Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1685
Peer-review started: December 20, 2023
First decision: January 4, 2024
Revised: February 22, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: March 26, 2024
Processing time: 95 Days and 22.2 Hours
Many patients with ulcerative colitis (UC) do not respond well to, or tolerate conventional and biological therapies. There is currently no consensus on the treatment of refractory UC. Studies have demonstrated that the selective Janus kinase 1 inhibitor upadacitinib, a small-molecule drug, is effective and safe for treating UC. However, no studies have revealed that upadacitinib is effective in treating refractory UC with primary nonresponse to infliximab and vedolizumab.
We report the case of a 44-year-old male patient with a chief complaint of bloody diarrhoea with mucus and pus, in addition to dizziness. The patient had recurrent disease after receiving mesalazine, prednisone, azathioprine, infliximab and vedolizumab over four years. Based on the endoscopic findings and pathological biopsy, the patient was diagnosed with refractory UC. In particular, the patient showed primary nonresponse to infliximab and vedolizumab. Based on the patient’s history and recurrent disease, we decided to administer upadacitinib. During hospitalisation, the patient was received upadacitinib under our guidance. Eight weeks after the initiation of upadacitinib treatment, the patient’s symptoms and endoscopic findings improved significantly. No notable adverse reactions have been reported to date.
Our case report suggests that upadacitinib may represent a valuable strategy for treating refractory UC with primary nonresponse.
Core Tip: Ulcerative colitis (UC) is a major type of inflammatory bowel disease. Many patients do not respond well to the current therapies. We report the case of a patient diagnosed with refractory UC, with primary nonresponse to infliximab and vedolizumab. The patient experienced recurrent symptoms after receiving mesalazine, prednisone, azathioprine, infliximab, and vedolizumab for more than four years. After optimising the upadacitinib treatment, UC remission was achieved. Our report suggests that the small-molecule upadacitinib may be a new treatment option that deserves to be reported and studied.
- Citation: Xu X, Jiang JW, Lu BY, Li XX. Upadacitinib for refractory ulcerative colitis with primary nonresponse to infliximab and vedolizumab: A case report. World J Clin Cases 2024; 12(9): 1685-1690
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1685.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1685
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) caused by multiple factors. However, its aetiology and pathogenesis are not completely understood. The disease often relapses and remits.
For UC treatment, 5-aminosalicylic acid preparations (5-ASA), glucocorticoids, and immunosuppressants are used. In recent years, various biological agents such as infliximab have shown good efficacy in treating IBD. However, many patients with UC do not respond well at all, or cannot tolerate conventional or biological therapies. Therefore, additional treatment options are needed for patients with refractory UC.
Here, we report a case of refractory UC successfully treated with upadacitinib, a small-molecule drug that inhibits Janus kinase (JAK) signalling. The patient failed to respond to infliximab and vedolizumab, two biological agents commonly used to treat IBD. We also reviewed the relevant literature on the use of JAK inhibitors for IBD and discussed the advantages of its oral administration and low immunogenicity.
The patient was a 44-year-old caucasian male with a body mass index of 26.5 (height, 178 cm; weight, 84 kg). He presented to the hospital on December 15, 2022, with a chief complaint of haematochezia and dizziness.
The patient had experienced frequent episodes of bloody diarrhoea with mucus and pus for more than 10 d.
He had a history of chronic intermittent small volume hematochezia for 5 years, with a frequency of more than 10 episodes per day.
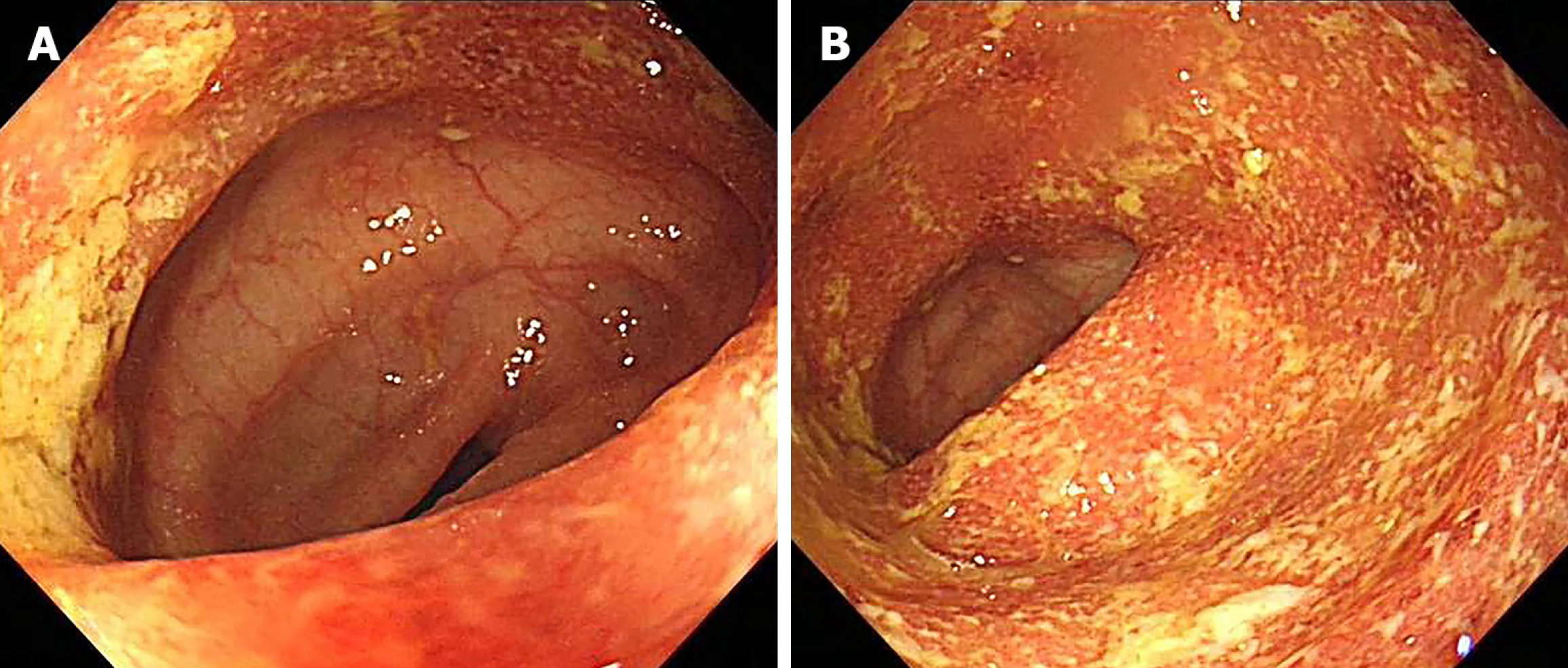
The patient underwent colonoscopy at another hospital and was diagnosed with UC (left-sided active phase) (Figure 1).
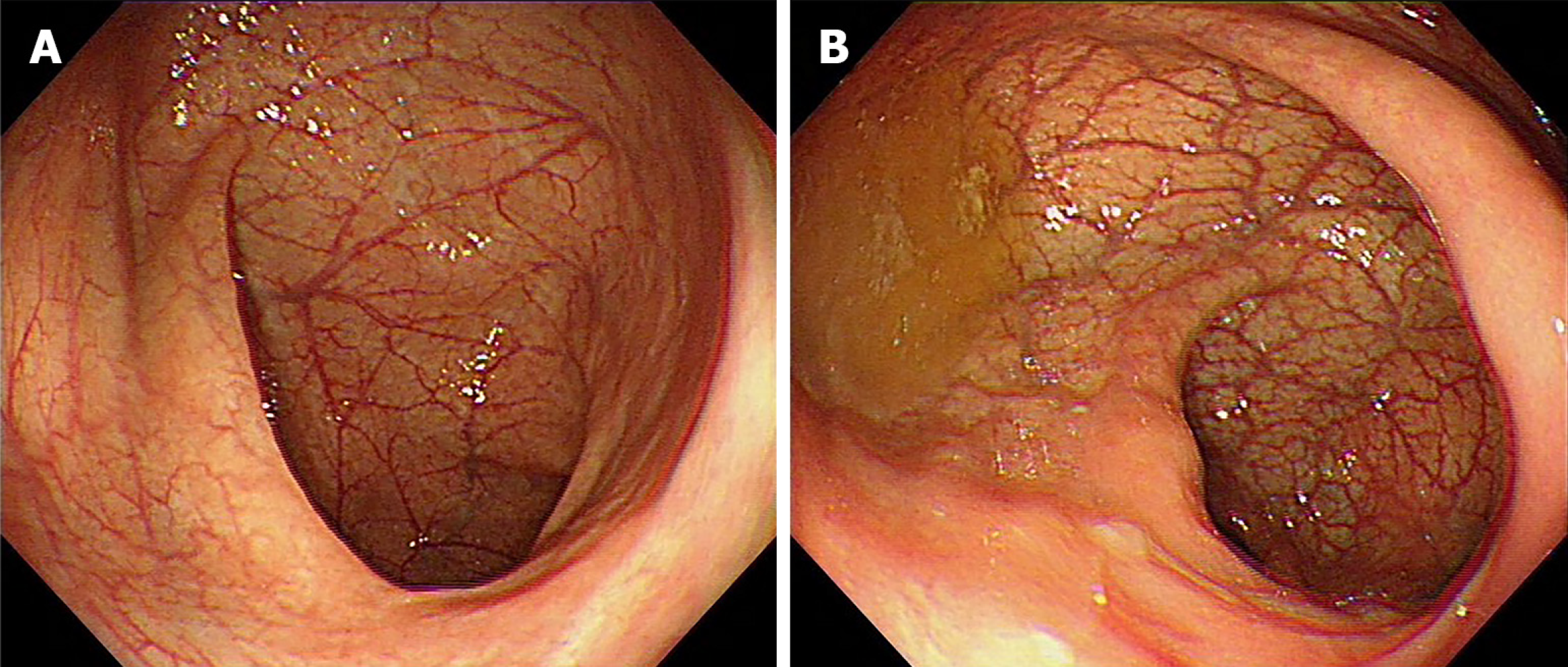
Despite receiving mesalazine and other symptomatic treatments, the patient experienced recurrent flare-ups. In November 2019, the patient received induction therapy with mesalazine (4 g) and prednisone (64 mg), which resulted in significant symptom relief, reduced the erythrocyte sedimentation rate (ESR) to 37 mm/h, and normalised stool frequency and quality (no mucus, pus, or blood). A follow-up colonoscopy performed in February 2021 revealed complete mucosal healing (Figure 2).
Mesalazine was maintained at 4 g/d after initial treatment. In January 2022, the patient again experienced recurrent symptoms of abdominal pain and bloody stool, ranging from two to five times a day and investigations revealed an elevated ESR of 93 mm/h. The symptoms were alleviated with oral methylprednisolone (64 mg), azathioprine (100 mg), and metronidazole. However, the symptoms recurred as bloody stools increased to 2–10 times a day. The patient was therefore, treated with infliximab (400 mg) and azathioprine (100 mg) in July 2022.
The patient experienced recurrent symptoms after the second infliximab infusion, such as passing > 10 stools per day with mucus, pus, or blood. The infliximab dose was increased to 500 mg at the fourth dose. However, there was no improvement in colonoscopic findings, which showed chronic relapsing UC involving the entire colon (Ulcerative Colitis Endoscopic Index of Severity score 6).
The patient exhibited primary nonresponse to infliximab and continued to experience symptoms.
The patient denied any family history of malignant tumours.
There were no obvious abnormalities except tenderness in the left lower abdomen.
Epstein-Barr virus DNA quantization was 1.28E+3 copies/mL. Haemoglobin level was 123 g/L, and ESR was 33 mm/h. Albumin 38.4g/L, and hepatorenal function was normal. Faecal erythrocyte count 4+, occult blood test +. Faecal and C. difficile cultures were negative. C-reactive protein, anti-neutrophil cytoplasmic antibodies, antinuclear antibodies, cytomegalovirus antibody, T-SPOT, thyroid function, tumour markers, eight infectious diseases [hepatitis B surface antigen, anti- hepatitis C virus, antigen-human immunodeficiency virus (HIV)/antibody-HIV, antibody-syphilis], coagulation function, D-dimers, and tuberculosis T-SPOT were all within the normal ranges.
Computed tomography showed that the mucosa of the rectum and sigmoid intestinal wall were uniformly thickened and significantly strengthened, indicating UC-related alterations. Pelvic magnetic resonance imaging revealed colorectal wall thickening.
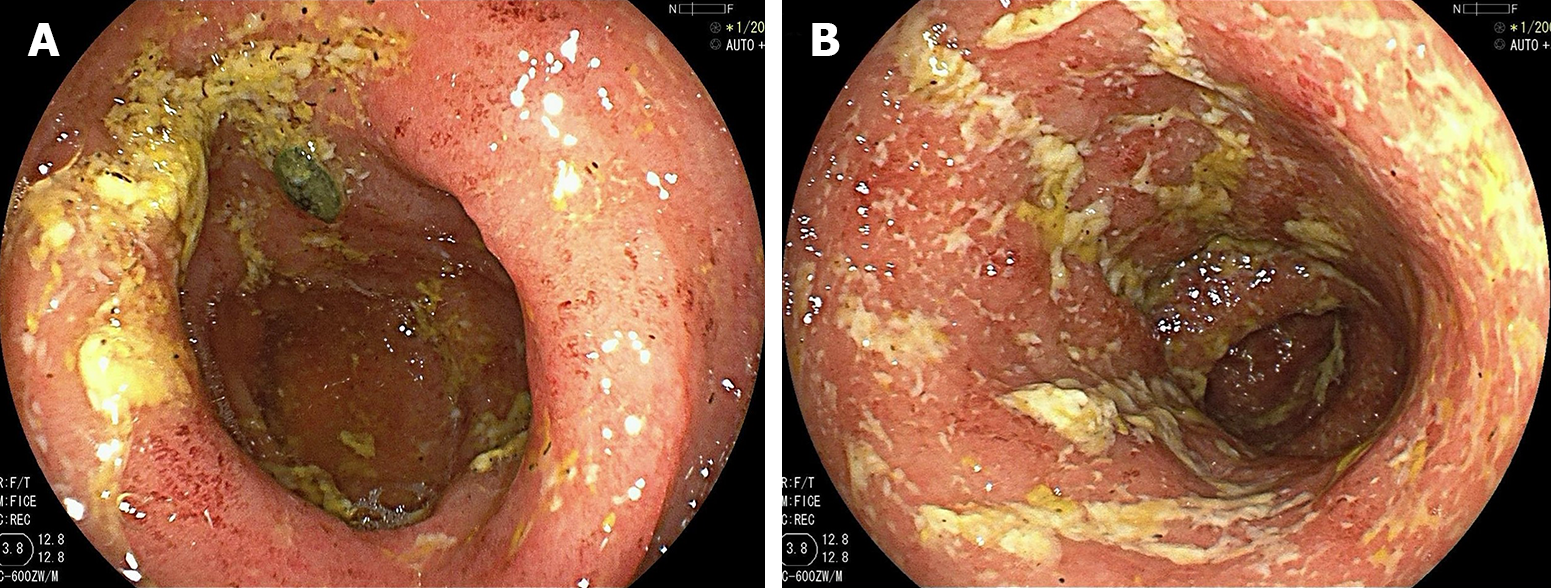
In December 2022, colonoscopy revealed extensive ulceration of both the sigmoid colon and rectum, and the diagnosis was of UC (E2, Ulcerative Colitis Endoscopic Index of Severity score 5–6, Mayo endoscopic score 2–3) (Figure 3).
The Pathology Department of our hospital confirmed UC on the patient’s biopsy specimens obtained from another hospital.
The final diagnosis was UC (chronic recurrent type, total colon type, active, moderate, Mayo score 6).
The patient declined glucocorticoid therapy after admission and received vedolizumab and azathioprine 100 mg/d, along with two daily enemas of Brinider suspension, starting on December 20, 2022. However, the symptoms persisted, with 5–10 stools per day, consisting mostly of pus and blood. The ESR was 42 mm/h after five days. The patient then received oral mesalazine, mesalazine enemas, faecal microbiota transplantation (FMT), and a second dose of vedolizumab.
Despite receiving a FMT on February 17-19, 2023, and a third dose of vedolizumab on February 18, 2023, the patient did not experience symptom relief. The patient received a fourth dose of vedolizumab on April 1, 2023, and a fifth dose on May 10, 2023, with no improvement in clinical outcomes.
The patient experienced recurrent symptoms of mucus, pus, and bloody stools 2–5 times per day on May 12, 2023, suggesting primary nonresponse to vedolizumab. The patient was then commenced on upadacitinib 45 mg/d on May 14, 2023.
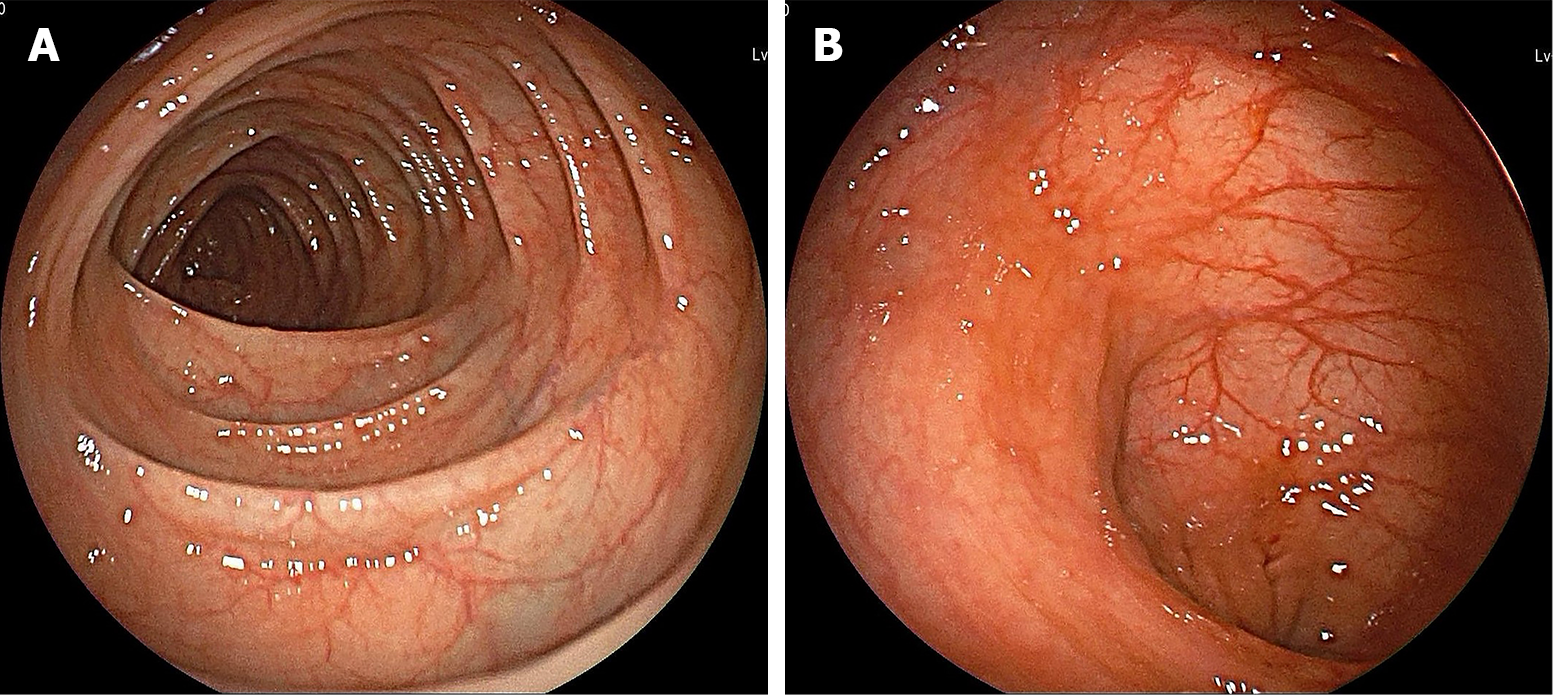
On June 9, 2023, the patient reported significant improvement in haematochezia symptoms, with no other discomfort. Follow-up colonoscopy after 8 wk (Figure 4) showed that the UC was in remission and the Mayo endoscopic score was 0. The patient is currently receiving upadacitinib (30 mg/d). We plan to review him in six months and reduce the dose to 15 mg/d, as appropriate.
UC is a major type of inflammatory bowel disease that was first described in 1895[1]. The main clinical feature of UC is bloody diarrhoea. Many patients experience left-sided abdominal pain, particularly if the inflammation is limited to the left colon. Patients with pancolitis, or inflammation of the entire colon, often experience diffuse abdominal pain and tenesmus[2]. Endoscopic examination reveals continuous inflammation of the colonic mucosa, starting from the rectum and extending proximally to the ileocecal valve[1].
Currently, the available treatments for UC include conventional drugs (such as 5-ASA, glucocorticoids and immuno
However, many patients with UC do not respond adequately, lose their response to conventional or biological therapies, or experience adverse effects from these treatments[4]. Previous studies have shown that even after switching to new biological agents, only one-third of patients achieve or maintain clinical remission at one year[5]. Patients who depend on glucocorticoids or are unresponsive or intolerant to at least one of 5-ASA, corticosteroids or immunosuppressants are considered to have refractory UC[6].
This patient had recurrent disease despite multiple courses of mesalazine, prednisone, azathioprine, and vedolizumab over four years, and showed primary nonresponse to infliximab and vedolizumab. These features met the criteria for refractory UC. The evidence for the efficacy of optimising vedolizumab or combining it with azathioprine is inconsistent across studies[7,8]. However, the patient’s symptoms did not improve significantly after optimising the vedolizumab treatment.
Upadacitinib is a selective small-molecule JAK1 inhibitor. In clinical trials, upadacitinib was shown to be highly effective in achieving remission in patients with moderate-to-severe UC[5].
We reviewed the relevant literature and found a few studies on upadacitinib treatment for intractable UC. A retrospective cohort study reported that most patients achieved steroid-free clinical remission and clinical response within 8-16 wk of starting upadacitinib and that most of them had previously received anti-tumor necrosis factors and vedolizumab[9]. Phase II and III trials demonstrated that a selective JAK1 inhibitor for UC was effective and safe.
The patients in this study previously failed to respond adequately, lost response to, or experienced adverse effects from, at least one conventional or biologic therapy[4].
The two previous studies did not specify the type of nonresponse (primary or secondary) of the patients included. In contrast, our patient showed primary nonresponse to vedulizumab and infliximab as well as an inadequate response to other drugs. His condition was more complex and severe than that of the patients in the two previous studies. Considering the patient’s lack of response to multiple therapies, and the efficacy of upadacitinib in phase II and III trials, we decided to administer upadacitinib only after obtaining informed consent.
We administered upadacitinib at a dose of 45 mg/d. Colonoscopy revealed that the patient achieved remission eight weeks later. The patient continued to receive a maintenance dose of 30 mg/d of upadacitinib monotherapy and remained in remission, demonstrating the efficacy of upadacitinib in this case.
In summary, our patient experienced recurrence of symptoms after receiving mesalazine and prednisone, and showed primary nonresponse to infliximab and vedolizumab. This case demonstrates that upadacitinib is effective in treating refractory UC, in a patient who failed to respond to infliximab and vedolizumab, with significant improvement in symptoms and endoscopic findings, and no notable adverse reactions have been observed to date. This provides an alternative option for patients with refractory UC and primary nonresponse. Moreover, small-molecule drugs may have advantages such as reduced immunogenicity and enhanced patient adherence. However, this was a single-case report. The efficacy of upadacitinib in refractory UC patients with primary nonresponse needs to be verified in further clinical studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal S-Editor: Zhang L L-Editor: A P-Editor: Chen YX
| 1. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 987] [Article Influence: 197.4] [Reference Citation Analysis (0)] |
| 2. | Kucharzik T, Koletzko S, Kannengiesser K, Dignass A. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch Arztebl Int. 2020;117:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: An update. Clin Med (Lond). 2021;21:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 4. | Goetsch A, D'Amico F, Allocca M, Fiorino G, Furfaro F, Zilli A, Parigi TL, Radice S, Peyrin-Biroulet L, Danese S. Advances in pharmacotherapy for ulcerative colitis: A focus on JAK1 inhibitors. Expert Opin Pharmacother. 2023;24:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 331] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 6. | Löwenberg M, de Boer NKh, Hoentjen F. Golimumab for the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014;7:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Attauabi M, Vind I, Pedersen G, Bendtsen F, Seidelin JB, Burisch J. Short and long-term effectiveness and safety of vedolizumab in treatment-refractory patients with ulcerative colitis and Crohn’s disease - a real-world two-center cohort study. Eur J Gastroenterol Hepatol. 2021;33:e709-e718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Samaan MA, Birdi S, Morales MS, Honap S, Tamilarasan AG, Cunningham G, Koumoutsos I, Ray S, Mawdsley J, Anderson SHC, Sanderson J, Irving PM. Effectiveness of vedolizumab dose intensification to achieve inflammatory bowel disease control in cases of suboptimal response. Frontline Gastroenterol. 2020;11:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Dalal RS, Kallumkal G, Cabral HJ, Bachour S, Barnes EL, Allegretti JR. Clinical and Endoscopic Outcomes After Upadacitinib Induction for Ulcerative Colitis: A Multicenter Retrospective Cohort Study. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |