Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1704
Peer-review started: January 24, 2024
First decision: February 8, 2024
Revised: February 21, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: March 26, 2024
Processing time: 61 Days and 7.4 Hours
Venous thromboembolism significantly contributes to patient deterioration and mortality. Management of its etiology and anticoagulation treatment is intricate, necessitating a comprehensive consideration of various factors, including the bleeding risk, dosage, specific anticoagulant medications, and duration of therapy. Herein, a case of lower extremity thrombosis with multiple primary malignant tumors and high risk of bleeding was reviewed to summarize the shortcomings of treatment and prudent anticoagulation experience.
An 83-year-old female patient was admitted to the hospital due to a 2-wk history of left lower extremity edema that had worsened over 2 d. Considering her medical history and relevant post-admission investigations, it was determined that the development of left lower extremity venous thrombosis and pulmonary embolism in this case could be attributed to a combination of factors, including multiple primary malignant tumors, iliac venous compression syndrome, pre
Anticoagulant prophylaxis should be promptly initiated in cases of high-risk thrombosis. Individualized anticoagulation therapy is required for complex thrombosis.
Core Tip: Lung cancer and pancreatic cancer form a rare combination of multiple primary malignant tumors. This patient had a rare lower extremity venous thrombosis complicated by pulmonary embolism. Its causes included a history of various malignant tumors, recent novel coronavirus infection, insufficient anticoagulant therapy for previous lower extremity thrombosis, and iliac vein compression syndrome. Anticoagulant therapy poses challenges to patients with active cancer and reduced fibrinogen levels; abnormally elevated D-dimer levels; and decreased platelet counts. This article provides a comprehensive overview of the therapeutic options for anticoagulation.
- Citation: Chen JX, Xu LL, Cheng JP, Xu XH. Challenging anticoagulation therapy for multiple primary malignant tumors combined with thrombosis: A case report and review of literature. World J Clin Cases 2024; 12(9): 1704-1711
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1704.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1704
Venous thromboembolism is a leading cause of patient deterioration and mortality. The management of anticoagulant therapy for this condition is inherently complex, requiring careful consideration of various factors including bleeding risk, dosage and type of anticoagulants, and duration of treatment. Patients at high risk of thrombosis should receive prompt anticoagulation prophylaxis.
We present a case of venous thrombosis in the lower extremities and pulmonary embolism caused by an active tumor, iliac vein compression syndrome, history of novel coronavirus infection, and previous thrombosis that was inadequately treated. This case poses challenges for anticoagulation treatment, and we have provided a review of the relevant literature.
An 83-year-old female patient complained of edema in her left lower extremity for the 2 wk, which had worsened over the last 2 d.
A 2-wk history of swelling in the left lower extremity, which worsened in the last 2 d without dyspnea or chest pain.
The patient had a history of novel coronavirus infection 6 months prior and venous thrombosis in the right lower extremity (administered oral edoxaban tablets for 2 months but discontinued on her own). No blood clots were observed on ultrasound examination of either lower limb after treatment discontinuation. Two months prior, she was diagnosed with non-small cell lung cancer, pancreatic ductal adenocarcinoma, and metastasis to the liver and right inguinal lymph node. Her current treatments included oral tegafur, gimeracil, and oteracil potassium + almonertinib mesilate tablets. Routine blood tests, liver function tests, renal function tests, and coagulation results were normal.
The patient denied any relevant family history.
Severe swelling in the left lower extremity. A few scattered petechiae were observed in the skin and mucosa.
The patient's platelet count was 75×109/L↓, D-dimer (DD) 80.55 mg/LFEU↑, fibrinogen (FIB) 1.86 g/L↓, creatinine 84.9 μmol/L↑, and her liver function was within normal limits.
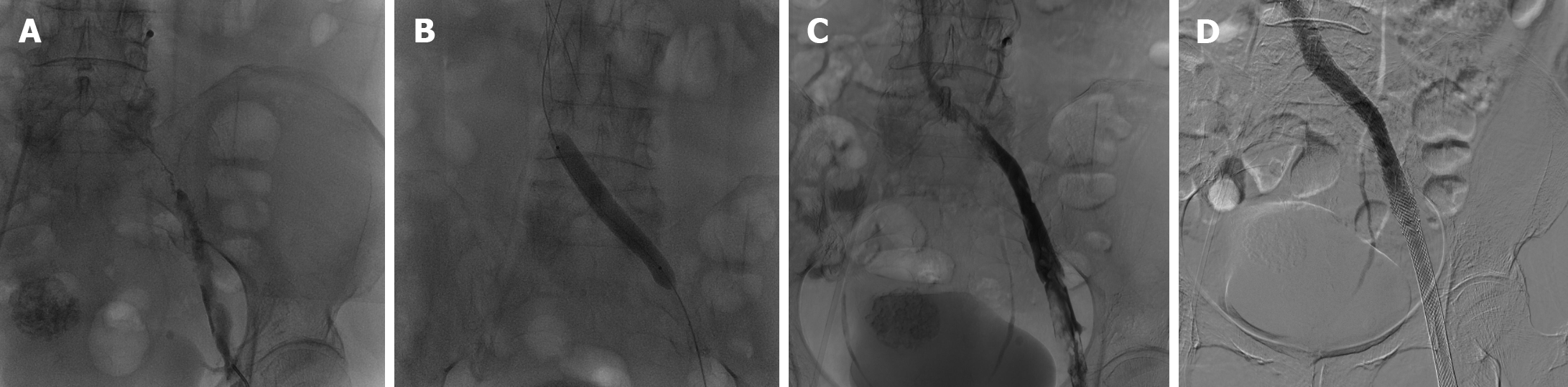
Double lower extremity vascular ultrasound indicated venous thrombosis in the left iliac, femoral, and popliteal veins. The patient underwent inferior vena cava venography, pulmonary arteriography, and lower extremity venography (Figure 1A).
Pulmonary artery embolism, left lower extremity venous thrombosis, left iliac vein compression syndrome, thrombocytopenia, renal insufficiency, non-small cell lung cancer, and pancreatic ductal adenocarcinoma.
The patient discontinued her current treatment (oral tegafur, gimeracil, and oteracil tablets) and underwent percutaneous transcatheter pulmonary artery aspiration thrombectomy, inferior vena cava filter placement, percutaneous transcatheter lower extremity vein aspiration thrombectomy, venous balloon dilation angioplasty of the lower extremity, and stenting of the iliac vein (Figure 1). The patient declined edoxaban tablets due to palpitations and elevated blood pressure. Consequently, she was prescribed apixaban tablets (2.5 mg/dose, Q12h, orally) as anticoagulation therapy following surgery.
Following postoperative re-examination, lower extremity vascular ultrasound revealed venous thrombosis in the distal end of the left femoral and popliteal veins. Additionally, the platelet count was 79×109/L, CA199 2.40 U/mL, and DD 8.67 mg/LFEU. The dose of apixaban was adjusted to 5 mg Q12h, and tegafur, gimeracil, and oteracil were continue discontinued. DD and FIB after 4 months were re-examined (Table 1).
| Date | May 30, 2023, 13:00 | May 30, 2023, 23:53 | May 31, 2023 | Operation | June 1, 2023 | June 2, 2023 | June 3, 2023 | June 5, 2023 | June 6, 2023 | … | October 27, 2023 |
| D-dimer (mg/L) | 80.55 | 64.91 | 53.64 | Operation | 30.89 | 19.55 | 13.67 | 9.02 | 8.67 | … | 3.00 |
| Fibrinogen quantification (g/L) | 1.86 | 1.76 | 2.08 | Operation | 2.14 | 1.87 | 1.92 | 1.79 | 1.96 | … | 2.57 |
Both coronavirus disease 2019 (COVID-19) and long COVID-19 (also known as "acute sequelae of COVID-19") patients are susceptible to thrombotic disease due to excessive inflammation, platelet activation, endothelial dysfunction, and stasis[1-4]. Thromboembolic complications resulting from COVID-19 have been extensively documented as primary contributors to sudden deterioration and mortality[5], highlighting the significance of prevention and early detection of thrombosis. The risk of thrombosis can be assessed by dynamically monitoring DD levels using various thrombus scoring tools, such as Caprini, Padua, and Improve[1,5,6]. Existing guidelines recommend that all COVID-19 patients who are not at a high risk of bleeding should receive anticoagulant prophylaxis[7-11]. The minimum duration of anticoagulant therapy for patients with venous thrombosis is 3 months[12].
In this case, the patient had COVID-19 infection 6 months prior. Anticoagulation prophylaxis was not administered during the infection period, and venous thrombosis of the lower extremities was identified within 1 month of recovery. The patient was prescribed anticoagulant therapy but discontinued after 2 months, which may have contributed to the occurrence of recurrent lower extremity venous thrombosis.
Iliac vein compression syndrome, also known as May-Thurner syndrome (MTS) or Cockett syndrome, is an anatomical variation resulting from compression of the left common iliac vein (LCIV) between the right common iliac artery and vertebrae[13]. While most cases are asymptomatic, compromised venous return and endothelial injury caused by chronic pulsatile compression of the LCIV by the right common iliac artery can occur, leading to subsequent obstruction and extensive deep vein thrombosis[14,15]. Venography is considered the gold standard for diagnosing MTS. MTS management involves alleviating LCIV compression and restoring normal blood flow through endovascular surgical intervention complemented by anticoagulation therapy[14,16]; this combination has been demonstrated to be an efficacious treatment for MTS[17]. The present patient was treated with this combination therapy.
Individuals with malignant tumors frequently have hypercoagulable blood and are prone to venous thrombotic events (VTEs). The incidence of VTEs varies across different cancers, with notably higher rates observed in pancreatic, gastric, and lung cancers[18]. Furthermore, chemotherapy, radiation, and surgical interventions all elevate the risk of deep vein thrombosis (DVT)[19]. Specifically, the use of systemic chemotherapy has been associated with an 18-19-fold increase in VTE risk[20]. Moreover, cancer patients are at a higher risk of complications, including VTE recurrence and bleeding during VTE treatment, than those without cancer[19,21]. The absolute risk of developing subsequent VTE in patients with cancer with a history of VTE is 6-7-fold higher than that in patients without prior thromboembolic events[18]. The prevalence of DVT accompanied by pulmonary embolism has been documented to be relatively low, ranging from 29 to 78 cases per 100000 individuals annually. This prevalence increases with the presence of active tumors[22,23].
Venous thromboembolism ranks as the second leading cause of mortality among individuals with cancer[18,20]. It is advisable to provide thromboprophylaxis to all hospitalized cancer patients and high-risk outpatients, as determined by risk assessment models and computerized tools, in a timely and targeted manner. This approach aims to reduce the incidence of thrombotic events, enhance the prognosis of cancer patients, and ultimately improve survival. A recent article deliberating on the appropriateness of thromboprophylaxis for cancer outpatients suggested that barring a high risk of bleeding, initial thromboprophylaxis is recommended for individuals with pancreatic cancer and lung cancer who may harbor anaplastic lymphoma kinase/ROS proto-oncogene 1, receptor tyrosine kinase translocations. Patients with upper gastrointestinal cancers are at a higher risk of VTE; however, a thorough evaluation of bleeding risk should precede decisions regarding antithrombotic prophylaxis. Notably, for cancer patients with a heightened risk of bleeding, such as those with brain cancer, moderate-to-severe thrombocytopenia, or severe renal impairment, it is not advisable to pursue primary prevention of VTE. In cases where patients present with absolute contraindications for anticoagulation therapy, such as active bleeding or severe long-term thrombocytopenia, inferior vena cava filter implantation may be considered based on specific circumstances[24].
Substantial evidence has accumulated regarding the advantages of anticoagulant therapy in individuals with highly thromboembolic tumors[25,26]. The Prospective Randomized Trial of Enoxaparin and Chemotherapy Concurrently for Pancreatic Cancer was formulated to assess the effectiveness of enoxaparin in patients with locally advanced or metastatic pancreatic cancer undergoing systemic chemotherapy. The findings indicated a reduction in the prevalence of VTEs from 87.1% to 25.3% at 9 months, and from 13.5% to 12% at 15 months[25]. The administration of anticoagulant intervention in patients with pancreatic cancer resulted in a significant reduction in the incidence of VTE, from 23% to 3.4%[26].
A review of multiple clinical guidelines from American society of clinical oncology (ASCO), European society for medical oncology (ESMO), and national comprehensive cancer network (NCCN) states that low molecular weight heparin (LMWH) or normal heparin is the recommended standard of care for the prevention and treatment of cancer-associated thrombosis (CAT)[19,27-29]. LMWH is the preferred choice due to its lower risk of heparin-induced thrombocytopenia and convenient administration[27,30,31]. However, patients may experience an injection burden after hospital discharge, and direct oral anticoagulants are approved as alternatives to LMWH for the treatment of CAT[21,29]. According to the 2023 ASCO guidelines, apixaban is effective in reducing the risk of recurrent VTE and has a lower risk of major bleeding. Additionally, a panel of experts agreed that apixaban could be recommended as an alternative treatment for CAT[19]. However, the CHEST guidelines update article published in 2021 indicated that oral Xa inhibitors (apixaban, edoxaban, and rivaroxaban) are more strongly recommended than LMWH for treatment initiation in patients with acute VTE and cancer-associated thrombosis (strong recommendation, moderate quality evidence)[32].
However, an article published in 2016 in Lancet suggested that direct oral anticoagulants should not be the first choice for VTE in patients with active cancer, although there are no contraindications[33]. The use of LMWH or oral anticoagulants in the acute phase remains controversial; further large-scale clinical trials are needed. In this case, an oral anticoagulant was used immediately after interventional therapy. However, the dose of anticoagulant was insufficient; the therapeutic effect on CAT could not achieve the ideal effect in theory.
Thrombocytopenia frequently leads to the discontinuation of anticoagulation therapy in cancer patients. Therefore, the guidelines recommend that patients with platelet counts ≥ 50×109/L receive full-dose anticoagulation (whether with using LMWH or an oral anticoagulant) without concomitant platelet transfusions[34-36]. CAT therapy typically consists of three phases: acute (occurring 5-10 d after diagnosis), maintenance (lasting 3-6 months), and extended phase (lasting > 6 months). In patients with active cancer undergoing cancer therapy, where the risk of recurrence outweighs bleeding complications, an extension of anticoagulation therapy for > 6 months may be considered. The recommended anticoagulation therapies for each period are presented in Table 2 Unfractionated heparin is recommended for patients with severe renal insufficiency (CrCl < 30 mL/min) because of the elevated risk of hemorrhage and recurrent venous thrombosis associated with anticoagulant therapy[29].
| Mode of administration | Initial therapeutic dose | Maintenance of therapeutic dose | Extended treatment dose | |
| Unfractionated heparin | Intravenous | Maintain APTT 1.5 times the upper limit of normal | / | / |
| Low molecular heparin | Subcutaneous | 200 IU/kg/d for 1 month | 150 IU/kg | / |
| Rivaroxaban | Oral | 15 mg each time, twice a day for 3 wk | 20 mg each time, once a day | 20 mg each time, once a day |
| Apixaban | Oral | 10 mg each time, twice a day for 1 wk | 5 mg each time, twice a day | 2.5 mg each time Twice a day |
| Eldosaban | Oral | At least 5 d of heparin introduction is required, with dose reduction after LMWH introduction, i.e. 30 mg each time, once a day | 60 mg each time, once a day | 60 mg each time, once a day |
In conclusion, the patient was in the acute stage of thrombosis at present. However, considering advanced age; thrombocytopenia; renal insufficiency; presence of a few scattered petechial dots on the skin and mucosa; and the slightly higher risk of anticoagulant bleeding in this patient, interventional therapy and apixaban (2.5 mg twice a day) were initially administered. After observation, petechiae in the skin and mucosa did not progress; the DD was 13.67 mg/L; and there was still thrombus on the reexamination using color Doppler ultrasound. The effect of anticoagulant therapy was considered to be unsatisfactory. Therefore, the dose of apixaban tablets was adjusted to 5 mg twice a day. The deficiencies in the treatment of the low-risk thrombocytopenia in this patient include: Receiving no anticoagulant prophylaxis. In addition, the dose of anticoagulant therapy was slightly lower than the recommended dose according to the guidelines. After follow-up, DD in the patient was significantly decreased. Furthermore, no more serious bleeding events occurred, which also indicated that the combined treatment scheme in this case was feasible; anticoagulant therapy was safe and effective.
FIB plays a crucial role as a reactive substrate in thrombosis and is implicated in critical stages[37]. DD, a small protein fragment resulting from fibrin breakdown, has been the subject of research as a predictive biomarker for VTE in cancer[18,38]. Elevated DD and FIB levels are commonly observed in patients with COVID-19 and those with malignancy[5]. The decrease in FIB is common in patients with primary and secondary hyperfibrinolysis, such as DIC. Additionally, impairment in liver cell function leads to the decrease in liver synthesis, snake venom therapy, and thrombolytic therapy. In conjunction with the present case, the patient in question had a tumor and experienced VTEs. In such cases, DD and FIB should be theoretically elevated; however, this patient exhibited abnormally elevated DD, low FIB levels, and a decreased platelet count. When considering the patient's history of a normal coagulation phase, it is reasonable to suspect the presence of DIC and a reduction in FIB due to the substantial consumption of FIB within the body.
In summary, patients with active cancer, chemotherapy, novel coronavirus infection, and iliac vein compression syndrome should be on high alert for venous thrombosis. This requires dynamic assessment of anticoagulation and bleeding risks; comprehensive management; reduction in thrombotic events; preventing bleeding complications and recurrence; and improvement in prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac & cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nicolae N, Romania S-Editor: Liu JH L-Editor: A P-Editor: Chen YX
| 1. | Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2178] [Cited by in RCA: 2191] [Article Influence: 438.2] [Reference Citation Analysis (0)] |
| 2. | Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 640] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 3. | Violi F, Harenberg J, Pignatelli P, Cammisotto V. COVID-19 and Long-COVID Thrombosis: From Clinical and Basic Science to Therapeutics. Thromb Haemost. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1688] [Cited by in RCA: 2167] [Article Influence: 1083.5] [Reference Citation Analysis (0)] |
| 5. | Gorog DA, Storey RF, Gurbel PA, Tantry US, Berger JS, Chan MY, Duerschmied D, Smyth SS, Parker WAE, Ajjan RA, Vilahur G, Badimon L, Berg JMT, Cate HT, Peyvandi F, Wang TT, Becker RC. Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. 2022;19:475-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, Zhang C, Li H, Xia X, Kong S, Liao J, Jia H, Pang X, Song Y, Tian Y, Wang B, Wu C, Yuan H, Zhang Y, Li Y, Sun W, Zhu S, Wang S, Xie Y, Ge S, Zhang L, Hu Y, Xie M. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation. 2020;142:114-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 7. | Xiang M, Jing H, Wang C, Novakovic VA, Shi J. Persistent Lung Injury and Prothrombotic State in Long COVID. Front Immunol. 2022;13:862522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment. J Thromb Haemost. 2020;18:2060-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 9. | Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 534] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 10. | Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol. 2020;76:122-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 746] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 11. | Donato AA. In non-critically ill patients with COVID-19, therapeutic anticoagulation improved survival to discharge without organ support. Ann Intern Med. 2021;174:JC134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Ali MAM, Spinler SA. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc Med. 2021;31:143-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 13. | Poyyamoli S, Mehta P, Cherian M, Anand RR, Patil SB, Kalva S, Salazar G. May-Thurner syndrome. Cardiovasc Diagn Ther. 2021;11:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Harbin MM, Lutsey PL. May-Thurner syndrome: History of understanding and need for defining population prevalence. J Thromb Haemost. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Mousa AY, AbuRahma AF. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013;27:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Mathur M, Cohen M, Bashir R. May-Thurner syndrome. Circulation. 2014;129:824-825. [PubMed] [DOI] [Full Text] |
| 17. | Ali MM, Hasan SA, Qaheri RS, Alkhozaae ZZ, Alharbi A. Endovascular Stenting for May-Thurner Syndrome: A Case Report. Cureus. 2023;15:e42525. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Gervaso L, Dave H, Khorana AA. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021;3:173-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 19. | Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Gates LE, Kakkar AK, Tempero MA, Gupta S, Lyman GH, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J Clin Oncol. 2023;41:3063-3071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 136] [Reference Citation Analysis (0)] |
| 20. | Donnellan E, Khorana AA. Cancer and Venous Thromboembolic Disease: A Review. Oncologist. 2017;22:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Riess H, Verhamme P, Weitz JI, Young A, Bauersachs R, Beyer-Westendorf J, Crowther M, Maraveyas A. Treatment of cancer-associated thrombosis: The evolution of anticoagulant choice and clinical insights into practical management. Crit Rev Oncol Hematol. 2021;157:103125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 702] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 23. | Yang G, Mi Y H, Xiao Y, Liang Y, Gong J, Zhang SY. Analysis of long-term prognosis and related factors in patients with malignant tumor complicated with acute pulmonary embolism. Xinfei Xueguanbing Zazhi. 2023;42:916-921. |
| 24. | Verso M, Muñoz A, Connors JM. Ambulatory cancer patients: who should definitely receive antithrombotic prophylaxis and who should never receive. Intern Emerg Med. 2023;18:1619-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 25. | Riess H, Pelzer U, Hilbig A, Stieler J, Opitz B, Scholten T, Kauschat-Brüning D, Bramlage P, Dörken B, Oettle H. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy). BMC Cancer. 2008;8:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, Sgouros J, Gardiner E, Wedgwood K, Ettelaie C, Bozas G. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 27. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 2615] [Article Influence: 653.8] [Reference Citation Analysis (1)] |
| 28. | Streiff MB, Holmstrom B, Angelini D, Ashrani A, Bockenstedt PL, Chesney C, Fanikos J, Fenninger RB, Fogerty AE, Gao S, Goldhaber SZ, Gundabolu K, Hendrie P, Lee AI, Lee JT, Mann J, McMahon B, Millenson MM, Morton C, Ortel TL, Ozair S, Paschal R, Shattil S, Siddiqi T, Smock KJ, Soff G, Wang TF, Williams E, Zakarija A, Hammond L, Dwyer MA, Engh AM. NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J Natl Compr Canc Netw. 2018;16:1289-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 29. | Falanga A, Ay C, Di Nisio M, Gerotziafas G, Jara-Palomares L, Langer F, Lecumberri R, Mandala M, Maraveyas A, Pabinger I, Sinn M, Syrigos K, Young A, Jordan K; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34:452-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 182] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 30. | Farge D, Frere C, Connors JM, Khorana AA, Kakkar A, Ay C, Muñoz A, Brenner B, Prata PH, Brilhante D, Antic D, Casais P, Guillermo Esposito MC, Ikezoe T, Abutalib SA, Meillon-García LA, Bounameaux H, Pabinger I, Douketis J; International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23:e334-e347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 31. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 694] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 32. | Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:2247-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 264] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 33. | Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 582] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 34. | Carrier M, Khorana AA, Zwicker JI, Noble S, Lee AY; Subcommittee on Haemostasis and Malignancy for the SSC of the ISTH. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH: a reply to a rebuttal. J Thromb Haemost. 2014;12:116-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Moik F, Pabinger I, Ay C. How I treat cancer-associated thrombosis. ESMO Open. 2020;5:e000610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Samuelson Bannow BT, Lee A, Khorana AA, Zwicker JI, Noble S, Ay C, Carrier M. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1246-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | Uitte de Willige S, Standeven KF, Philippou H, Ariëns RA. The pleiotropic role of the fibrinogen gamma' chain in hemostasis. Blood. 2009;114:3994-4001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |