplanar capability, is considered the best method of assessment, potentially indicating hematoma or migrating fat grafts. Therefore, postoperative examinations should be performed with caution.
CES is commonly indicated for emergency surgery and decompression. In the same reason, many researchers concur that the treatment of postoperative CES is wide decompression[5,10]. Jensen[1] suggested that repeated exploration is reasonable in negative imaging for the mass effect. However, in Duncan’s report, no other compressive etiology could be elucidated in five patients with postoperative CES, and further decompression in three patients was not effective[5]. In the series of Henriques’ report, a good clinical outcome was observed when decompression was performed within a few hours from the development of CES and when the treatment was postponed up to 48 hours, suggesting that the time factor seems to be of less importance[11]. Hence, radiographic investigations are required to identify the etiology, particularly compressive lesion. Conversely, Jain reported that two cases were managed conservatively without re-exploration and eventually resulted in favorable outcomes[2]. Consequently, repeat operations should not be routinely performed in cases of postoperative CES, especially in noncompressive CES.
This case report highlights postoperative CES with neurogenic bladder as a potential complication of spinal surgery. When CES is identified, emergency imaging is essential to rule out spinal cord compression. Further exploration is required if stenosis persists. However, these compressive lesions may not contribute to the patient’s symptoms; thus, vascular insufficiency may be indicated. If noncompressive CES is diagnosed, conservative management should be indicated. However, research on postoperative CES remains limited, and further studies are needed to predict, assess, and carefully manage this rare complication.
| 1. | Jensen RL. Cauda equina syndrome as a postoperative complication of lumbar spine surgery. Neurosurg Focus. 2004;16:e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Jain M, Das SS, Behera S, Tirpude A. Non-compressive postoperative cauda equina syndrome following decompression and transforaminal interbody fusion surgery. BMJ Case Rep. 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Nadeau M, Rosas-Arellano MP, Gurr KR, Bailey SI, Taylor DC, Grewal R, Lawlor DK, Bailey CS. The reliability of differentiating neurogenic claudication from vascular claudication based on symptomatic presentation. Can J Surg. 2013;56:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Ramirez LF, Thisted R. Complications and demographic characteristics of patients undergoing lumbar discectomy in community hospitals. Neurosurgery. 1989;25:226-30; discussion 230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Duncan JW, Bailey RA. Cauda equina syndrome following decompression for spinal stenosis. Global Spine J. 2011;1:15-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Olmarker K. Spinal nerve root compression. Nutrition and function of the porcine cauda equina compressed in vivo. Acta Orthop Scand Suppl. 1991;242:1-27. [PubMed] |
| 7. | Olmarker K, Rydevik B. Single- versus double-level nerve root compression. An experimental study on the porcine cauda equina with analyses of nerve impulse conduction properties. Clin Orthop Relat Res. 1992;35-39. [PubMed] |
| 8. | Montaldi S, Fankhauser H, Schnyder P, de Tribolet N. Computed tomography of the postoperative intervertebral disc and lumbar spinal canal: investigation of twenty-five patients after successful operation for lumbar disc herniation. Neurosurgery. 1988;22:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Annertz M, Jönsson B, Strömqvist B, Holtås S. Serial MRI in the early postoperative period after lumbar discectomy. Neuroradiology. 1995;37:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Yuan T, Zhang J, Yang L, Wu J, Tian H, Wan T, Xu D, Liu Q. Cauda equina syndrome without motor dysfunction following lumbar spinal stenosis surgery: A case report. Medicine (Baltimore). 2019;98:e16396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Henriques T, Olerud C, Petrén-Mallmin M, Ahl T. Cauda equina syndrome as a postoperative complication in five patients operated for lumbar disc herniation. Spine (Phila Pa 1976). 2001;26:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |