Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1649
Peer-review started: October 29, 2023
First decision: December 15, 2023
Revised: December 26, 2023
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: March 26, 2024
Processing time: 147 Days and 16.4 Hours
Postoperative pancreatic fistula (POPF) contributes significantly to morbidity and mortality after pancreaticoduodenectomy (PD). However, the underlying mechanisms remain unclear. This study explored this pathology in the pancreatic stumps and elucidated the mechanisms of POPF following PD.
Pathological analysis and 16S rRNA gene sequencing were performed on spe
The mechanisms underlying POPF include high biochemical activity in the pan
Core Tip: Postoperative pancreatic fistula (POPF) is a major complication of pancreaticoduodenectomy. However, the underlying mechanisms remain unclear. Compared to the relatively simple histological structure of the gastrointestinal wall, pancreatic stump is undoubtedly the crucial factor in the occurrence of POPF. This study systemically investigated the pathology in pancreatic stumps and provided insights into the underlying mechanisms of POPF. Gradient inflammation and digestive reflux are present in the pancreatic stumps. As the understanding of the role of inflammation in POPF increases, effectively managing the side effects of inflammation will bring about a significant possibility of terminating POPF.
- Citation: Wang TG, Tian L, Zhang XL, Zhang L, Zhao XL, Kong DS. Gradient inflammation in the pancreatic stump after pancreaticoduodenectomy: Two case reports and review of literature. World J Clin Cases 2024; 12(9): 1649-1659
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1649.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1649
Postoperative pancreatic fistula (POPF) is a mechanical issue caused by the leakage of fluid from the pancreatic remnant into the abdominal cavity when pancreatic anastomosis fails[1]. It is a significant contributor to postoperative morbidity and mortality after pancreaticoduodenectomy (PD) and results in high social and financial costs. Currently, no effective strategies have been developed to prevent POPF[2,3].
Long-term clinical observations have suggested that postoperative pancreatitis (POP) correlates with the occurrence of POPF after PD[4,5]. Analysis of drained fluids and animal studies provide indirect evidence linking trypsinogen acti
The diagnosis and classification of pancreatic fistula in this study followed the guidelines of the International Study Group on Pancreatic Fistula[9]. A grade A POPF is characterized as a leak with no significant clinical effects, while grades B and C POPFs are considered clinically relevant due to their impact on the patients’ health.
Case 1: A 60-year-old female who reported yellow urine for 20 d.
Case 2: A 66-year-old male who found jaundice for three months.
Case 1: 20 d ago, the patient noticed yellow urine and sought treatment at a local hospital for gastric disease, but there was no improvement. The patient began experiencing poor appetite, accompanied by an aversion to greasy food, upper abdominal bloating, occasional clay-colored stools, and itching skin. There were no symptoms of fever, acid reflux, nau
Case 2: The patient first presented with generalized jaundice without any obvious cause three months ago, accompanied by yellowing of the eyes and urine. There was no nausea, vomiting, abdominal pain, or bloating. The patient did not experience fever, chills, diarrhea, urgency after defecation, or loss of appetite.
Case 1: The patient underwent an appendectomy 43 years ago; has a three-year history of diabetes and hyperlipidemia.
Case 2: Two months ago, the patient experienced a lacunar stroke.
Neither patient had a personal or family history of similar diseases.
Those two patients had similar performance. The patients are lucid and in good spirits. The skin and sclera of the entire body are jaundiced. The abdomen is flat, with no visible peristalsis or wave-like movements. There is no tenderness, rebound tenderness, or muscular tension throughout the abdomen. The liver and spleen are not palpable, and Murphy’s sign is negative. Abdominal percussion produces a tympanic sound, and shifting dullness is negative. There is no per
The laboratory test results for these two patients both indicate obstructive jaundice, accompanied by elevated levels of carbohydrate antigen 199.
Pathology and apoptosis: Two experienced pathologists independently evaluated the pathology without prior know
The inflammatory index was calculated using a histological scoring system for acute pancreatitis, including edema, inflammatory cell infiltration, and necrosis[10]. Furthermore, the occurrence of apoptosis was assessed using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay with a Cell Death Detection Kit from Roche. The cell nuclei were stained with TUNEL to visualize apoptosis. Pancreatic head tissue was used as a control to define the normal level of inflammation and apoptosis.
Microbiome and 16S rRNA bacterial gene sequencing: DNA was extracted from each sample, preserved in formalin, and embedded in paraffin (Norgen BioTek, Thorold, ON, Canada). Thirty nanograms of DNA were obtained from each sample and subjected to polymerase chain reaction (PCR) amplification. The hypervariable V3-V4 regions were targeted using primers 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) for ampli
Purified PCR products were sequenced using a MiSeq platform (Illumina, United States), and image analysis, base calling, and error estimation were performed using the Illumina Analysis Pipeline (Version 2.6). Sequences shorter than 230 bp with low-quality scores, containing ambiguous bases, or those that did not match the primer sequences and bar
Statistical analysis: Statistical analysis was conducted to determine the significance of the results. One sample t-test was employed in the microbial analysis. A P-value of less than 0.05 was considered statistically significant, and all calculations were carried out using IBM SPSS statistics version 26.0.
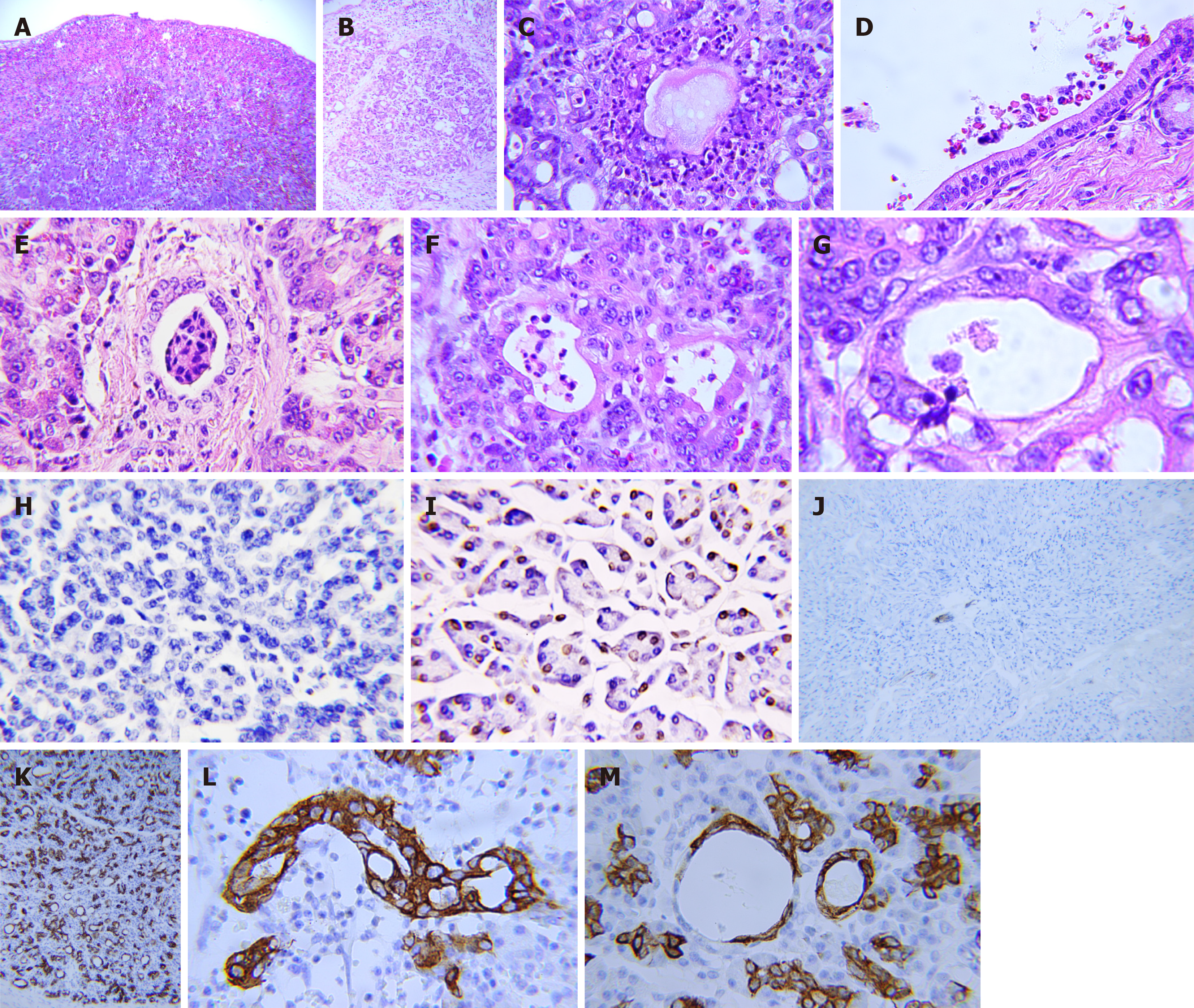
Pathology in the pancreatic stumps: The pancreatic stumps exhibit widespread inflammation and other pathological alterations. Subcapsular hematoma is common and extends deep into the parenchyma surrounding the suture, whereas it is relatively sparse in the tail. Neutrophils were the dominant inflammatory cells that infiltrated the glandular and inter
Apoptotic activity in the pancreatic head decreased or disappeared after POPF, resulting in cell proliferation throu
Inflammatory and red blood cells (RBC) were also concentrated in the ductal system, including the main pancreatic duct, interlobular duct, and abnormal ducts formed by acinar duct metaplasia (ADM) (Figure 1D and E). Notably, the concentration of neutrophils in the ductal system was significantly higher than that in the local vasculature. The ducts formed by the ADM are the weakest parts of the entire ductal network, where the blood-duct barrier is destroyed. The increased permeability of the ductal system allows the migration of blood cells, particularly neutrophils, from the blood vessels into the space between the acinar cells and basal membrane and then into the lumen of the ductal system (Figure 1G). ADM could be detected throughout the entire pancreatic stump, exhibiting no discernible pattern in its distribution. Moreover, the ductal system was unfavorable for the survival of blood cells as they decomposed (Figure 1E and F). RBCs lose their membranes, and neutrophils are decomposed, leaving fragmented nuclei.
The expression of CK19 becomes strongly positive in the pancreatic stump compared with that in the normal pa
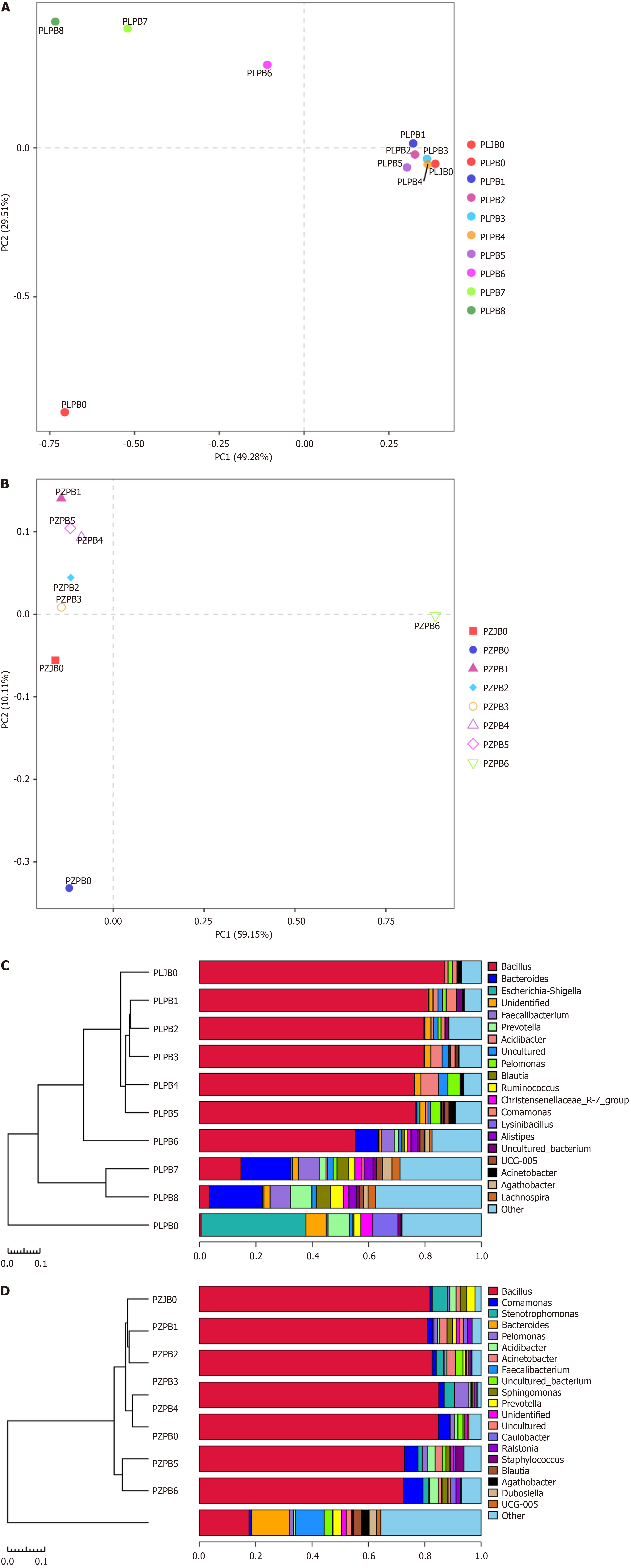
Microbial distribution and digestive reflux in pancreatic stumps: We conducted microbial analyses, including bacterial and fungal analyses, on both patients. Unfortunately, the vast majority of the fungal sequences could not be identified, so we focused solely on the bacteria. The length of the sequence was 400-440 bp. For further analysis, we selected a subset of 26301 tags from each sample in the patient 1 and 31277 tags in the patient 2. The alpha diversity indices are presented in Table 1. Our analysis revealed a statistically significant increase in OTUs in the stump compared to those in the duode
| Sample ID | Clean_Tags | Final_Tags | Chao’s index | Shannon-Wiener’s index | Simpson’s index | OTUs |
| PLDB0 | 158140 | 26301 | 52.20 | 1.01 | 0.24 | 34 |
| PLPB0 | 33717 | 26301 | 332.00 | 5.08 | 0.85 | 318 |
| PLPB1 | 54270 | 26301 | 522.35 | 1.68 | 0.34 | 324 |
| PLPB2 | 59038 | 26301 | 422.91 | 1.81 | 0.36 | 249 |
| PLPB3 | 27489 | 26301 | 339.00 | 1.72 | 0.36 | 250 |
| PLPB4 | 33657 | 26301 | 207.33 | 1.62 | 0.41 | 109 |
| PLPB5 | 29017 | 26301 | 405.02 | 2.05 | 0.41 | 310 |
| PLPB6 | 44928 | 26301 | 487.23 | 4.08 | 0.69 | 439 |
| PLPB7 | 115402 | 26301 | 444.89 | 6.06 | 0.96 | 338 |
| PLPB8 | 168118 | 26301 | 537.00 | 6.48 | 0.97 | 418 |
| PZDB0 | 165233 | 31277 | 140.00 | 1.25 | 0.32 | 35 |
| PZPB0 | 183971 | 31277 | 100.50 | 2.00 | 0.47 | 89 |
| PZPB1 | 47561 | 31277 | 68.00 | 1.48 | 0.34 | 44 |
| PZPB2 | 38512 | 31277 | 241.87 | 1.45 | 0.31 | 189 |
| PZPB3 | 48707 | 31277 | 86.10 | 1.04 | 0.27 | 49 |
| PZPB4 | 32320 | 31277 | 241.97 | 1.27 | 0.28 | 184 |
| PZPB5 | 34351 | 31277 | 275.18 | 2.04 | 0.47 | 183 |
| PZPB6 | 65726 | 31277 | 408.28 | 6.00 | 0.95 | 381 |
At the genus level, the most common bacteria found in the patient 1 samples are Bacillus, Bacteroides, Escherichia-Shige
Furthermore, the dominant bacterial species exhibited a declining distribution with increasing distance from the transection plane. The dissimilarity and variability of the samples are shown in Figure 2A and B. The distances between the samples demonstrated a much more clustered distribution in the patient 1 than in the patient 2 through PCA. However, the proportion of bacterial DNA and microbiome in the samples varied in the jejunum, pancreatic head, and pancreatic stump. The UPGMA clustering analysis indicated that the microbial community distribution adjacent to the transection plane in these two patients was similar to that in the jejunum (Figure 2C and D). The preoperative distribution of bacterial DNA in the pancreatic head was comparable to that in the pancreatic tail, where the inflammatory response was relatively slow.
Case 1: Computed tomography (CT) examination showed that there was a mass in the middle and lower sections of the common bile duct, with dilation of the intrahepatic and extrahepatic bile ducts.
Case 2: Magnetic resonance imaging suggested a duodenal mass, and a biopsy confirmed it as duodenal adenocarcinoma.
Biliary carcinoma.
Duodenal carcinoma.
Those two patients accepted conventional PDs. The pancreas was transected at the neck in front of the portal vein, and a modified Blumgart anastomosis was performed in all cases. A U-suture was placed 1 cm from the transection plane. Then, 3-0 polypropylene and 5-0 polydioxanone (both from Ethicon) were used in the outer and inner layers. Catheters with a range of 5-8 Fr were placed in the stump and fixed using anchoring sutures.
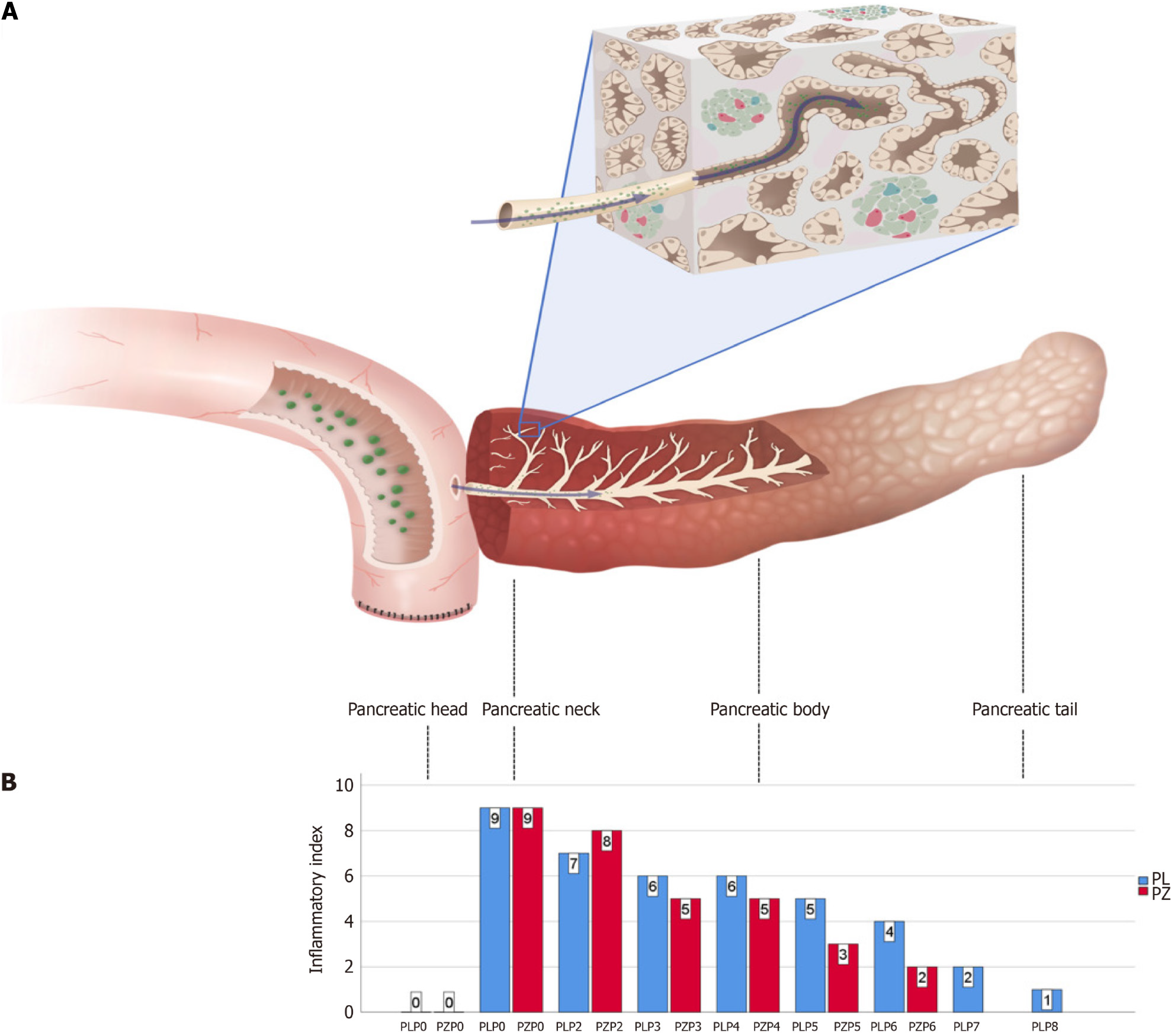
POPF happened in both cases within 6 d postoperatively. And those two patients accepted total pancreatectomy after life-threatening and repeat abdominal bleeding. We collected two pancreatic stump samples measuring 12 cm × 6 cm × 3 cm and 12 cm × 4 cm × 2 cm. Eight and six slices of the specimens were sequentially obtained with an interval of 1.5-2.0 cm from the transection plane to the pancreatic tail. In addition, patient-matched specimens from the jejunum and pan
This patient passed away in the seventh month after surgery due to severe malnutrition and multiple organ disfunction syndrome.
This patient passed away on the day 11 after the first surgery due to multiple infections and multiple organ disfunction syndrome.
POPF is a major and unresolved complication of PD. Recent studies have indicated that POPF is associated with POP; however, the exact mechanism remains unknown. One reasonable hypothesis suggests that the local activation of pancreatic enzymes triggers POPF, leading to increased damage to acinar cells, ischemia, manipulation of the gland, and blockage of the pancreatic duct[15]. However, no solid evidence for this has been reported to date.
No consensus exists currently regarding the definition of POP. Although serum amylase, lipase, and urinary tryp
Adults’ acinar cells are highly plastic and can undergo trans-differentiation into a progenitor-like cell type with ductal characteristics, which has been linked to multiple mechanisms, including ductal ectasia[19], the activation of nuclear fac
Inflammation may drive ADM in pancreatitis cases[20]. In our study, ADM-induced ducts exhibited distinct morphological features that differentiated them from normal ducts, including increased lumen size and elongated ductal cells. Moreover, no previous studies have reported increased ductal permeability in patients with pancreatitis. However, a case study has documented the presence of neutrophil-predominant inflammatory cell infiltration, ADM, and fibrosis in a patient with pancreatitis induced by pembrolizumab[24]. Our study showed that the blood-duct barrier was destroyed, and the ducts formed by ADM served as sites for the transmigration of neutrophils and RBCs through the duct wall.
The significant accumulation of neutrophil-dominant inflammatory cells within the ductal system and the gradient distribution of bacteria from the intestine suggest that inflammatory agents originate from the intestine, traverse the site of anastomosis, and accumulate in the ductal system. Further evidence of this reflux is the decomposition of blood cells in the ductal system, as the activated enzymes in the system are not conducive to the survival of blood cells. Intra-acinar trypsin activation within the pancreas is enough to cause acute pancreatitis[25]. The reflux of digestive fluid could exa
Theoretically, intestinal fluid can flow through the anastomosis site or stent into the stump and has the potential to activate trypsin during Blumgart anastomosis. However, the small size of the jejunal loop incision (2-3 mm) minimizes enteric fluid reflux. In contrast, invagination with pancreaticojejunostomy is associated with increased reflux[26]. This difference in reflux may explain why Blumgart anastomosis minimizes severe complications after PD[27]. Additionally, gastric juice performs less biologically than intestinal fluid, making pancreaticogastrostomy a safer alternative to pancreaticojejunostomy[28].
The main distinction between pancreaticojejunostomy and other digestive anastomoses is the high level of biological activity of the pancreas, which is characterized by its enzymatic secretory capacity[29]. Some researchers have suspected that suture placement is induced by the placement of sutures[6]. Our study found that suture placement can lead to pa
Based on these findings, the underlying mechanisms of POPF include high-level biochemical activity in the pancreas, mechanical injury, and digestive reflux. These three mechanisms contribute to the increased incidence and prolonged healing of POPF. The inflammatory response in the stump reached its peak on day 4 after PD and did not heal even 30 d after surgery.
Additionally, various risk factors, including soft tissue texture, small pancreatic duct[30], ischemia, ductal obstruction, excessive blood loss, high intraoperative fluid intake[31], elevated bilirubin level, large body mass index[32], low fibrosis[33], high acinar cell density, and acinar marginal content[34], increase the complexity of surgical maneuvers, resulting in anastomotic failure due to the heightened local tension created by inflammation and reflux.
Regardless of the surgical techniques employed, including stents[35], various methods of pancreaticojejunostomy and pancreaticogastrostomy[26], fibrin sealants, autologous tissue patches, bioabsorbable mesh[36], externally draining of pancreatic fluid[37], and the application of somatostatin[38], mechanical damage to the pancreas and digestive reflux cannot be entirely avoided. Therefore, surgeons have limited flexibility when treating each patient.
Due to the unavoidable suture and POPF, a paradox arises. Preventing pancreatitis in the stump or achieving a flaw
POPF is a complex condition that is caused by increased biochemical activity, mechanical damage, and digestive reflux. Currently, manipulation of the pancreatic stump and reflux into the pancreatic duct cannot be avoided. Based on these findings, stopping reflux and reducing inflammation in the pancreatic stump can decrease the occurrence of pancreatic fistulas. However, a more practical approach is to allow for the presence of inflammation and anastomotic dehiscence while controlling the proper flow of pancreatic juice, thereby breaking the logical relationship between anastomotic dehiscence and POPF.
The corresponding author expresses gratitude to William R. Jarnagin (Memorial Sloan-Kettering Cancer Center) and Kevin C Soares (Memorial Sloan-Kettering Cancer Center) for their valuable assistance at the onset of this project amid the challenges posed by the COVID-19 pandemic in New York in 2020. The author also extends appreciation to Joshua Jolissaint (Memorial Sloan-Kettering Cancer Center) for his careful review of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagaya M, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 2. | Shrikhande SV, Sivasanker M, Vollmer CM, Friess H, Besselink MG, Fingerhut A, Yeo CJ, Fernandez-delCastillo C, Dervenis C, Halloran C, Gouma DJ, Radenkovic D, Asbun HJ, Neoptolemos JP, Izbicki JR, Lillemoe KD, Conlon KC, Fernandez-Cruz L, Montorsi M, Bockhorn M, Adham M, Charnley R, Carter R, Hackert T, Hartwig W, Miao Y, Sarr M, Bassi C, Büchler MW; International Study Group of Pancreatic Surgery (ISGPS). Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2017;161:1221-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 3. | Tzeng CW, Katz MH, Fleming JB, Lee JE, Pisters PW, Holmes HM, Varadhachary GR, Wolff RA, Abbruzzese JL, Vauthey JN, Aloia TA. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg. 2014;18:146-55; discussion 155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Globke B, Timmermann L, Klein F, Fehrenbach U, Pratschke J, Bahra M, Malinka T. Postoperative acute necrotizing pancreatitis of the pancreatic remnant (POANP): a new definition of severe pancreatitis following pancreaticoduodenectomy. HPB (Oxford). 2020;22:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Bannone E, Andrianello S, Marchegiani G, Masini G, Malleo G, Bassi C, Salvia R. Postoperative Acute Pancreatitis Following Pancreaticoduodenectomy: A Determinant of Fistula Potentially Driven by the Intraoperative Fluid Management. Ann Surg. 2018;268:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Wüster C, Shi H, Kühlbrey CM, Biesel EA, Hopt UT, Fichtner-Feigl S, Wittel UA. Pancreatic Inflammation and Proenzyme Activation Are Associated With Clinically Relevant Postoperative Pancreatic Fistulas After Pancreas Resection. Ann Surg. 2020;272:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Shrikhande SV. Invited commentary: Evolving landscape of postoperative hyperamylasemia, postoperative acute pancreatitis, and postoperative pancreatic fistula: Time for a unifying definition. Surgery. 2021;169:740-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Ikenaga N, Ohtsuka T, Nakata K, Watanabe Y, Mori Y, Nakamura M. Clinical significance of postoperative acute pancreatitis after pancreatoduodenectomy and distal pancreatectomy. Surgery. 2021;169:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2959] [Article Influence: 369.9] [Reference Citation Analysis (35)] |
| 10. | Moreno C, Nicaise C, Gustot T, Quertinmont E, Nagy N, Parmentier M, Louis H, Devière J. Chemokine receptor CCR5 deficiency exacerbates cerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1089-G1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Jackson MA, Bell JT, Spector TD, Steves CJ. A heritability-based comparison of methods used to cluster 16S rRNA gene sequences into operational taxonomic units. PeerJ. 2016;4:e2341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Sheng HF, He Y, Wu JY, Jiang YX, Tam NF, Zhou HW. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl Environ Microbiol. 2012;78:8264-8271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 13. | Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7107] [Cited by in RCA: 7391] [Article Influence: 389.0] [Reference Citation Analysis (0)] |
| 14. | Jiang XT, Peng X, Deng GH, Sheng HF, Wang Y, Zhou HW, Tam NF. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb Ecol. 2013;66:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Nahm CB, Connor SJ, Samra JS, Mittal A. Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin Exp Gastroenterol. 2018;11:105-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 16. | Palani Velu LK, Chandrabalan VV, Jabbar S, McMillan DC, McKay CJ, Carter CR, Jamieson NB, Dickson EJ. Serum amylase on the night of surgery predicts clinically significant pancreatic fistula after pancreaticoduodenectomy. HPB (Oxford). 2014;16:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Dalla Valle R, De Bellis M, Pedrazzi G, Lamecchi L, Bianchi G, Pellegrino C, Iaria M. Can early serum lipase measurement be routinely implemented to rule out clinically significant pancreatic fistula after pancreaticoduodenectomy? Int J Surg. 2015;21 Suppl 1:S50-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Connor S. Defining post-operative pancreatitis as a new pancreatic specific complication following pancreatic resection. HPB (Oxford). 2016;18:642-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Ferreira MJ, McKenna LB, Zhang J, Reichert M, Bakir B, Buza EL, Furth EE, Bogue CW, Rustgi AK, Kaestner KH. Spontaneous Pancreatitis Caused by Tissue-Specific Gene Ablation of Hhex in Mice. Cell Mol Gastroenterol Hepatol. 2015;1:550-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Liou GY, Döppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs. J Cell Biol. 2013;202:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 21. | Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci U S A. 2007;104:19327-19332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Means AL, Ray KC, Singh AB, Washington MK, Whitehead RH, Harris RC Jr, Wright CV, Coffey RJ Jr, Leach SD. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology. 2003;124:1020-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | da Silva L, Jiang J, Perkins C, Atanasova KR, Bray JK, Bulut G, Azevedo-Pouly A, Campbell-Thompson M, Yang X, Hakimjavadi H, Chamala S, Ratnayake R, Gharaibeh RZ, Li C, Luesch H, Schmittgen TD. Pharmacological inhibition and reversal of pancreatic acinar ductal metaplasia. Cell Death Discov. 2022;8:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Hirota M, Murakami K, Koiwai A, Kawamura K, Yoshino Y, Takasu A, Kin R, Katayama T, Endo K, Kogure T, Meguro T, Tabata T, Satoh K. Neutrophil Infiltration and Acinar-ductal Metaplasia Are the Main Pathological Findings in Pembrolizumab-induced Pancreatitis. Intern Med. 2022;61:3675-3682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Gaiser S, Daniluk J, Liu Y, Tsou L, Chu J, Lee W, Longnecker DS, Logsdon CD, Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Bai X, Zhang Q, Gao S, Lou J, Li G, Zhang Y, Ma T, Xu Y, Liang T. Duct-to-Mucosa vs Invagination for Pancreaticojejunostomy after Pancreaticoduodenectomy: A Prospective, Randomized Controlled Trial from a Single Surgeon. J Am Coll Surg. 2016;222:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Casadei R, Ricci C, Ingaldi C, Alberici L, De Raffele E, Minni F. Comparison of Blumgart Anastomosis with Duct-to-Mucosa Anastomosis and Invagination Pancreaticojejunostomy After Pancreaticoduodenectomy: A Single-Center Propensity Score Matching Analysis. J Gastrointest Surg. 2021;25:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Figueras J, Sabater L, Planellas P, Muñoz-Forner E, Lopez-Ben S, Falgueras L, Sala-Palau C, Albiol M, Ortega-Serrano J, Castro-Gutierrez E. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013;100:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Vollmer CM, Trudeau M, Casciani F. Objectifying Pancreatic Fistula Risk: On the Right Track, but More yet to Do. J Am Coll Surg. 2021;232:945-947. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Chen JS, Liu G, Li TR, Chen JY, Xu QM, Guo YZ, Li M, Yang L. Pancreatic fistula after pancreaticoduodenectomy: Risk factors and preventive strategies. J Cancer Res Ther. 2019;15:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Han IW, Kim H, Heo J, Oh MG, Choi YS, Lee SE, Lim CS. Excess intraoperative fluid volume administration is associated with pancreatic fistula after pancreaticoduodenectomy: A retrospective multicenter study. Medicine (Baltimore). 2017;96:e6893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Nong K, Zhang Y, Liu S, Yang Y, Sun D, Chen X. Analysis of pancreatic fistula risk in patients with laparoscopic pancreatoduodenectomy: what matters. J Int Med Res. 2020;48:300060520943422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Deng Y, Zhao B, Yang M, Li C, Zhang L. Association Between the Incidence of Pancreatic Fistula After Pancreaticoduodenectomy and the Degree of Pancreatic Fibrosis. J Gastrointest Surg. 2018;22:438-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Umezaki N, Hashimoto D, Nakagawa S, Kitano Y, Yamamura K, Chikamoto A, Matsumura F, Baba H. Number of acinar cells at the pancreatic stump predicts pancreatic fistula after pancreaticoduodenectomy. Surg Today. 2018;48:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Guo C, Xie B, Guo D. Does pancreatic duct stent placement lead to decreased postoperative pancreatic fistula rates after pancreaticoduodenectomy? A meta-analysis. Int J Surg. 2022;103:106707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM, Zaheer A, Wolfgang C, Hruban R, Marchegiani G, Fernández Del Castillo C, Brugge W, Ha Y, Kim MH, Oh D, Hirai I, Kimura W, Jang JY, Kim SW, Jung W, Kang H, Song SY, Kang CM, Lee WJ, Crippa S, Falconi M, Gomatos I, Neoptolemos J, Milanetto AC, Sperti C, Ricci C, Casadei R, Bissolati M, Balzano G, Frigerio I, Girelli R, Delhaye M, Bernier B, Wang H, Jang KT, Song DH, Huggett MT, Oppong KW, Pererva L, Kopchak KV, Del Chiaro M, Segersvard R, Lee LS, Conwell D, Osvaldt A, Campos V, Aguero Garcete G, Napoleon B, Matsumoto I, Shinzeki M, Bolado F, Fernandez JM, Keane MG, Pereira SP, Acuna IA, Vaquero EC, Angiolini MR, Zerbi A, Tang J, Leong RW, Faccinetto A, Morana G, Petrone MC, Arcidiacono PG, Moon JH, Choi HJ, Gill RS, Pavey D, Ouaïssi M, Sastre B, Spandre M, De Angelis CG, Rios-Vives MA, Concepcion-Martin M, Ikeura T, Okazaki K, Frulloni L, Messina O, Lévy P. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 37. | Patel K, Teta A, Sukharamwala P, Thoens J, Szuchmacher M, DeVito P. External pancreatic duct stent reduces pancreatic fistula: a meta-analysis and systematic review. Int J Surg. 2014;12:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Yoon SJ, Lee O, Jung JH, Shin SH, Heo JS, Han IW. Prophylactic octreotide for postoperative pancreatic fistula in patients with pancreatoduodenectomy: Risk-stratified analysis. Medicine (Baltimore). 2022;101:e29303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |