Published online Apr 6, 2025. doi: 10.12998/wjcc.v13.i10.101719
Revised: November 11, 2024
Accepted: December 5, 2024
Published online: April 6, 2025
Processing time: 85 Days and 15.3 Hours
Precision medicine is an emerging field that includes tumor-targeted delivery and tumor microenvironment. This review explores the synergistic potential of combining nano-drug delivery systems with low radiation doses to achieve optimized therapeutic outcomes, particularly in the context of cancer treatment. Nanoparticle-based drug carriers offer precise and targeted delivery, enhancing the therapeutic index of anticancer agents. The use of lower radiation doses has become a focus in radiation oncology to minimize off-target effects on healthy tissues in palliation treatment with high-target volume lesions.
To conduct a bibliometric review of nanomedicine and glioblastoma (GBM), all relevant studies from the last two decades were included.
The search strategy comprised the keywords ”nanomedicine “and “glioblastoma” in the title and/or abstract. All English-language documents from 1 January 2000 to 31 December 2023 were considered for the analysis. R code (version 4.2.0) with R Studio (version 2022.12.0-353) and the Bibliometrix package (version 4.0.1) were used for the analysis. A total of 680 documents were collected.
We analyzed the bibliometric features of nanomedicine in glioma. With the limitations of the research, our analysis aims to highlight the increasing interest of researchers in the precision medicine field in GBM treatment and lead us to suggest further studies focusing on the association between nanomedicine and radiotherapy.
Due to the poor prognosis associated with GBM, new therapeutic approaches are necessary. There is an increasing interest in precision medicine, which includes nanomedicine and radiotherapy, for GBM treatment. This integration enhances the efficacy of targeted treatments and provides a promising avenue for reducing adverse effects, signifying a notable advancement in precision oncology.
Core Tip: Glioblastoma is a highly aggressive brain cancer, which main treatment consists of surgical resection or biopsy followed by radiotherapy and chemotherapy (STUPP protocol). Precision medicine is an emerging field that includes tumor-targeted delivery and tumor microenvironment. The integration of nano drug delivery systems with low radiation doses may offer a promising avenue for maximizing therapeutic efficacy and minimizing systemic toxicity.
- Citation: Pontoriero A, Critelli P, Zeppieri M, Bosurgi A, Guercio S, Caffo M, Angileri FF, Parisi S, Lavalle S, Pergolizzi S. Nano-drug delivery systems integrated with low radiation doses for enhanced therapeutic efficacy in cancer treatment. World J Clin Cases 2025; 13(10): 101719
- URL: https://www.wjgnet.com/2307-8960/full/v13/i10/101719.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i10.101719
Glioblastoma (GBM) continues to be one of the most formidable malignancies owing to its invasive proliferation and resistance to standard treatments[1-3]. Despite the STUPP procedure, which integrates radiation and chemotherapy, enhancing results, the blood-brain barrier (BBB) remains a continual impediment to efficient drug delivery[4-6]. Nanomedicine offers a promising strategy to circumvent the BBB and deliver medicines precisely, utilizing increased permeability and retention (EPR) effects in malignancies[7-9]. Furthermore, low-dose radiation has been examined to mitigate detrimental effects on healthy tissues, indicating a potentially synergistic strategy when integrated with targeted nanomedicine[10,11]. Accordingly, to improve the EPR effect, nanomaterials accumulate and retain in higher amounts in tumors than in healthy tissue due to the erratic and leaky vasculature and poor lymphatic drainage of the tumor tissue[5,6]. Nano-drug delivery platforms, including liposomes, polymeric nanoparticles, and nanogels, are investigated for their ability to facilitate controlled drug release and improve the bioavailability of therapeutic agents. Liposomes, known for their biocompatibility and versatility, offer a promising avenue for precise drug delivery[12]. Polymeric nanoparticles, with their tunable properties, allow for controlled release kinetics[13]. Nanogels, characterized by their three-dimensional networks, exhibit the potential for sustained and targeted drug delivery[14]. The diverse range of nano-drug delivery systems with unique physicochemical properties underscores the multifaceted nature of precision nanomedicine[8,12-14]. Considering the tumor microenvironment (TME), precision nanomedicine strategies aim to address the challenges posed by the complex interactions within the TME[15]. Designed nanoparticles play a pivotal role in modulating the TME, enhancing drug penetration, reducing immunosuppression, and overall augmenting the effectiveness of both nanomedicine and radiotherapy within the intricate context of the TME[16].
Concurrently, advancements in radiation therapy planning and delivery techniques have enabled the exploration of lower radiation doses, seeking to strike a delicate balance between effective tumor control and reduced damage to surrounding healthy tissues[10]. The integration of nano-drug delivery systems with low radiation doses may offer a promising avenue for maximizing therapeutic efficacy and minimizing systemic toxicity[11]. Notwithstanding advancements in nanomedicine, deficiencies persist in comprehending the interactions between nano-drugs and low-dose radiotherapy within the TME. This review examines the possible synergy of different modalities, intending to build a basis for future research aimed at optimizing treatment effects while reducing systemic toxicity. This bibliometric analysis aims to highlight the increasing interest of researchers in the precision medicine field in glioblastoma treatment. It suggests evaluating the synergistic potential of combining nano-drug delivery systems with radiotherapy to optimize therapeutic outcomes in the treatment of GBM. The precision offered by nanoparticle-based drug carriers, ensuring targeted delivery, coupled with the focus on minimizing off-target effects through lower radiation doses, may represent a paradigm shift in the field of oncology[17,18].
The Web of Science (WoS) electronic documents database was used as a data source (consulted in January 2024). Using the key words nanomedicine and GBM, the authors searched for records that included the same in their titles and abstracts.
Records published from 2000 to 2023 were included in this study. Papers not published in the English language were discarded. The entire metadata for the selected records was obtained in Bib TeX format. These records were then classified and assessed for relevance by Antonio Pontoriero and Paola Critelli. A total of 12 articles were retrieved. References used by these records in also searched for relevant documentation.
The analysis was carried out with R code (version4.2.0), R Studio (version 2022.12.0-353), and the Bibliometrix package (version 4.0.1)[19]. The “convert2df” function was used to extra data frames from the modified BibTeXfiles, subsequently utilizing the “biblioAnalysis” command and the “Summary ()” function from the Bibliometrix package. This facilitated an exploratory analysis, emphasizing critical attributes such as the annual publication count, growth rate, leading active countries, and their respective output based on the institution the publication lead is affiliated to, the most cited papers, and the most prominent journals.
The command “Biblioshiny ()” was engaged to further analyze sampled studies by converting them to a pictorial representation, a graphical user interface, and generating maps for national scientific cooperation, institutional collaboration networks, and a comprehensive co-occurrence network.
Keywords were evaluated using clustering analyses in machine learning, and trending topics were identified. The co-occurrence network of authors' keywords was examined using the walk trap clustering technique[7].
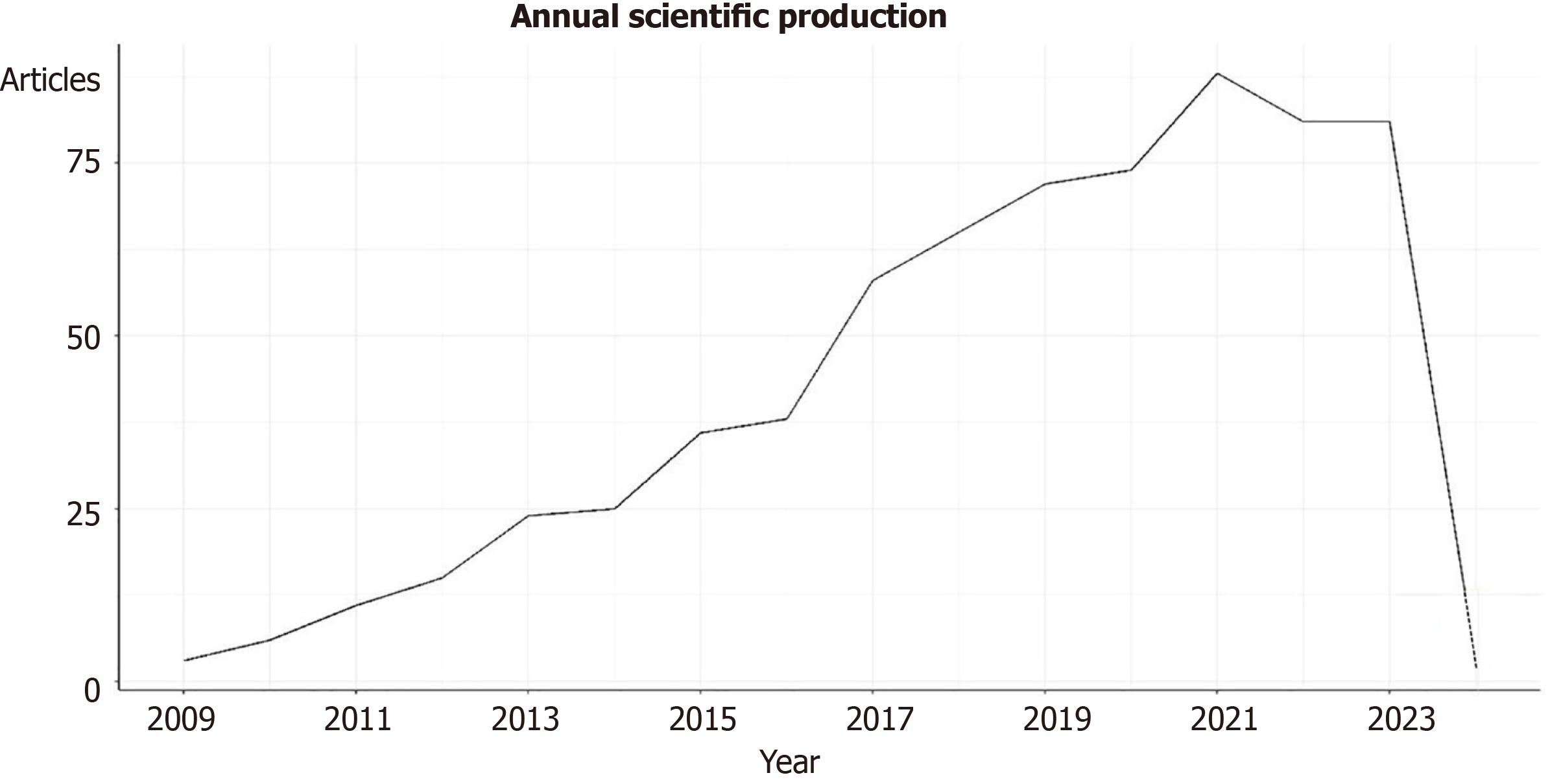
A total of 680 publications about nanomedicine and GBM were collected from 2000 to 2024. Among them, 514 were articles (74%), 143reviews (20%), 9 editorial material (1%), 8 meeting abstracts (1%), 7 early access (1%), six book chapters (1%), 6 Proceeding Paper (1%), 4 Corrections (1%) and 2retractions (0%). Figure 1 shows the trend of the publications in the years for the period 2009-2023. Since 2009, an increase in the production of documents has been observed. The most productive year was 2021, with 88 articles, followed by 2021 and 2022, with 81 documents. In 2013, the highest mean of total citations per article and per year was observed (Supplementary Figure 1).
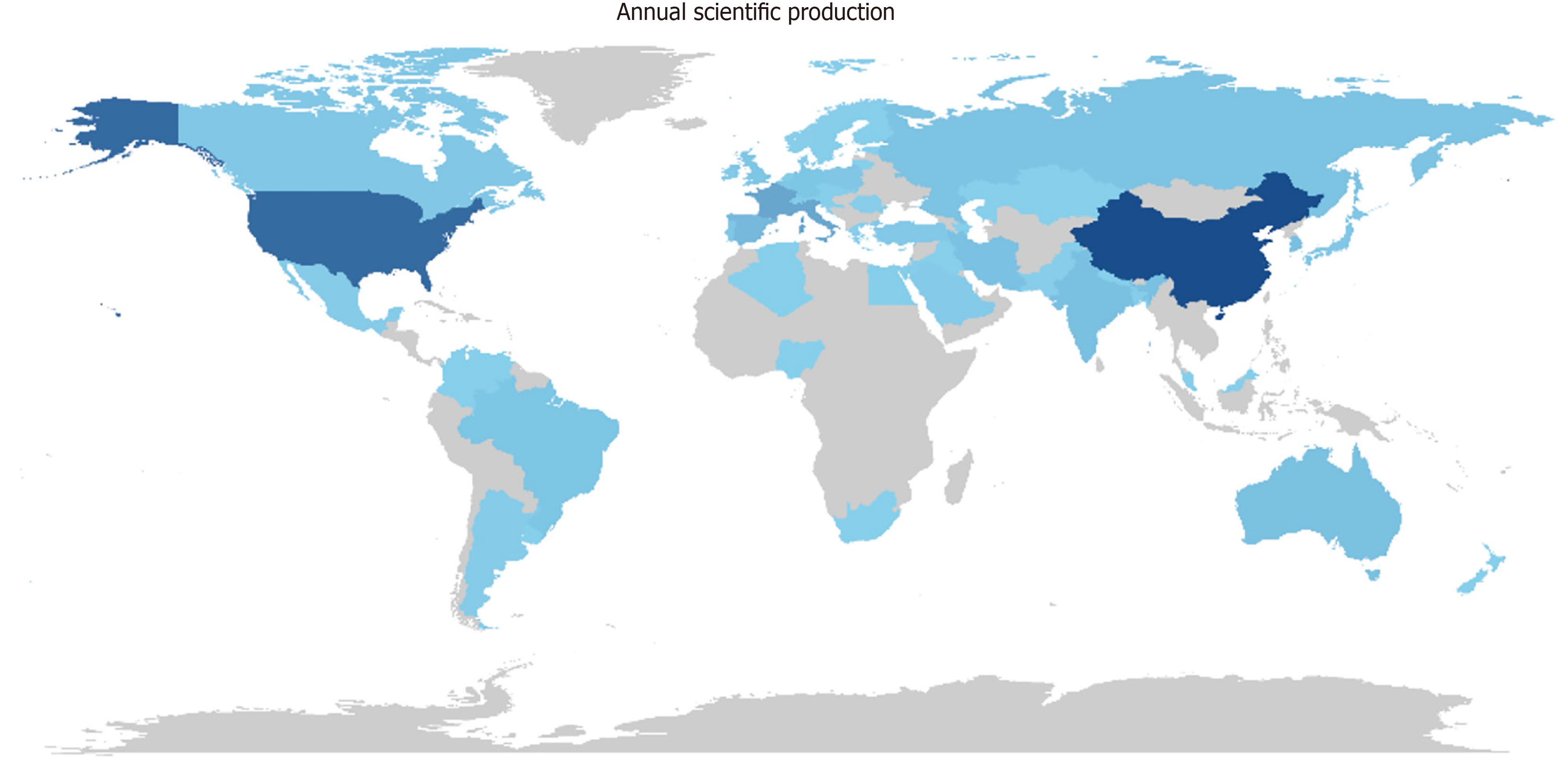
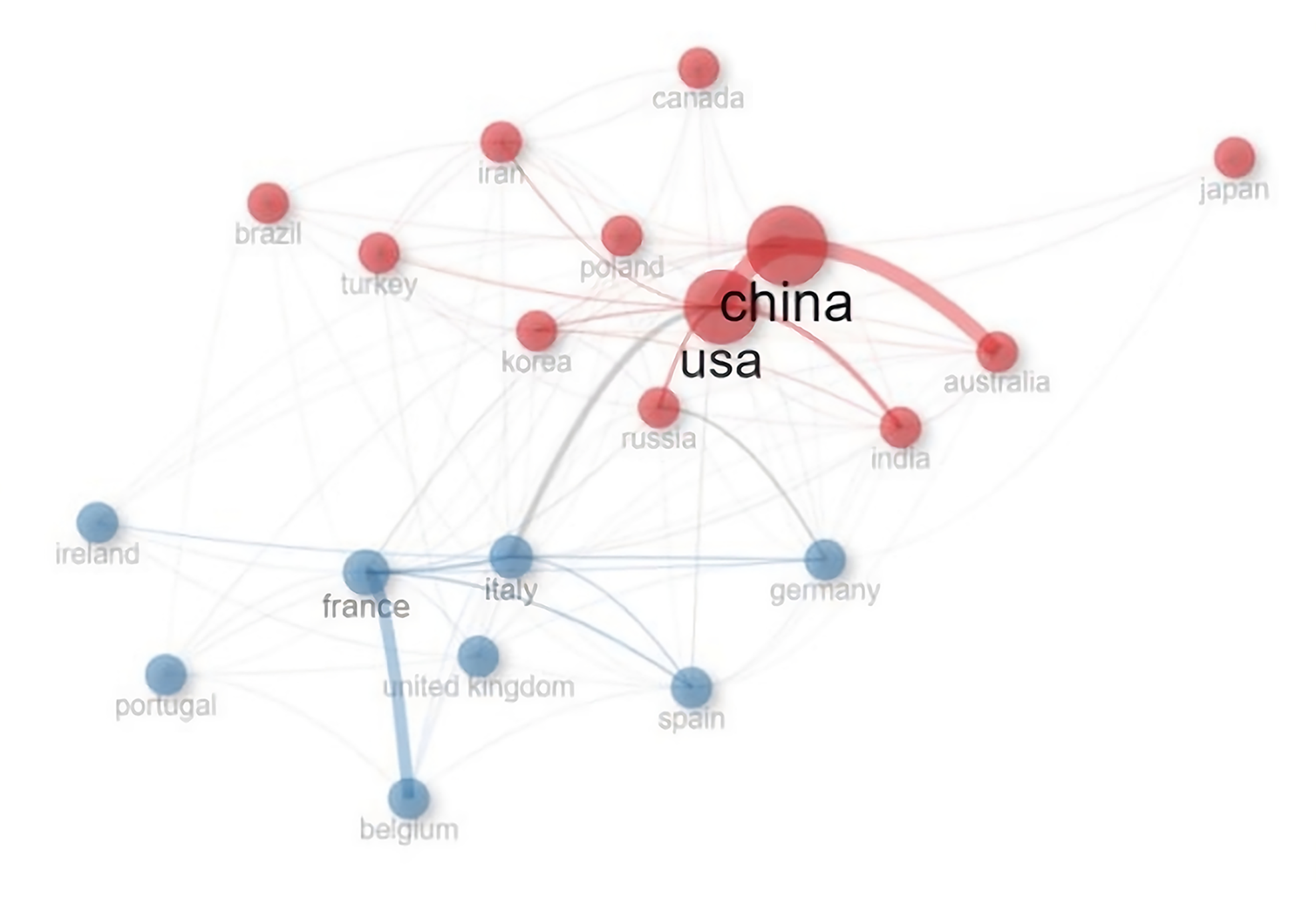
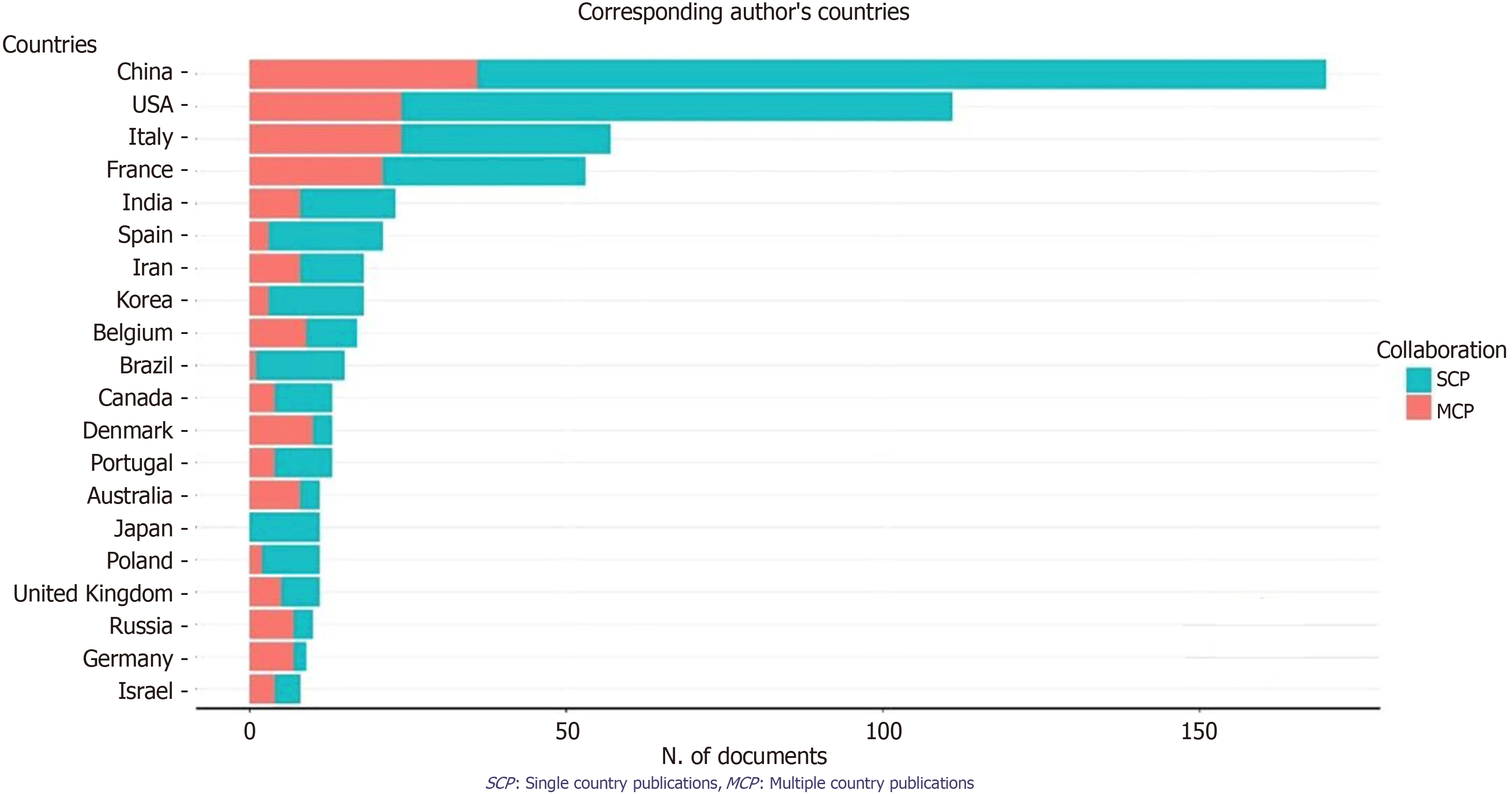
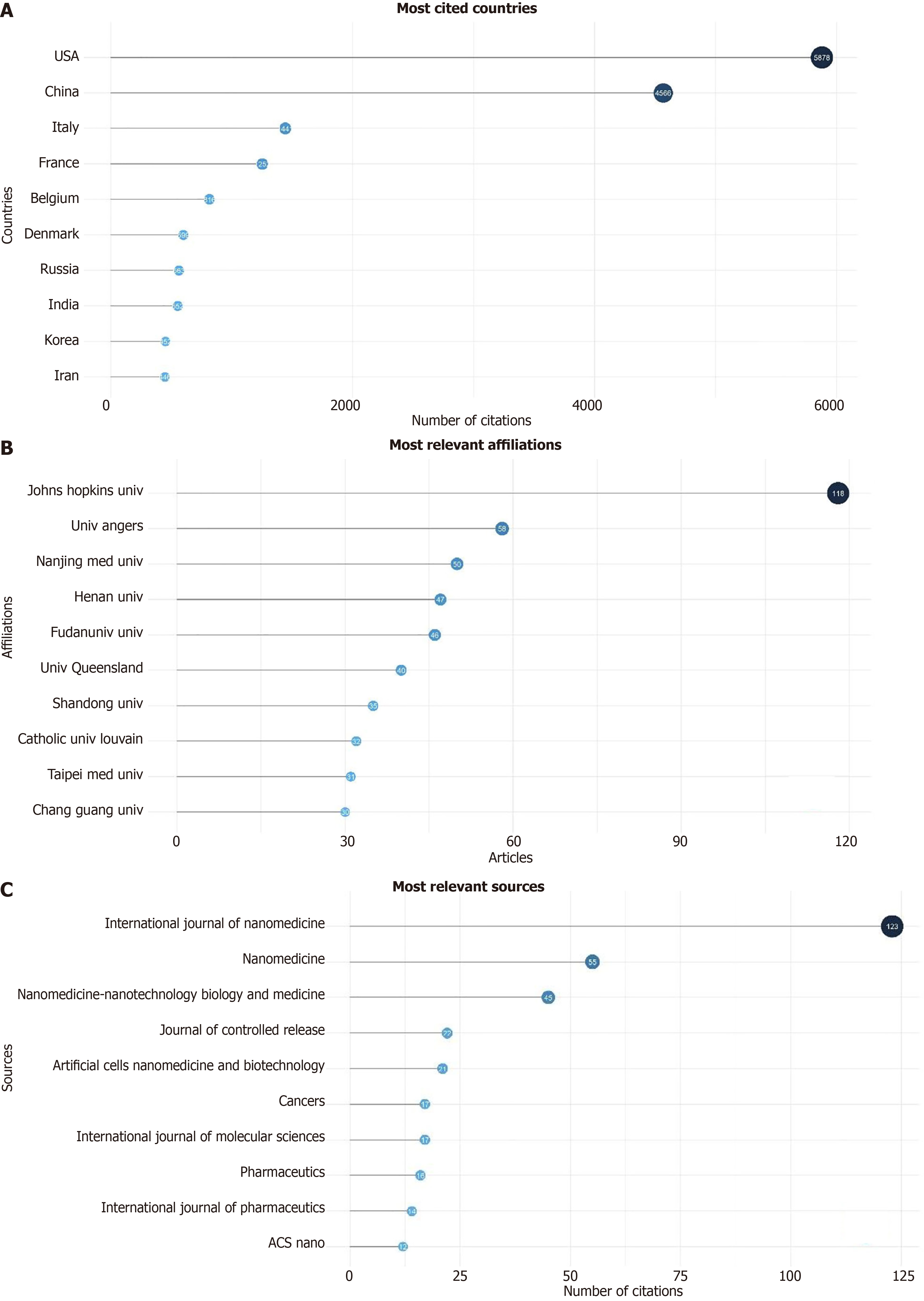
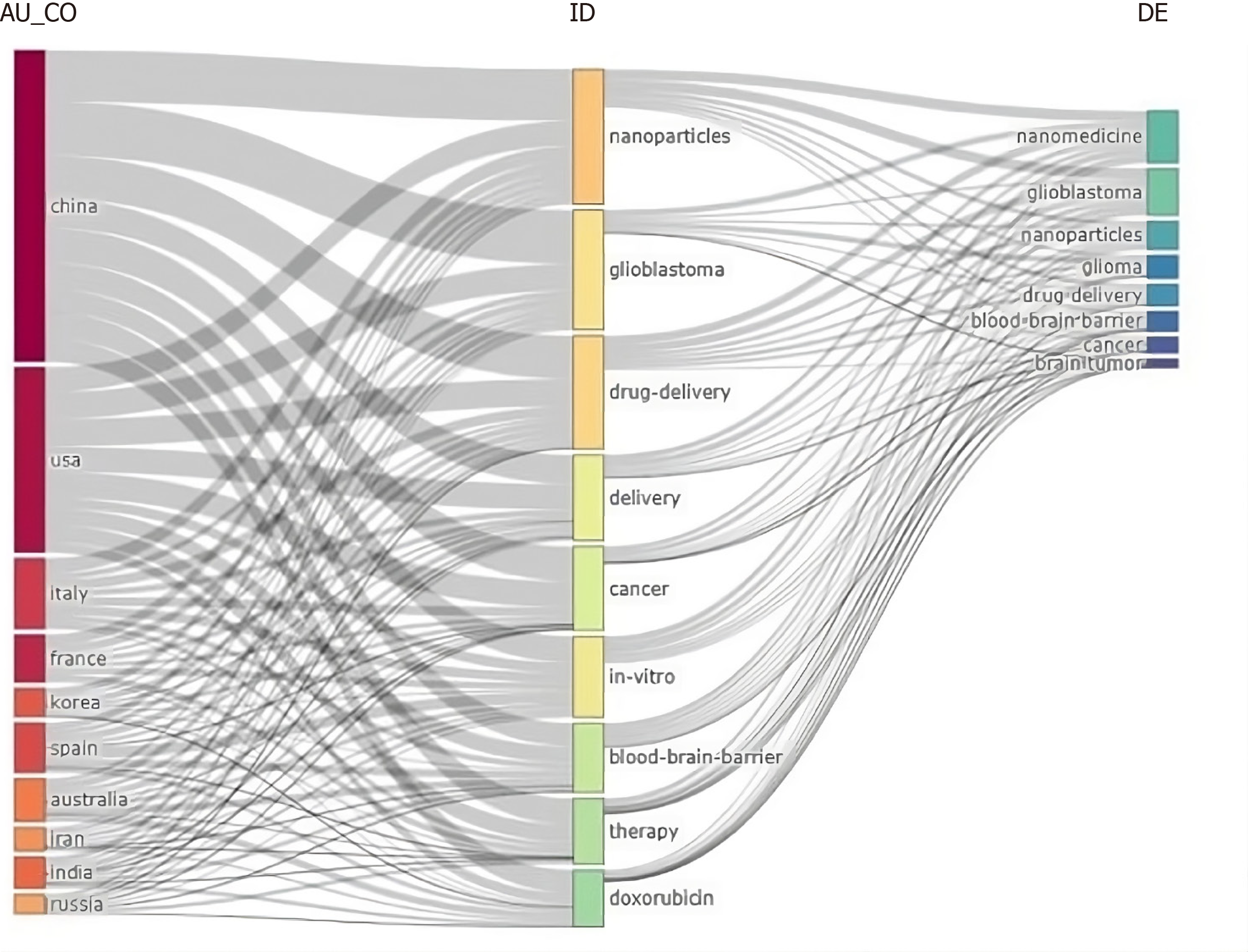
A total of 680 publications from 8 countries and 10 institutions. The two main Countries that contributed to production are China and the United States, with 999 and 755 articles, respectively, followed by France (293 articles) and Italy (287 articles) (Figure 2, Supplementary Figure 2 and Table 1). The top ten countries in terms of publication volume are reported in Table 2. The highest number of publications was from China, with 999 articles. In Figure 3 and Supplementary Figure 3, we analyzed the relationship network among countries. Each vertex represents an item, and its size is proportional to the item’s occurrence. Each cluster can be considered as a topical macro-area, and the colors represent the cluster to which each word belongs. China has a strong connection with the United States and Australia, and there is a clear tendency for European countries (blue) to collaborate. China, the United States, and Italy generated the greatest quantities of multi-national documents, characterized as publications with authors from a minimum of two countries. China and the United States were often the foremost contributors to single-country publications. In most cases, China and the United States participated in publications (Figure 4). Furthermore, the United States and China are the most cited countries, respectively, with 5878 and 4566 citations (Figure 5A). The principal institutions in terms of production were Johns Hopkins University, with 118 articles, followed by the University of Anger, with 58 articles (Figure 5B, Supplementary Figure 4).
| Year | China | United States | France | Italy |
| 2009 | 0 | 5 | 0 | 1 |
| 2010 | 0 | 16 | 5 | 4 |
| 2011 | 8 | 23 | 5 | 6 |
| 2012 | 32 | 31 | 11 | 18 |
| 2013 | 47 | 88 | 21 | 22 |
| 2014 | 51 | 126 | 36 | 36 |
| 2015 | 88 | 182 | 62 | 45 |
| 2016 | 114 | 254 | 80 | 53 |
| 2017 | 208 | 320 | 115 | 74 |
| 2018 | 304 | 428 | 132 | 85 |
| 2019 | 397 | 508 | 155 | 132 |
| 2020 | 519 | 600 | 178 | 176 |
| 2021 | 653 | 662 | 218 | 206 |
| 2022 | 815 | 724 | 250 | 240 |
| 2023 | 991 | 755 | 290 | 284 |
| 2024 | 999 | 755 | 293 | 287 |
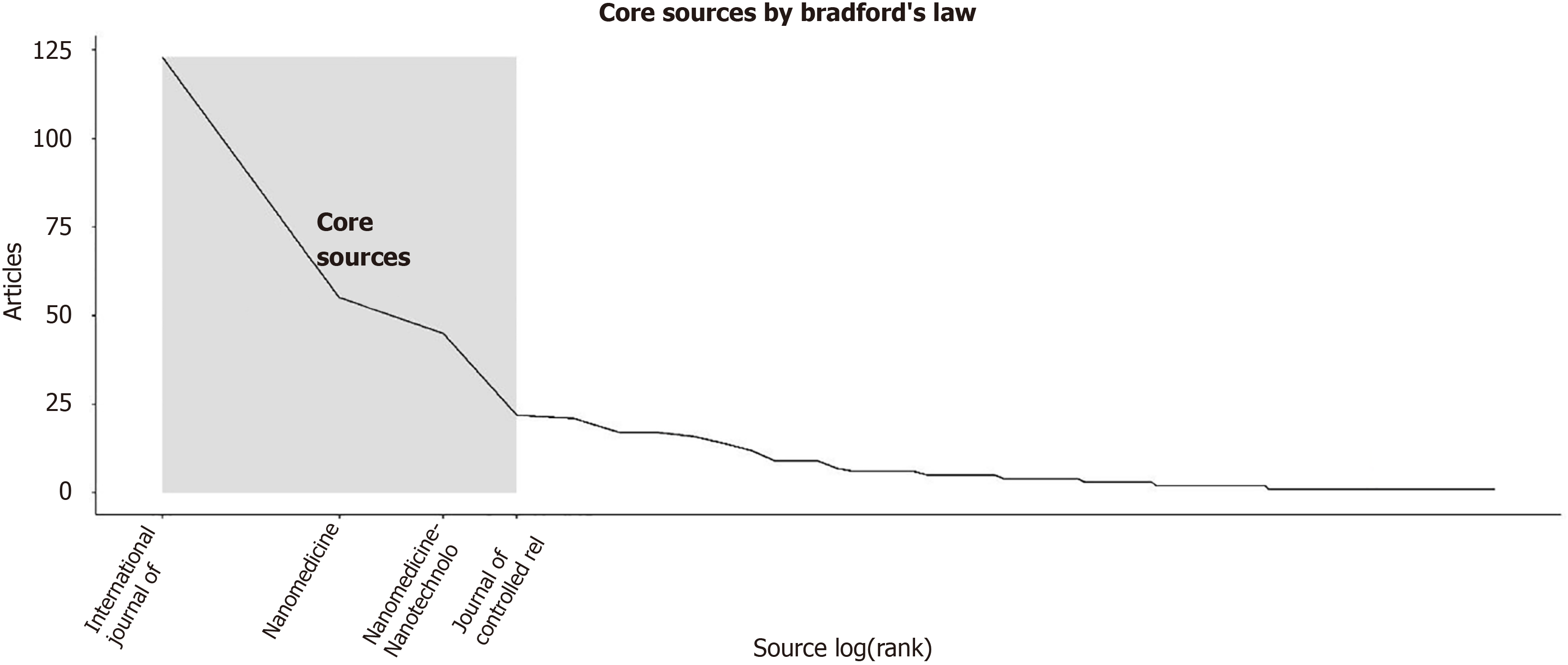
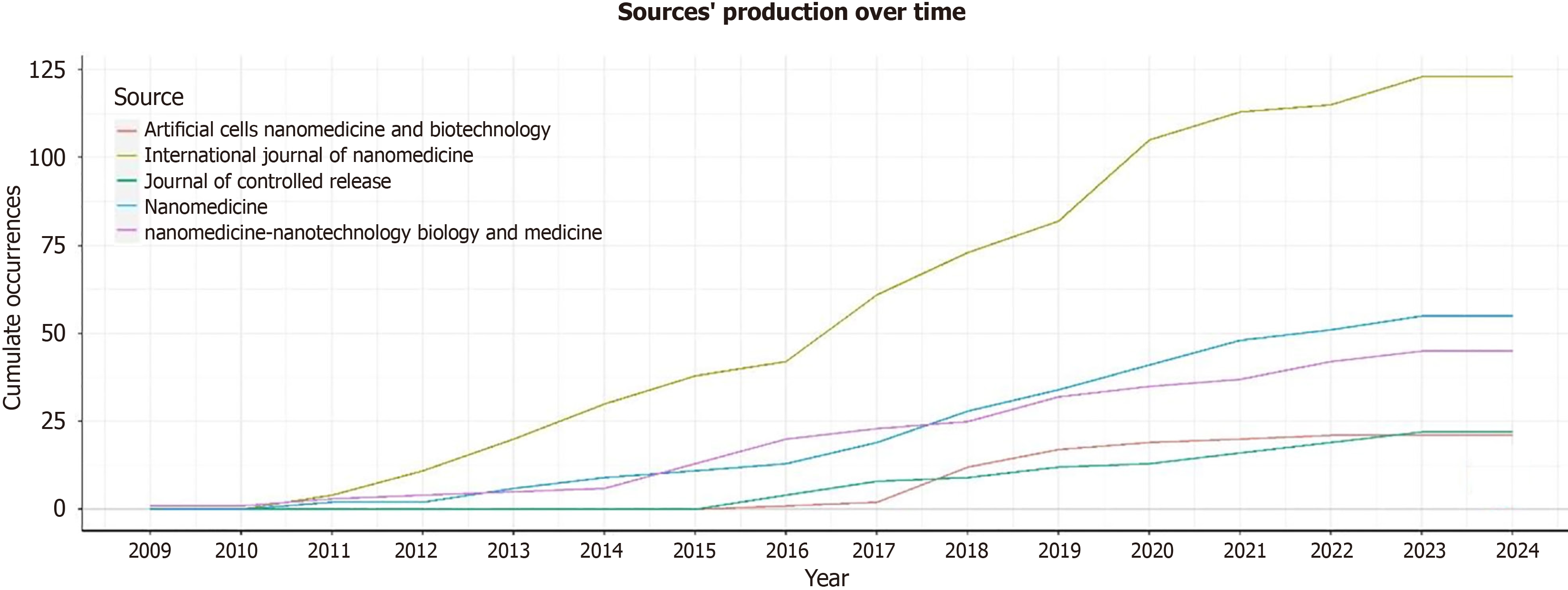
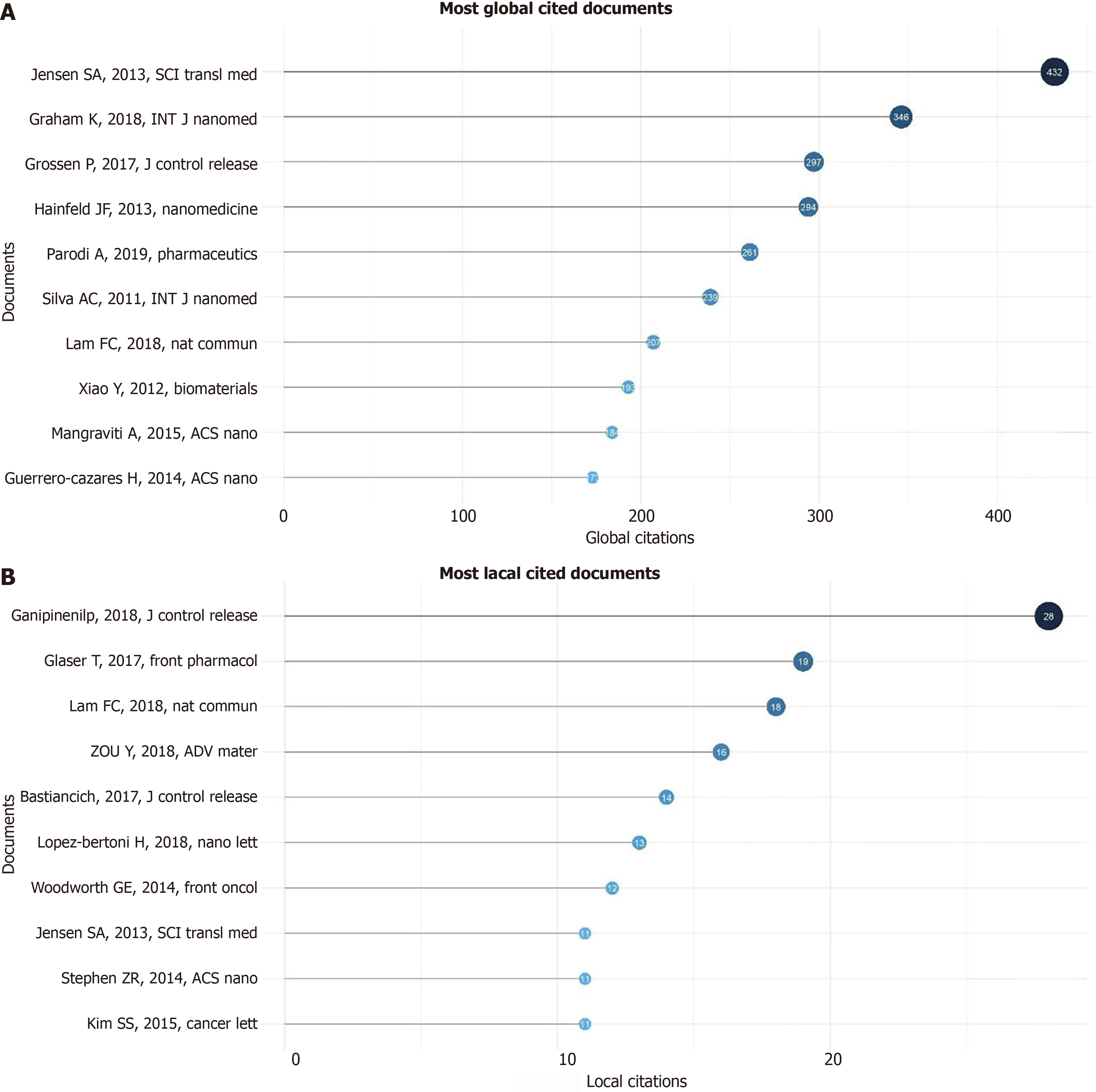
The most relevant source is represented by the “International Journal of Nanomedicine” with 123 articles, followed by “Nanomedicine” and “Nanomedicine Nanotechnology Biology and Medicine” with 55 and 45 articles, respectively (Figure 5C). The most locally cited sources are Journal Control Release and Biomaterial, with 1725 and 1343 articles, respectively (Supplementary Figure 5). The core sources are represented by “The International Journal of Nanomedicine” Nanomedicine and Nanomedicine Nanotechnology Biology and Medicine” and “Journal of Controlled Release” (Figure 6). The source production over time revealed the “International Journal of Nanomedicine” as the principal source with increasing production from 2009 to 2024 (Figure 7). The most local global cited reference was “Radiotherapy plus concomitant and adjuvant temozolomide for GBM” with 146 followed by “Effects of radio therapy with concomitant and adjuvant temozolomide vs radio therapy alone on survival in GBM in a randomized phase III study: 5-yearanalysis of the EORTC-NCIC trial” with 52 citations (Supplementary Figure 6). The most global cited document was Jensen et al[8] with 432 citations followed by Graham et al[9] with 346 citations; the most local cited document was Ganipineni et al[20] with “Drug delivery challenges and future of chemotherapeutic nanomedicine for GBM treatment” obtaining with 28 citations (Figure 8).
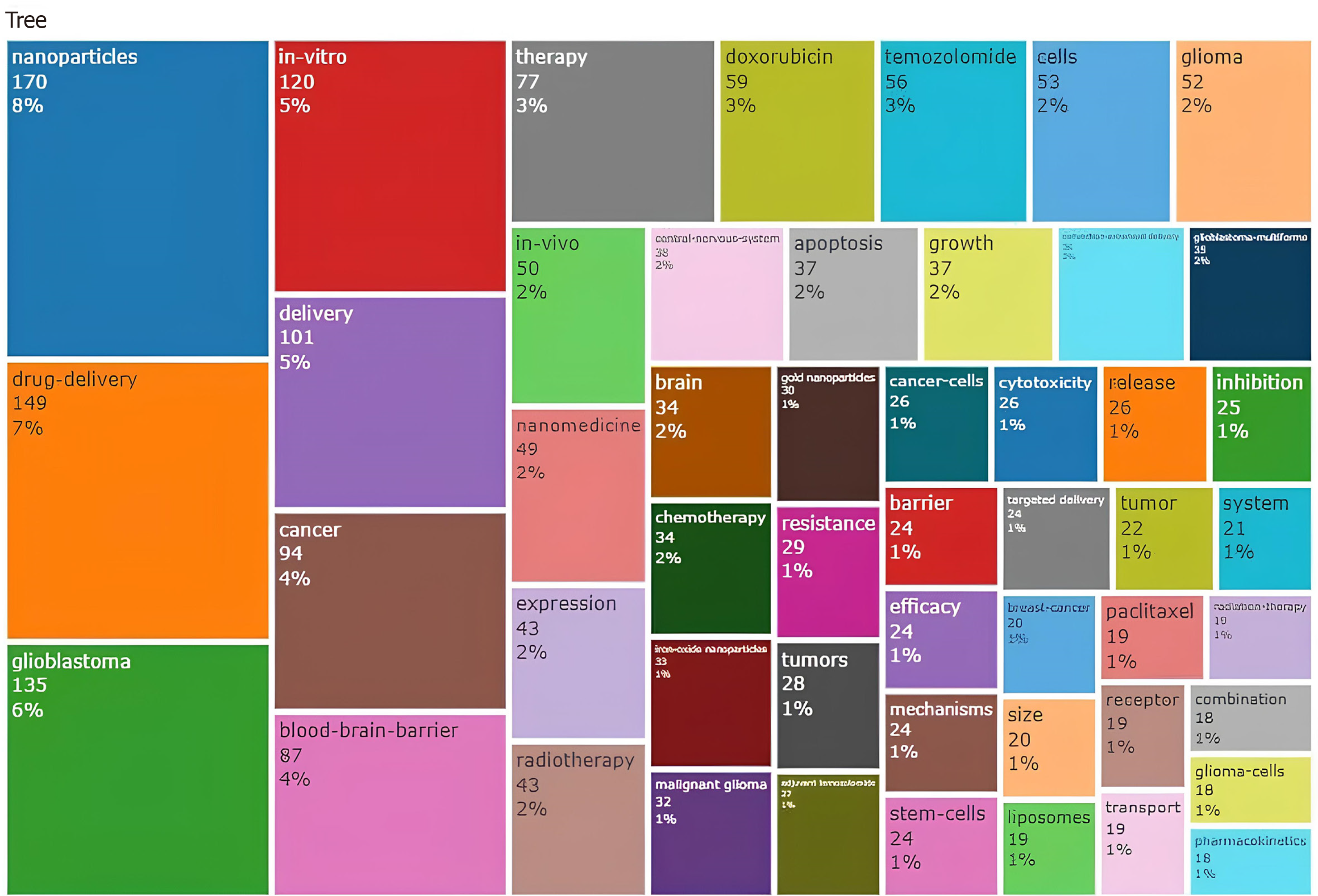
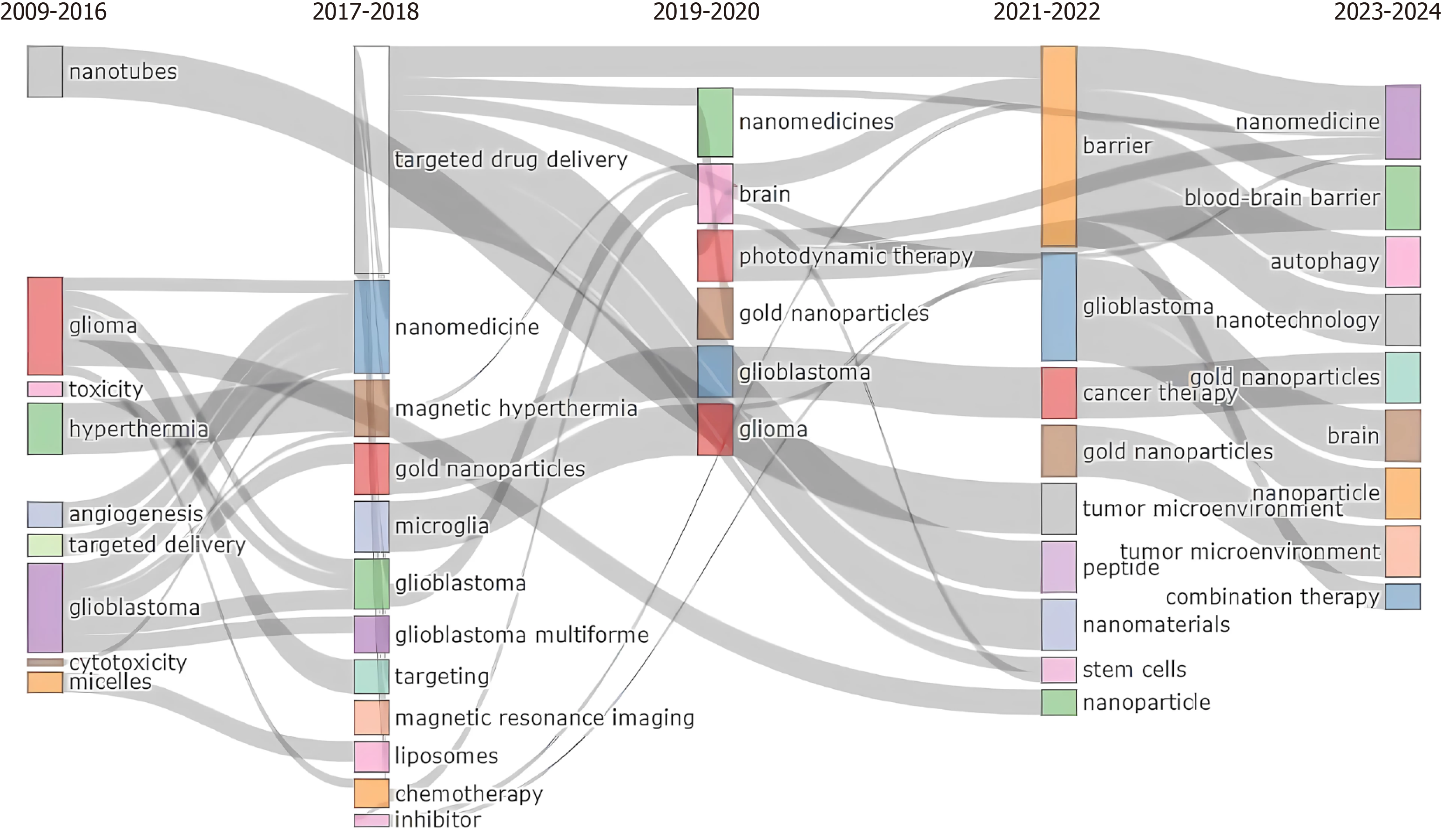
A total of 51 keywords were identified from documents. The main keyword’s authors (right side) resulted in GBM, nanomedicine, nanoparticles, glioma, drug delivery, BBB, nanoparticles, GBM multiforme, brain tumors, cancer, temozolomide, nanotechnology, chemotherapy, delivery, radiotherapy, brain, targeted, brain cancer (Supplementary Figure 7). The top ten keywords are nanomedicine, GBM, nanoparticles, glioma drug delivery, BBB, cancer, and brain tumor, and are reported in Figure 9. Among the keywords used by authors, the top three are nanoparticles (8%), drug delivery (7%), and GBM (6%) (Figure 10). The keywords network (Figure 11) confirmed two different clusters and GBM, nanoparticle, and drug delivery are the principal words. In the period 2011–2023 a change in trend topics demonstrated an evolution of the research. The principal themes were “drug delivery” “GBM” and “in vitro”. The trending topics developed from 2017 to 2021, “nanoparticle” and “blood-barrier” saw their main development during 2017–2023. The most recent topic (2020-2023) regards “loaded liposomes” “blood” “loaded page nanoparticles” and “drug-delivery system” (Supplementary Figure 8). Figure 12 represents the evolution of the thematic areas and their relationships during the considered periods (2009-2016, 2017–2018, 2019–2020, 2021-2022, 2023-2024). Several niches appeared in 2009-2016. Among them, “nanotubes”, “glioma”, “toxicity”, “hyperthermia”, “angiogenesis”, “target delivery”, “glioblastoma”, “cytotoxicity” and “micelles” in 2017-2018 emerged “nanomedicine”, “magnetic hyperthermia”, “gold nanoparticles”, “microglia”, “magnetic resonance imaging”, “liposomes”, “chemotherapy” and “inhibitor”; in 2019-2020 appeared “photodynamic therapy” while in 2021-22 appeared “barrier”, “tumor microenvironment”, “peptide” and “stem cells”. The niches that have appeared in the last year are “blood barrier”, “autophagy”, “nanotechnology” and “combination therapy”.
The quantity of publications is a vital indicator for evaluating the progression and trends in academic research on subjects over time. The speed of the publications in a specific period is important for assessing the trend of academic research. Our bibliometric research encompassed 680 documents published from 2000 to 2023 across 59 distinct nations. The annual volume of literature pertaining to nanomedicine and GBM has markedly escalated, reflecting an increasing interest in the adjunctive treatment of GBM. The works of Stupp et al[1,2] garnered the most citations (n = 146 and 52, respectively)[1,2].
Concerning countries and geographical distribution, the United States and China had the highest volume of publications and citations. The most efficient academic institution is situated in the United States (Johns Hopkins University). Italy has demonstrated a rise in document creation from 2009 to the present, ranking fourth (n = 287) after the United States, China, and France in output, and third in citation frequency. The primary cluster of involving the United States with Italy and India, as well as France with Belgium.
An examination of the most referenced papers was conducted to ascertain the nature and significance of articles that have profoundly influenced scholarly literature in the field. The initial and most frequently referenced paper is regarded as the foundation of adjuvant therapy in GBM. A report by Stupp et al[1] published in 2005 shown that incorporating temozolomide with radiotherapy for newly diagnosed GBM yielded clinical benefits regarding survival and toxicity.
The evolution of precision medicine with the necessity of new therapeutic approaches introduces new treatment strategies over time. This progression is evident in the analysis of trend topics. From 2009 the main interest was focused on “nanotubes”, “glioma”, “toxicity”, “hyperthermia”, “angiogenesis”, “target delivery”, “glioblastoma”, “cytotoxicity” and “micelles” while in the last 3 years, the principal collaboration transpired between the United States and China, succeeded by lesser clusters topics included “barrier”, “tumor microenvironment”, “peptide” and “stem cells” and “blood-barrier”, “autophagy”, “nanotechnology” and “combination therapy”.
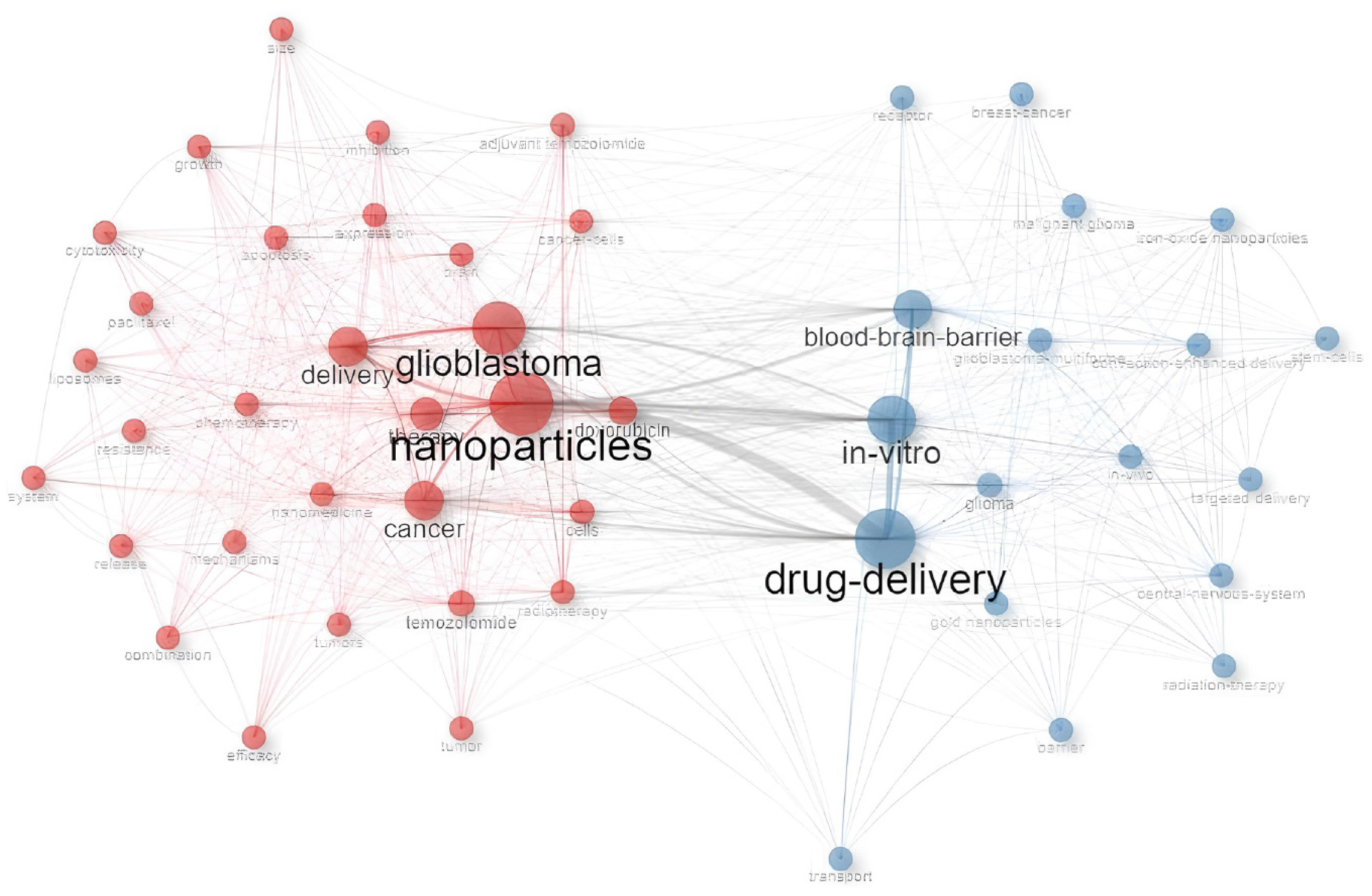
The cluster analysis results of the machine-learning-based revealed two main groupings of co-occurring words, highlighting the key areas of focus within the field and the interconnections between them.
The first cluster, with nanoparticles as the main contributor, presents networks focused on systemic treatment as “temozolomide”, “doxorubicin” and “paclitaxel”. The second cluster, centered on “drug delivery” presents networks with the main mechanisms of actions such as “in vitro”, “blood-brain barrier”, “in vivo” and “receptor”. Both clusters present a small network with “radiotherapy” terms, indicating the lack of studies that deepen the combination between “nanomedicine” and “radiotherapy” in the treatment of GBM.
The word “radiotherapy” occurs 43 times and represents only 2% of the words cited in the tree map among 680 documents. Furthermore, “radiotherapy” isn’t present in the trend topic theme from 2009 to 2024. Recent literature data show that the different fractionations of radiation dose and low-dose radiotherapy can induce effects on the TME and support the delivery of drugs[10,11].
Radiotherapy and nanomedicine could be combined to enhance the overall effectiveness of cancer treatment. An association between radiotherapy and nanomedicine could determine the following. Increase tumor-targeting: Radiotherapy could modify the tumor environment, enhancing the EPR effect and improving the accumulation of nanoparticles[17]; enhance drug delivery: Radiotherapy induces a change in tumor physiology, making it more susceptible to drug penetration, improving, in turn, the overall therapeutic effect[17]; radio sensitivity: Nanomedicines have intrinsic radio-sensitizing properties; when combined with radiotherapy, they can improve the cancer sensitivity of therapeutic agents in response to specific stimuli, including radiation, providing a cell to radiation, leading to increased cell death; low radiation doses could exploit this synergistic effect[18]; nanoparticles can serve as imaging agents to visualize tumors and monitor the response to radiotherapy. Real-time imaging can help guide the radiation treatment, ensuring accurate targeting. Additionally, nanoparticles can be planned to controlled and targeted drug release[19]; reduced side effects: By improving the specificity of drug delivery to tumor cells, the combination of radiotherapy and nanomedicine may help reduce systemic toxicity. This is particularly important in minimizing side effects associated with traditional chemotherapy, where healthy tissues can be affected[20]; overcoming treatment resistance: Nanoparticles can be engineered to overcome resistance mechanisms in cancer cells. By combining nanomedicine with radiotherapy, it may be possible to address different resistance pathways, improving the likelihood of treatment success[21]; personalized medicine: The combination of radiotherapy and nanomedicine permits a more personalized approach to cancer treatment. Tailoring the treatment strategy based on the individual characteristics of the patient and their tumor can optimize therapeutic outcomes[22].
Young researchers should concentrate on enhancing nanoparticle formulations to optimize the therapeutic index in GBM treatment. Research on low-dose radiation and its potential to augment the EPR effect and drug infiltration is also an interesting domain. Furthermore, interdisciplinary research on nanoparticle interactions in the TME may provide essential insights for clinical use.
The frequency of citations reflects the research hotspots and development trends about the discipline. The research hotspots and trends of nanomedicine in glioma were:
Nanomedicine platform RNA interference-based with nanoparticle conjugates of spherical nucleic acid: Jensen et al[8] assess, with 432 citations, a preclinical Nanomedicine platform RNA interference (RNAi)-based with nanoparticle conjugates of spherical nucleic acid (SNA) to inhibit oncogene expression in GBM. SNAs are gold nanoparticles covalently modified with arranged densely, strongly orientated small interfering RNA (Bcl2 L12) an effector caspase and p53 inhibitor that is overexpressed in GBM duplexes. The small nucleolar RNAs (SNAs) against of the oncoprotein Bcl2 Like12 compared to normal brain tissue and low-grade astrocytoma reduced endogenous Bcl2 L12 mRNA and protein levels. The sensitizing glioma cells to therapy-induced apoptosis by augmenting impediment to effective cancer therapy effector caspase and p53 activity. Consequently, inhibiting antiapoptotic signaling through SNAs constitutes a novel strategy for systemic RNAi therapy targeting GBM and maybe other fatal cancers.
Tumor hypoxia impairment to cancer therapy: The hyperbaric oxygen therapy, artificial hemoglobin, allosteric hemoglobin modifiers, hypoxia-activated prodrugs, and fluorocarbons (FCs) have been employed. Graham et al[9], with 346 citations, assess oxygen treatments utilizing liquid fluorocarbons, which may enhance the oxygen-carrying capacity of blood to mitigate tumor hypoxia. At present, a minimum of two pharmaceuticals are undergoing clinical trials aimed at reversing tumor hypoxia; one is intended to enhance oxygen permeability within tumor tissue, while the other utilizes a low boiling point fluorocarbon that delivers greater quantities of oxygen per gram than previously evaluated fluorocarbons.
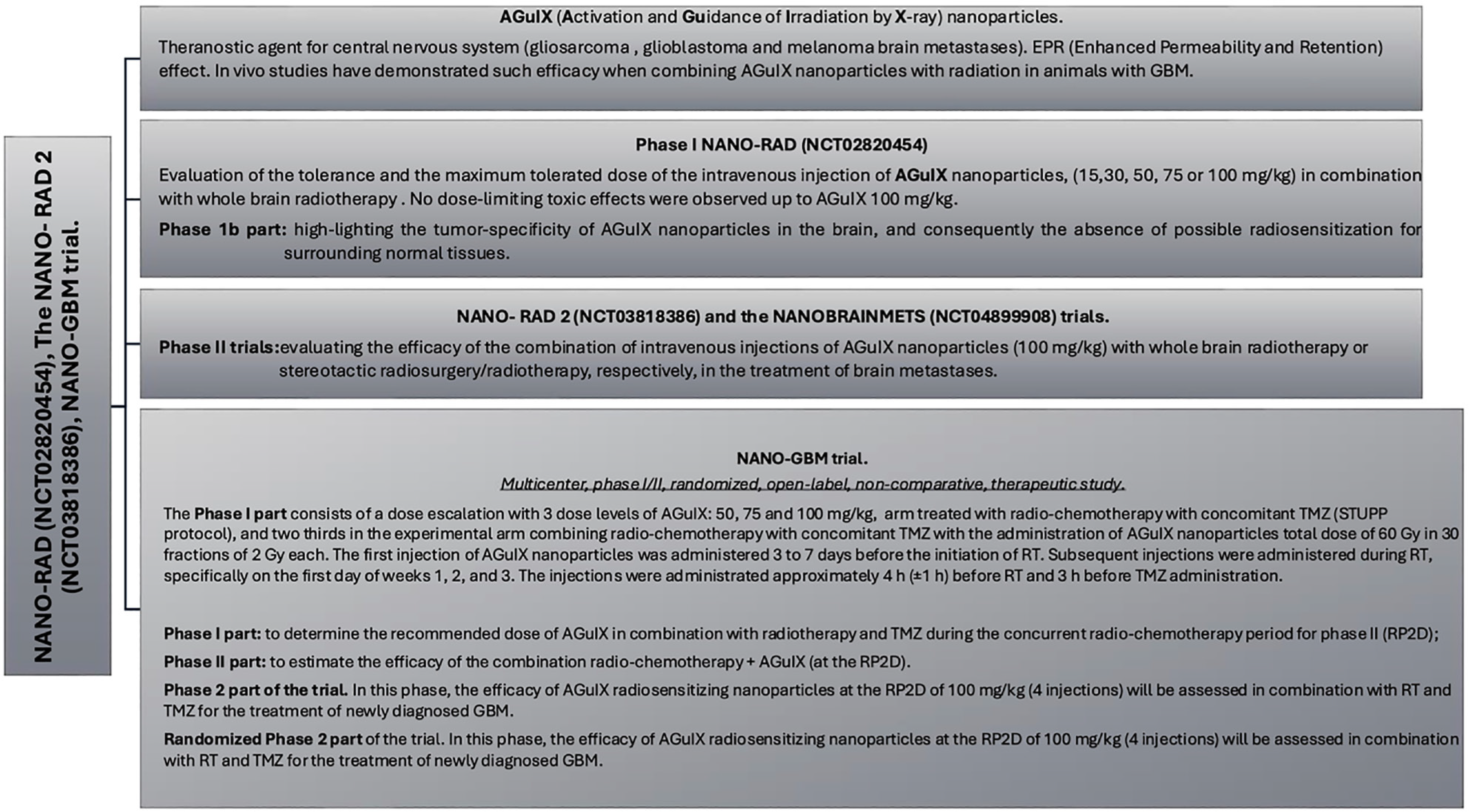
The prominence of nanomedicine clinical translation: Biau et al[23] proposed the results of the first human use of the theragnostic nanoparticles based on a polysiloxane network surrounded by gadolinium chelates (AGuIX) with radiotherapy and chemotherapy in the newly diagnosed GBM (Figure 13)[24].
The data were derived from WOS only. Failure by reviewers to manually remove irrelevant publications can lead to selection bias[25-28].
We analyzed the bibliometric features of nanomedicine in glioma. A comprehensive analysis was conducted to evaluate the research hotspots in the nanomedicine field. Integrating nano-drug delivery systems with minimal radiation doses presents a revolutionary method for treating cancer, especially GBM. This review emphasizes notable progress in nanomedicine, underlines the effectiveness of targeted medication administration, and calls for additional research on the synergistic potential with low-dose radiation. This method enhances therapeutic efficacy while aiming to reduce systemic toxicity, indicating a promising direction in precision oncology. However, new therapeutic approaches are necessary due to the poor prognosis associated with GBM. With the limitations of the research, our analysis aims are to highlight the increasing interest of researchers in the precision medicine field in GBM treatment and lead us to suggest further studies focusing on the association between nanomedicine and radiotherapy.
| 1. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 15775] [Article Influence: 788.8] [Reference Citation Analysis (0)] |
| 2. | Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4968] [Cited by in RCA: 5873] [Article Influence: 367.1] [Reference Citation Analysis (1)] |
| 3. | Mattix B, Moore T, Uvarov O, Pollard S, O'Donnell L, Park K, Horne D, Dhulekar J, Reese L, Nguyen D, Kraveka J, Burg K, Alexis F. Effect of polymer nanoparticle surface properties on interaction with the brain tumor environment. Nano Life. 2013;3:1343003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Zhao M, Li A, Chang J, Fu X, Zhang Z, Yan R, Wang H, Liang S. Develop a novel superparamagnetic nano-carrier for drug delivery to brain glioma. Drug Deliv. 2013;20:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2662] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 6. | Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-6392. [PubMed] |
| 7. | Grafals-Ruiz N, Rios-Vicil CI, Lozada-Delgado EL, Quiñones-Díaz BI, Noriega-Rivera RA, Martínez-Zayas G, Santana-Rivera Y, Santiago-Sánchez GS, Valiyeva F, Vivas-Mejía PE. Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. Int J Nanomedicine. 2020;15:2809-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:209ra152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 9. | Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomedicine. 2018;13:6049-6058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 395] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 10. | Ferini G, Parisi S, Lillo S, Viola A, Minutoli F, Critelli P, Valenti V, Illari SI, Brogna A, Umana GE, Ferrantelli G, Lo Giudice G, Carrubba C, Zagardo V, Santacaterina A, Leotta S, Cacciola A, Pontoriero A, Pergolizzi S. Impressive Results after "Metabolism-Guided" Lattice Irradiation in Patients Submitted to Palliative Radiation Therapy: Preliminary Results of LATTICE_01 Multicenter Study. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Pontoriero A, Critelli P, Chillari F, Ferrantelli G, Sciacca M, Brogna A, Parisi S, Pergolizzi S. Modulation of Radiation Doses and Chimeric Antigen Receptor T Cells: A Promising New Weapon in Solid Tumors-A Narrative Review. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Yasaswi PS, Shetty K, Yadav KS. Temozolomide nano enabled medicine: promises made by the nanocarriers in glioblastoma therapy. J Control Release. 2021;336:549-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Bhardwaj H, Jangde RK. Current updated review on preparation of polymeric nanoparticles for drug delivery and biomedical applications. Next Nanotechnology. 2023;2:100013. [DOI] [Full Text] |
| 14. | Singh S, Drude N, Blank L, Desai PB, Königs H, Rütten S, Langen KJ, Möller M, Mottaghy FM, Morgenroth A. Protease Responsive Nanogels for Transcytosis across the Blood-Brain Barrier and Intracellular Delivery of Radiopharmaceuticals to Brain Tumor Cells. Adv Healthc Mater. 2021;10:e2100812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Li TF, Li K, Wang C, Liu X, Wen Y, Xu YH, Zhang Q, Zhao QY, Shao M, Li YZ, Han M, Komatsu N, Zhao L, Chen X. Harnessing the cross-talk between tumor cells and tumor-associated macrophages with a nano-drug for modulation of glioblastoma immune microenvironment. J Control Release. 2017;268:128-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Zhu Y, Yu X, Thamphiwatana SD, Zheng Y, Pang Z. Nanomedicines modulating tumor immunosuppressive cells to enhance cancer immunotherapy. Acta Pharm Sin B. 2020;10:2054-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Liu W, Chen B, Zheng H, Xing Y, Chen G, Zhou P, Qian L, Min Y. Advances of Nanomedicine in Radiotherapy. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Song X, Sun Z, Li L, Zhou L, Yuan S. Application of nanomedicine in radiotherapy sensitization. Front Oncol. 2023;13:1088878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 19. | Man F, Lammers T, T M de Rosales R. Imaging Nanomedicine-Based Drug Delivery: a Review of Clinical Studies. Mol Imaging Biol. 2018;20:683-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Ganipineni LP, Danhier F, Préat V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J Control Release. 2018;281:42-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Xu M, Han X, Xiong H, Gao Y, Xu B, Zhu G, Li J. Cancer Nanomedicine: Emerging Strategies and Therapeutic Potentials. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 22. | Ortíz R, Quiñonero F, García-Pinel B, Fuel M, Mesas C, Cabeza L, Melguizo C, Prados J. Nanomedicine to Overcome Multidrug Resistance Mechanisms in Colon and Pancreatic Cancer: Recent Progress. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Biau J, Durando X, Boux F, Molnar I, Moreau J, Leyrat B, Guillemin F, Lavielle A, Cremillieux Y, Seddik K, Dufort S, De Beaumont O, Thivat E, Le Duc G. NANO-GBM trial of AGuIX nanoparticles with radiotherapy and temozolomide in the treatment of newly diagnosed Glioblastoma: Phase 1b outcomes and MRI-based biodistribution. Clin Transl Radiat Oncol. 2024;48:100833. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | DuRoss AN, Neufeld MJ, Rana S, Thomas CR Jr, Sun C. Integrating nanomedicine into clinical radiotherapy regimens. Adv Drug Deliv Rev. 2019;144:35-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Verry C, Sancey L, Dufort S, Le Duc G, Mendoza C, Lux F, Grand S, Arnaud J, Quesada JL, Villa J, Tillement O, Balosso J. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open. 2019;9:e023591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Verry C, Dufort S, Villa J, Gavard M, Iriart C, Grand S, Charles J, Chovelon B, Cracowski JL, Quesada JL, Mendoza C, Sancey L, Lehmann A, Jover F, Giraud JY, Lux F, Crémillieux Y, McMahon S, Pauwels PJ, Cagney D, Berbeco R, Aizer A, Deutsch E, Loeffler M, Le Duc G, Tillement O, Balosso J. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother Oncol. 2021;160:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 27. | Bennett S, Verry C, Kaza E, Miao X, Dufort S, Boux F, Crémillieux Y, de Beaumont O, Le Duc G, Berbeco R, Sudhyadhom A. Quantifying gadolinium-based nanoparticle uptake distributions in brain metastases via magnetic resonance imaging. Sci Rep. 2024;14:11959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 28. | Thivat E, Casile M, Moreau J, Molnar I, Dufort S, Seddik K, Le Duc G, De Beaumont O, Loeffler M, Durando X, Biau J. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC Cancer. 2023;23:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |