Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1644
Peer-review started: October 20, 2023
First decision: January 5, 2024
Revised: January 20, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: March 26, 2024
Processing time: 156 Days and 23.7 Hours
Pulmonary alveolar proteinosis (PAP) and X-linked agammaglobulinemia (XLA) are rare diseases in children. Many theories infer that immunodeficiency can induce PAP, but these reports are almost all review articles, and there is little clinical evidence. We report the case of a child with both PAP and XLA.
A 4-month-old boy sought medical treatment due to coughing and difficulty in breathing for > 2 wk. He had been hospitalized multiple times due to respiratory infections and diarrhea. Chest computed tomography and alveolar lavage fluid showed typical PAP-related manifestations. Genetic testing confirmed that the boy also had XLA. Following total lung alveolar lavage and intravenous immunoglobulin replacement therapy, the boy recovered and was discharged. During the follow-up period, the number of respiratory infections was significantly reduced, and PAP did not recur.
XLA can induce PAP and improving immune function contributes to the prognosis of children with this type of PAP.
Core Tip: Pulmonary alveolar proteinosis (PAP) and X-linked agammaglobulinemia (XLA) are both rare diseases in children. This article shares the diagnosis and treatment process of a special case to confirm that XLA was a secondary cause of PAP which improved with intravenous immunoglobulin treatment.
- Citation: Zhang T, Li M, Tan L, Li X. Pulmonary alveolar proteinosis induced by X-linked agammaglobulinemia: A case report. World J Clin Cases 2024; 12(9): 1644-1648
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1644.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1644
Pulmonary alveolar proteinosis (PAP) and X-linked agammaglobulinemia (XLA) are rare diseases in children. Many studies have suggested that immunodeficiency can induce PAP. However, only two cases of PAP induced by immune deficiency have been reported[1-3], and the remaining studies are almost all review articles. Therefore, more clinical data are needed to prove the correlation between PAP and immunodeficiency.
XLA is an X-linked recessive genetic disease. This is due to a defect in Bruton’s tyrosine kinase (BTK). The differentiation of primitive B lymphocytes to mature B lymphocytes is impaired. There is a lack of B lymphocytes and plasma cells in the peripheral blood, and this leads to insufficient immunoglobulin synthesis. Therefore, children with XLA are prone to repeated bacterial infections and autoimmune diseases[4,5].
According to the etiology, PAP can be divided into congenital, acquired and idiopathic PAP[6]. Congenital PAP is an autosomal recessive disorder. It is caused by a defect in the surface-active substance protein or the gene encoding granulocyte–macrophage colony-stimulating factor (GM-CSF)[7,8]. Idiopathic PAP is caused by a large amount of GM-CSF self-neutralizing antibodies in the body, which block the function of GM-CSF. Therefore, the function of alveolar macrophages is severely affected, resulting in decreased alveolar surfactant clearance[9]. The pathogenesis of acquired PAP is unclear. It is currently believed to be related to various autoimmune, infectious, malignant and environmental etiologies[10].
The 4-month-old male patient had an acute cough, expectoration and difficulty breathing for > 10 d.
The child developed paroxysmal cough and phlegm 2 wk previously, along with difficulty breathing, runny nose, diarrhea, and cyanosis around the mouth. The symptoms did not improve after receiving penicillin infusion at the local hospital.
The child is gravida 2 para 2 and was a full-term birth. The birth process was smooth, with no asphyxia or respiratory distress. His birth weight was ~3 kg. He has suffered pneumonia and diarrhea many times since birth. However, no abnormalities were found on chest imaging except for pulmonary infection.
There was no other relevant personal or family history.
The patient’s body temperature was 36.5C, heart rate 136 bpm, respiratory rate 62 breaths/min, fingertip oxygen saturation (SpO2) 86%, and body weight 7.2 kg. Upon examination, his lips were cyanotic, no swollen superficial lymph nodes were noted throughout the body, respiratory sounds in both lungs were rough, and fine moist rales were heard in both lungs. Physical examination of the heart, abdomen and nervous system did not reveal any abnormalities.
Blood tests revealed that his erythrocyte sedimentation rate, liver and kidney function, and C-reactive protein level were normal. The myocardial enzyme spectrum was normal except for lactate dehydrogenase (704 U/L). Humoral immunity showed the following: Ig 14.0 g/L, IgG 0.93 g/L, IgM 0.29 g/L, IgA 0.0 g/L, complement C3 0.40 g/L, complement C4 0.11 g/L, and total IgE 1 U/mL. Lymphocyte subpopulation determination was as follows: CD3+ 95.49%, CD3+CD4+ 65.58%, CD16+CD56+ 3.58% and CD19+ 0.03%. No pathogens were found in blood and sputum tests.
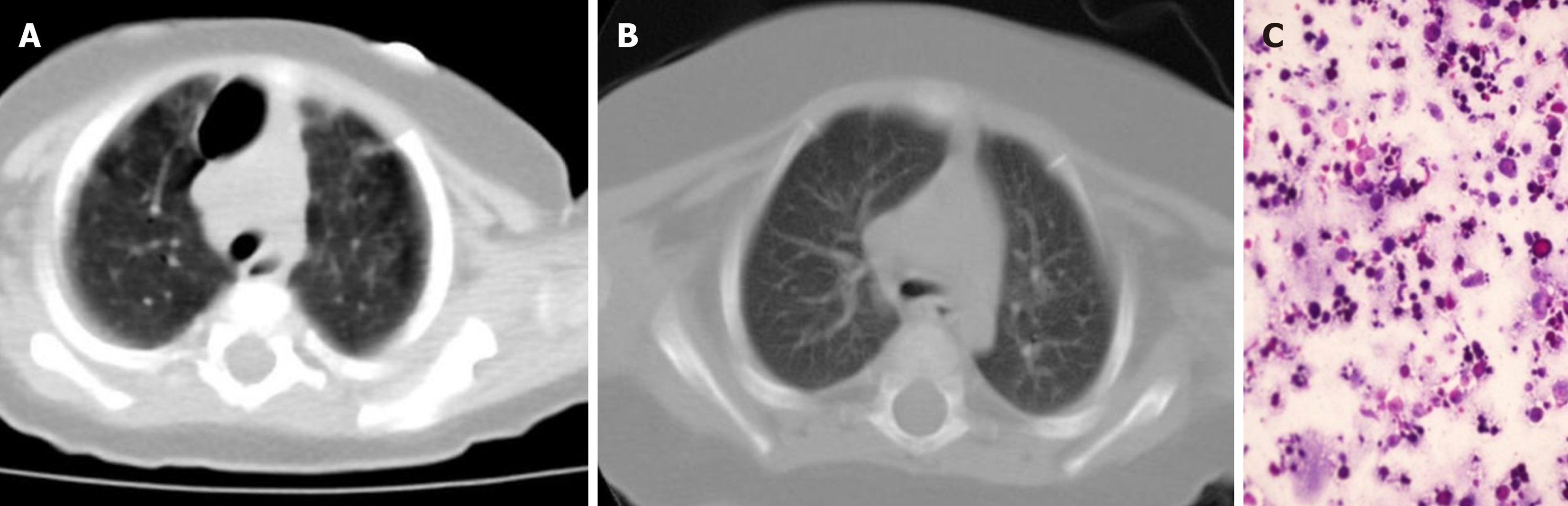
His bronchoalveolar lavage fluid was milky white and Periodic acid–Schiff staining was positive (Figure 1). Genetic testing [whole exome sequencing revealed that the BTK gene (located at chrX:100608340) had a hemizygous mutation (c.1751-1g>A)]. Thus, he was diagnosed with PAP and XLA. Genetic testing did not find gene mutations related to PAP[2] [such as surfactant protein B, surfactant protein C, ATP-binding cassette subfamily A member 3 (ABCA3), Nkx homeobox-1 gene (NKX2-1), and granulocyte-monocyte colony stimulating factor receptor genes]. No respiratory pathogens were detected in blood, sputum and alveolar lavage fluid.
Chest spiral computed tomography plain scan showed a significant decrease in the transparency of both lungs, with ground glass or butterfly-shaped changes in both lungs, and a large bubble in the upper lobe of the right lung (Figure 1). Tracheoscopy revealed that his alveolar lavage fluid was pale milky white.
PAP and XLA.
The patient received ceftriaxone, total lung lavage, and infusion of intravenous immunoglobulin (IVIG).
After discharge, the patient received regular IVIG replacement therapy. During 1 year of follow-up, his respiratory tract infection frequency significantly decreased, and all were mild infections. He did not exhibit symptoms of PAP such as excessive phlegm or difficulty breathing, and there were no special features on chest imaging. Therefore, the child has not yet undergone a second bronchoscopy examination.
Current studies suggest that PAP is related to a decrease in macrophage clearance. The Toll-like receptor (TLR) pathway is an important signal pathway regulating the function of macrophages. BTK is an important regulatory molecule in the TLR pathway, which is involved in the regulation of cytokine production, phagocytosis, differentiation and the function of macrophages after TLR activation[11,12]. Therefore, XLA may directly induce PAP. At the same time, XLA children can be prone to repeated pulmonary infections, leading to PAP. Based on the above theory, we believe that XLA is one of the potential causes of PAP. Therefore, improving immune function may benefit the prognosis of children with PAP caused by immunodeficiency.
The child in this report had a history of multiple respiratory infections and diarrhea. Based on his genetic examination, chest imaging, and alveolar lavage fluid examination, he met the diagnostic criteria for PAP and XLA. In addition, his laboratory test results did not find GM-CSF autoantibodies, and genetic testing did not identify gene mutations related to PAP[2] (such as surfactant protein B, surfactant protein C, ABCA3, NKX2-1, and GM-CSF receptor genes). No respiratory pathogens were detected in blood, sputum and alveolar lavage fluid. His mother, brother, father and neighbors did not have PAP-related symptoms. Moreover, his residence was free from industrial pollution. Based on a previous analysis of the etiology of PAP, this patient’s PAP was considered to be secondary to immunodeficiency.
At present, the most effective treatment for PAP is massive whole lung lavage[13]. Large volume whole lung lavage can directly remove the protein-like substances deposited in the alveoli, reduce macrophage inhibitory factors in the alveoli and distal bronchioles, improve the function of alveolar macrophages, and thus improve lung ventilation and function. However, for acquired PAP, we believe that the treatment of primary disease might be equally important. Tanaka-Kubota et al[3] studied children with PAP secondary to immunosuppression. In their study, the children only received lung lavage at the beginning, and the children’s PAP symptoms improved, but soon relapsed. After cell transplantation, long-term relief of the symptoms of PAP was achieved. All cases (our report and Tanaka–Kubota’s studies) suggest that XLA was a secondary cause of PAP which improved with IVIG treatment.
We describe a child with XLA and PAP. It is suggested that XLA may cause PAP, and immunotherapy was helpful in improving the prognosis of this child with PAP acquired due to immunodeficiency.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Polat SE, Turkey S-Editor: Luo ML L-Editor: Kerr C P-Editor: Cai YX
| 1. | Zhang FZ, Yuan JX, Qin L, Tang LF. Pulmonary Alveolar Proteinosis Due to Pneumocystis carinii in Type 1 Hyper-IgM Syndrome: A Case Report. Front Pediatr. 2020;8:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Gallagher J, Adams J, Hintermeyer M, Torgerson TR, Lopez-Guisa J, Ochs HD, Szabo S, Salib M, Verbsky J, Routes J. X-linked Hyper IgM Syndrome Presenting as Pulmonary Alveolar Proteinosis. J Clin Immunol. 2016;36:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Tanaka-Kubota M, Shinozaki K, Miyamoto S, Yanagimachi M, Okano T, Mitsuiki N, Ueki M, Yamada M, Imai K, Takagi M, Agematsu K, Kanegane H, Morio T. Hematopoietic stem cell transplantation for pulmonary alveolar proteinosis associated with primary immunodeficiency disease. Int J Hematol. 2018;107:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton's tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 5. | Ochs HD, Smith CI. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine (Baltimore). 1996;75:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Jouneau S, Ménard C, Lederlin M. Pulmonary alveolar proteinosis. Respirology. 2020;25:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Hildebrandt J, Yalcin E, Bresser HG, Cinel G, Gappa M, Haghighi A, Kiper N, Khalilzadeh S, Reiter K, Sayer J, Schwerk N, Sibbersen A, Van Daele S, Nübling G, Lohse P, Griese M. Characterization of CSF2RA mutation related juvenile pulmonary alveolar proteinosis. Orphanet J Rare Dis. 2014;9:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Al-Haidary AS, Alotaibi W, Alhaider SA, Al-Saleh S. A newly identified novel variant in the CSF2RA gene in a child with pulmonary alveolar proteinosis: a case report. J Med Case Rep. 2017;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J, Wang T, Morgan C, Cottin V, McCarthy C. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. 2019;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 10. | Patel SM, Sekiguchi H, Reynolds JP, Krowka MJ. Pulmonary alveolar proteinosis. Can Respir J. 2012;19:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Xu B, Luo Q, Gong Y, Li J, Cao J. TLR7 Expression Aggravates Invasive Pulmonary Aspergillosis by Suppressing Anti-Aspergillus Immunity of Macrophages. Infect Immun. 2021;89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bush A, Pabary R. Pulmonary alveolarproteinosis in children. Breathe (Sheff). 2020;16:200001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Smith BB, Torres NE, Hyder JA, Barbara DW, Gillespie SM, Wylam ME, Smith MM. Whole-lung Lavage and Pulmonary Alveolar Proteinosis: Review of Clinical and Patient-centered Outcomes. J Cardiothorac Vasc Anesth. 2019;33:2453-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |