Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1585
Peer-review started: November 29, 2023
First decision: December 12, 2023
Revised: January 11, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: March 26, 2024
Processing time: 116 Days and 8.3 Hours
Cerebral palsy (CP) describes a group of disorders affecting movement, balance, and posture. Disturbances in motor functions constitute the main body of CP symptoms. These symptoms surface in early childhood and patients are affected for the rest of their lives. Currently, treatment involves various pharmacothe
To analyze the efficacy and safety of MSCT in CP patients.
Our sample consists of four CP patients who cannot stand or walk without external support. All of these cases received allogeneic MSCT six times as 1 × 106/kg intrathecally, intravenously, and intramuscularly using umbilical cord-derived MSCs (UC-MSC). We monitored and assessed the patients pre- and post-treatment using the Wee Functional Independence Measure (WeeFIM), Gross Motor Function Classification System (GMFCS), and Manual Ability Classification Scale (MACS) instruments. We utilized the Modified Ashworth Scale (MAS) to measure spasticity.
We found significant improvements in MAS scores after the intervention on both sides. Two months: Right χ2 = 4000, P = 0.046, left χ2 = 4000, P = 0.046; four months: Right χ2 = 4000, P = 0.046, left χ2 = 4000, P = 0.046; 12 months: Right χ2 = 4000, P = 0.046, left χ2 = 4000, P = 0.046. However, there was no significant difference in motor functions based on WeeFIM results (P > 0.05). GMFCS and MACS scores differed significantly at 12 months after the in
In light of our findings, we believe that UC-MSC therapy has a positive effect on spasticity, and it partially improves motor functions.
Core Tip: Cerebral palsy (CP) describes a group of non-progressive disorders affecting movement, balance, posture, and motor function. Research suggests that stem cell therapy may be a new treatment option in CP. We monitored four CP patients who underwent mesenchymal stem cell therapy (MSCT) for 12 months and analyzed treatment efficacy. MSCT resulted in significantly improved Modified Ashworth Scale, Gross Motor Function Classification System, and Manual Ability Classification System scores.
- Citation: Boyalı O, Kabatas S, Civelek E, Ozdemir O, Bahar-Ozdemir Y, Kaplan N, Savrunlu EC, Karaöz E. Allogeneic mesenchymal stem cells may be a viable treatment modality in cerebral palsy. World J Clin Cases 2024; 12(9): 1585-1596
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1585.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1585
Cerebral palsy (CP) describes a group of disorders affecting movement, balance, posture, and motor functions. These symptoms surface in early childhood and patients are affected for the rest of their lives[1,2]. While motor dysfunctions constitute the main body of CP symptoms, patients often suffer from other pathologies such as epilepsy, musculoskeletal diseases, and cognitive, perceptive, communicative, sensory, and behavioral disorders[3]. Research reports the incidence rate of CP as 0.15%-0.25% in developed countries[4]. Given the complex etiology (perinatal stroke, gestational age, low birth weight, birth complications, etc.) and symptom variations, CP comprises a wide spectrum[5,6]. This has led re
Stem cell therapy (SCT) is a novel treatment option that has been researched in over 250 studies involving a wide variety of disorders. Accordingly, SCT boasts significant potential and versatility and is promising for ameliorating CP symptoms[10-12].
The therapeutic efficacy of mesenchymal SCT (MSCT) is evaluated based on its anti-inflammatory effects, neuroregeneration, and neural protection. According to early studies, the mechanism of action of MSCT involves stem cells mi
One study focused on the neuroprotective activity of MSCs and reported a lower count of apoptotic neurons after intravenous treatment in a stroke model created in female rats. The stem cells migrated to the injury site, increased the expression of basic fibroblast growth factor (bFGF), and promoted endogenous proliferation, providing functional re
Many researchers have reported the anti-inflammatory activity of MSCs through paracrine mechanisms. Huang et al[17] found that interleukin (IL)-6 and VEGF had an important role in the anti-inflammatory activity of MSCs in vitro. The anti-inflammatory activity of IL-6 occurs through its inhibitory effect on tumor necrosis factor α and IL-1[17]. The roles of IL-6 in this mechanism have been supported by other studies. MSC implantation increases IL-6 production through resident neuronal stem cells NFkB activation, independent of the PI3/Akt pathway, thus reducing apoptosis and pro
Given this variety of mechanisms of action, MSCT offers a new treatment perspective not only in CP, but also in many central nervous system disorders such as hypoxic ischemic encephalopathy, Alzheimer’s disease, stroke, Parkinson’s disease, multiple sclerosis, and spinal cord injury[20,21].
Lee et al[22] examined a murine model of Alzheimer’s disease and found that human umbilical cord-derived MSCs (UC-MSC) inhibited the release of pro-inflammatory cytokines from microglia and reduced apoptosis and amyloid pla
One study used the experimental autoimmune encephalitis (EAE) model, which is the most common model in animal research on multiple sclerosis; the authors found that treatment reduced inflammatory infiltrates and demyelination[12]. Other clinical trials on EAE have also shown some clinical improvements and have deemed EAE as a safe and effective model[24].
In 2012, Lalu et al[25] published a meta-analysis on the possible risks of MSCT and highlighted that no statistically significant side effects were involved other than transient fever[25]. Similarly, another meta-analysis published in 2021 revealed that, despite the expanding patient population in the intervening years, the only side effect that could be associated with MSCT was transient fever. Transient fever is more common in women and less common in the North American population. Despite the tumorigenic potential of MSCs, only one malignancy was detected in the entire po
In the present research, we monitored four CP patients who underwent MSCT for 12 months and analyzed treatment efficacy.
This was a multi-center, longitudinal, open-label phase I trial. Our sample included four CP patients with severe dis
| Frequency | % | ||
| Age, yr | 1 | 1 | 25.0 |
| 4 | 1 | 25.0 | |
| 9 | 2 | 50.0 | |
| Sex | Female | 1 | 25.0 |
| Male | 3 | 75.0 | |
| Etiology of cerebral palsy | Hypoxia during birth | 2 | 50.0 |
| Hypoxia with bleeding in the surgical field after tonsillectomy operation | 1 | 25.0 | |
| Cardiac arrest | 1 | 25.0 | |
| Comorbidity | None | 4 | 100.0 |
| Cerebral palsy duration and first transplantation | 1 year | 1 | 25.0 |
| 13 months | 1 | 25.0 | |
| 7 years | 1 | 25.0 | |
| 9 years | 1 | 25.0 | |
The study protocol was approved by the local Ethics Committee. We obtained the informed consent of each participant and/or their caregivers in written form. The trial was conducted in accordance with the principles of the Declaration of Helsinki.
Ethical information and donor consent: The present study was approved by the medical ethics committee of the authors’ institution (protocol No. 56733164-203-E.3178). Umbilical Cords (UCs) were obtained from the Good Manufacturing Practices facility of LivMedCell (Istanbul, Turkey). In accordance with the approval of an institutional regulatory board (LivMedCell); the donors donated UCs after being informed of the aim of the current study and gave their informed consent in written form.
Processing and quality control of UCs: The UCs were washed with phosphate-buffered saline (Invitrogen/Gibco, Paisley, United Kingdom). Before removing blood vessels, the tissue samples were cut into 5-10 mm3 pieces as explants. These explants were placed in dishes and cultured under humanized culture conditions at 37 °C with 5% CO2 until the cells displaced. The resulting cells were collected when they reached 70% to 80% confluence and subjected to characterization tests at Passage 3[27]. Quality control and assurance was performed by the Pharmaceuticals and Medical Devices Agency.
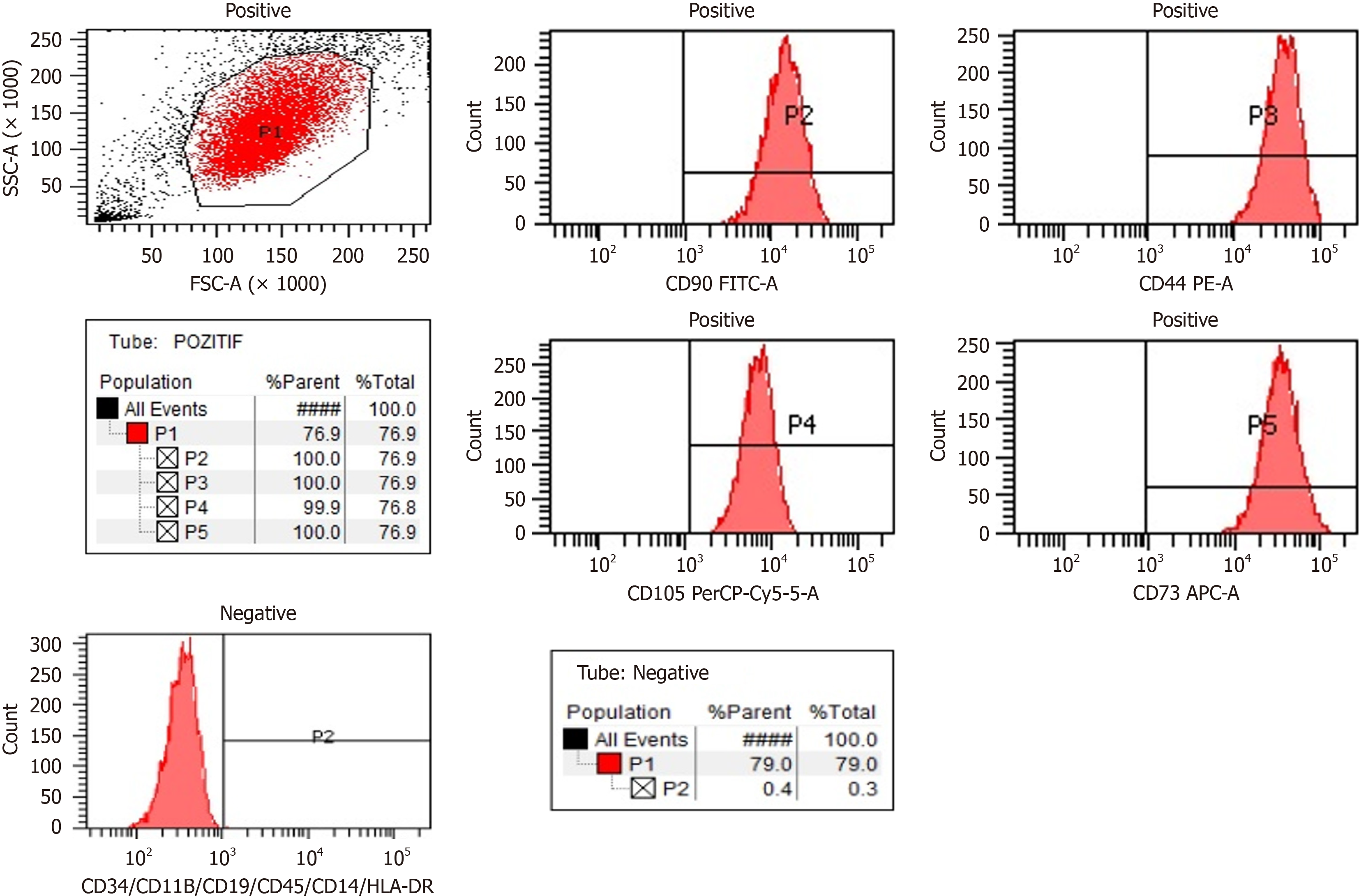
Determination of UC-MSCs by flow cytometry: Expressed surface antigens were analyzed by flow cytometry, which revealed that cells were consistently positive for CD44, CD73, CD90, and CD105 and negative for hematopoietic lineage markers of CD34, CD45, and human leukocyte antigen DR2 (Figure 1). Telomerase activity was stable in culture con
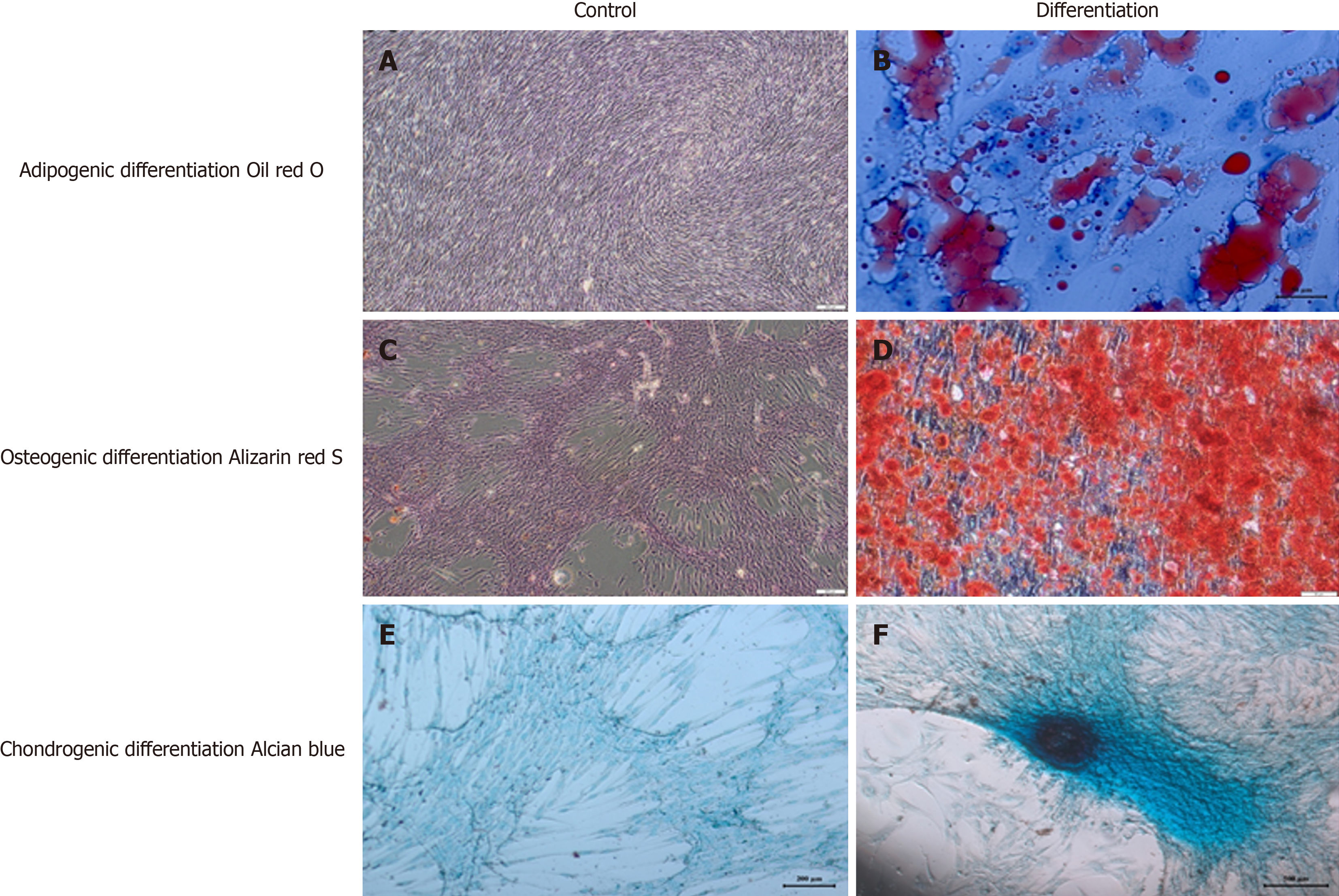
Cell differentiation and karyotyping: We identified some stem cell expressions and the differentiation markers of TERT, SOX2, POU5F1, CD44, ZFP42, VIM, ICAM1, THY1, VCAM1, BMP2, RUNX-1, and NES. Differentiation analyses confirmed that these cells had a trilineage (chondrocytes, osteoblasts, and adipocytes) differentiation capacity[27]. Karyotyping studies revealed no numerical or structural chromosomal abnormalities.
In vitro adipogenic differentiation and oil red O staining: To induce adipogenic differentiation, cells from Passage 3 (3000 cells/cm2) were seeded onto coated type I collagen coverslips (BD Biosciences) in 6-well plates. The adipogenic medium Dulbecco’s Modified Eagle’s Medium, Low Glucose (DMEM-LG, Invitrogen) was supplemented with 10% FBS (Invitrogen/Gibco), 0.5 mmol/L isobutyl-methylxanthine (IBMX-Sigma-Aldrich), 10-6 M dexamethasone (Sigma-Aldrich, Fluka Chemie AG, Buchs, Switzerland), 0.02% insulin (Invitrogen/Gibco), 200 µM indomethacin (Sigma-Aldrich), and 1% penicillin-streptomycin (Invitrogen/Gibco) for three weeks. The medium was replaced twice a week. Intracellular lipid droplets indicating adipogenic differentiation were confirmed by oil red O (Sigma-Aldrich) staining with 0.5% oil red O in methanol. The cells were then allowed to dry completely and mounted in a mounting medium.
In vitro osteogenic differentiation and Alizarin red S staining: Cells from Passage 3 (3000 cells/cm2) were seeded onto type I collagen-coated coverslips (BD Biosciences) in 6-well plates. The osteogenic medium DMEM-LG (Invitrogen) was supplemented with 10-8 M dexamethasone (Sigma-Aldrich), 50 µg/mL ascorbate-2- phosphate (Wako Chemicals, Richmond, VA, United States), 10 mmol/L b-glycerophosphate (Sigma-Aldrich), 1% penicillin-streptomycin, and 10% FBS (Invitrogen/Gibco) for three weeks. The medium was replaced twice a week. At the end of the third week, osteogenic differentiation was assessed by staining with Alizarin Red (Sigma-Aldrich, Fluka Chemie AG, Buchs, Switzerland). The medium was discarded and the cells were washed with PBS. Cells were incubated with ethanol for 5 min at room temperature and then allowed to dry completely. Cells were washed with distilled water and stained with alizarin red solution comprising 2% alizarin red S for one minute. Stained cells were dehydrated in acetone (20 dips), fixed in acetone–xylene (1:1) solution (20 dips), cleared with xylene (20 dips), and then allowed to dry completely and mounted in a mounting medium.
In vitro chondrogenic differentiation and Alcian blue staining: In the chondrogenesis mechanism, high cell density and cell-cell interaction play an important role. Therefore, for chondrogenic differentiation, cells were seeded as droplets onto type I collagen-coated coverslips (BD Biosciences) in 6-well plates. The medium was added after the cells adhered to the coverslips. The cells were incubated in chondrogenic medium Dulbecco’s Modified Eagle’s Medium, High (4.5 g/L) Glucose (DMEM-HG, Invitrogen) supplemented with 100 nM dexamethasone (Sigma-Aldrich), 50 µg/mL ascorbate-2-phosphate (Wako Chemicals, Richmond, VA, United States), 10 ng/mL transforming growth factor-beta 1 (TGF-β1, Peprotech, Rocky Hill, NJ, United States), 1% sodium pyruvate (Invitrogen), 50 mg/mL ITS (Sigma-Aldrich), 40 µg/mL proline (Sigma-Aldrich), 1% penicillin-streptomycin, and 10% FBS (Invitrogen/Gibco) for three weeks. The medium was replaced twice a week. Cells were fixed with formaldehyde for 10 min at room temperature, washed with distilled water, and then allowed to dry completely. Chondrogenic differentiation was confirmed by Alcian Blue (Abcam) staining. The cells were allowed to dry completely and mounted in a mounting medium (Figure 2).
Pre-transfer process: Final UC-MSCs preparations before implantation were collected from Passage 3 and maintained in normal saline at final concentrations of 1 × 106 in 3 mL, 1 × 106 in 20 mL, and 1 × 106 in 30 mL.
Transfer of UC-MSCs and operational procedures: Before starting the treatment, we performed a multidisciplinary approach with a team of pediatricians, pediatric neurologists, neurosurgeons, anesthesia and reanimation specialists, and physical medicine and rehabilitation specialists. Prior to implantation, we evaluated the patients for contraindications to sedoanalgesia or general anesthesia[13]. Intrathecal (IT) administration was performed with a 22-gauge spinal needle through the lumbar 3/4 vertebra. For intravenous (IV) administration, 1 × 106 cells/kg were administered with 250 cc isotonic slow infusion in 60 min intramuscularly (IM) and the patients stayed in the hospital for one day. After these applications, movement restrictions were applied for two days, and the patients’ relatives were warned to avoid water contact around the injection sites.
On the third day of application, we initiated an intense physiotherapy and exercise program. A rehabilitation session included warm-up, neck-trunk stabilization, and postural control exercises. Exercises were performed in a pool three days a week; stretching exercises were practiced for a longer time for extremities with severe spasticity. We also included exercises to improve fine motor skills[8,28].
Patient assessment: We categorized the patients according to their functional levels using the Gross Motor Function Classification System (GMFCS) and the Manual Ability Classification System (MACS). MACS describes how children with CP use their hands during daily activities. We used the Modified Ashworth Scale (MAS) to evaluate spasticity and the Wee Functional Independence Measure (WeeFIM) scale to assess quality of life in terms of independence in daily activities[27].
Patient assessment took place preoperatively and postoperatively (one week, one month, two months, four months, and 12 months). Postoperative improvement in neurological and functional evaluations based on GMFSC, MAS, and WeeFIM scores was accepted as treatment success.
Statistical analysis: We used the Friedman Test to measure the changes in WeeFIM, MAS, GMFCS, and MACS scores after the intervention. We chose this nonparametric test as the number of obtained data was not sufficient for parametric tests. All analyses were carried out using SPSS 22 (IBM Corp., Armonk, NY, United States).
Case 1: The first case was a one-year-old female diagnosed with CP due to hypoxia at birth. Before treatment, case 1 had a WeeFIM score of 18, a MAS score of 14 (both sides), a GMFCS score of five, and a MACS score of five. The patient received allogeneic MSCT six times as 1 × 106/kg IT, IV, and IM using UC-MSCs. Four months after the treatment, her WeeFIM cognitive score increased by one and her MAS score (both sides) decreased by one. Moreover, her GMFCS and MACS scores went down to three. The patient showed no side effects after the second and third applications, except subfebrile fever, which lasted approximately 12 h and regressed with cold application.
Case 2: The second case was a four-year-old male diagnosed with CP due to hypoxia that developed secondary to bleeding during tonsillectomy approximately one year before our trial. Before treatment, the patient had a WeeFIM score of 18, a MAS score of 21 (both sides), a GMFCS score of five, and a MACS score of five. This case had severe spasticity. He received allogeneic MSCT six times as 1 × 106/kg IT, IV, and IM using UC-MSCs. His WeeFIM score did not change after the intervention, but his MAS score started to decrease from the first postoperative week, eventually reaching 18 on both sides. His GMFCS and MACS scores decreased to three. This patient showed no side effects during the entire follow-up period.
Case 3: This was a nine-year-old male diagnosed with CP due to cardiac arrest of unknown cause 11 months after birth. Before treatment, this case had a WeeFIM score of 18, a MAS score of 28 (both sides), a GMFCS score of five, and a MACS score of five. Aside from limb spasticity, the patient demonstrated significant truncal spasticity and had an extensor posture. He received allogeneic MSCT six times as 1 × 106/kg IT, IV, and IM using UC-MSCs. His WeeFIM score did not change after the intervention, but his MAS score went down by three points (both sides) at four months. The patient showed no improvement in GMFCS and MACS scores. Similarly, this case did not experience any side effects after three applications, other than subfebrile fever, which regressed within approximately 12 h with cold application.
Case 4: The final case was a nine-year-old male diagnosed with CP that developed due to hypoxia during birth. Before treatment, he had a WeeFIM score of 48, a MAS score of 17 (both sides), a GMFCS score of four, and a MACS score of four. He underwent allogeneic MSCT six times as 1 × 106/kg IT, IV, and IM using UC-MSCs. This patient showed a significant increase of 32 points in his WeeFIM motor score and a decrease of two points in his MAS score (both sides) starting from the second month. Also, his GMFCS and MACS scores went down to two points after the intervention. Regarding side effects, the patient only suffered temporary pain in the injection sites after the application.
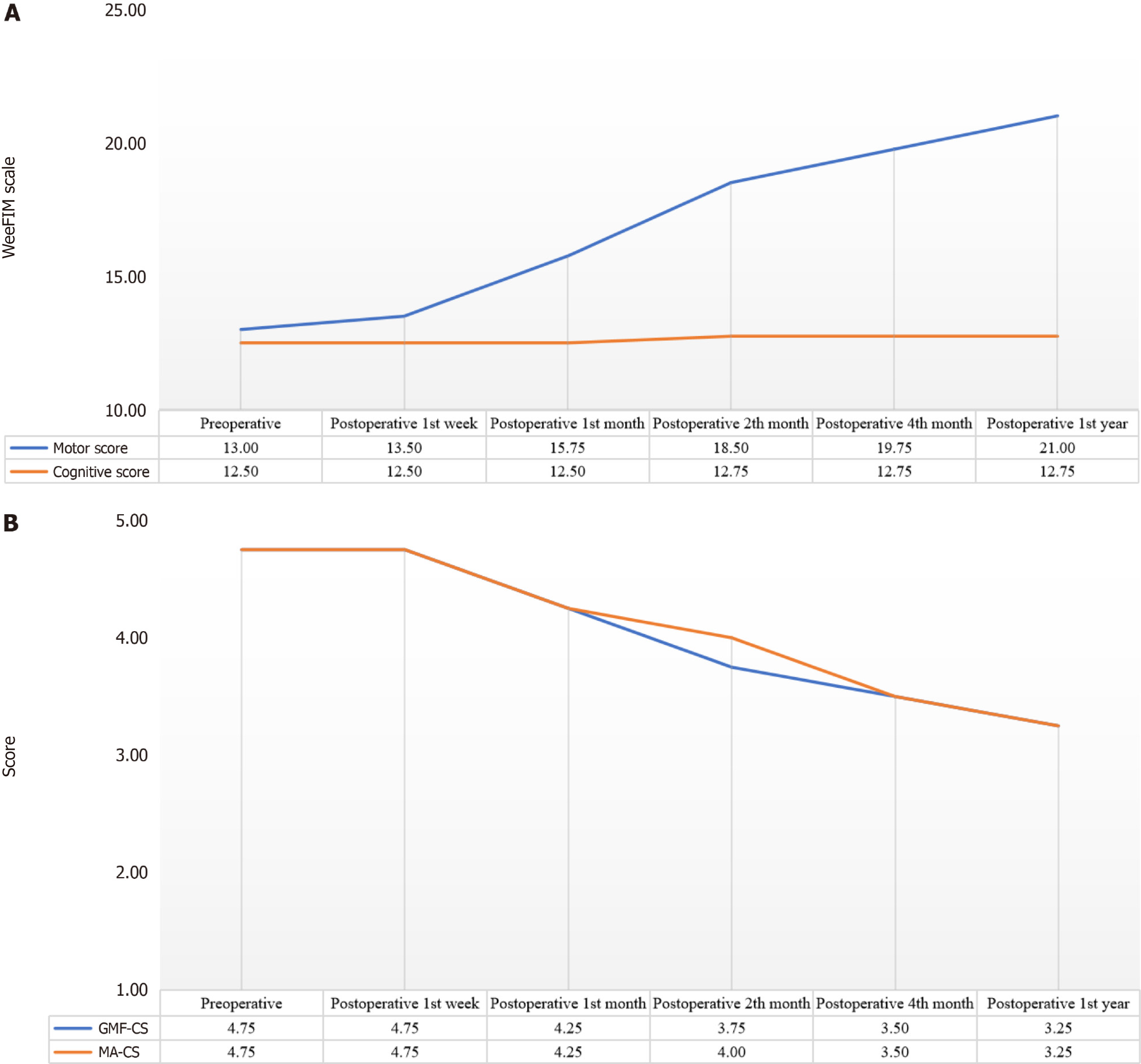
Figure 3A shows the changes in WeeFIM motor and cognitive subtests at different times. Accordingly, these scores did not show a statistically significant change between preoperative and postoperative periods (P = 0.42).
Table 2 lists the changes in the patients’ mean MAS scores at different times. Accordingly, MAS scores for both sides decreased significantly after the intervention (two months: Right χ2 = 4000, P = 0.046, left χ2 = 4000, P = 0.046; four mon
| n | Mean | SD | Mean rank | χ2 | df | P value | ||
| Right | Preoperative | 4 | 20.00 | 6.06 | 5.50 | 17.414 | 5 | 0.004 |
| Postoperative 1-wk | 4 | 19.75 | 6.02 | 5.13 | ||||
| Postoperative 1-month | 4 | 19.00 | 5.48 | 3.88 | ||||
| Postoperative 2-month | 4 | 18.25 | 5.74 | 2.75 | ||||
| Postoperative 4-month | 4 | 17.75 | 5.25 | 1.88 | ||||
| Postoperative 1-year | 4 | 17.75 | 5.25 | 1.88 | ||||
| Left | Preoperative | 4 | 20.00 | 6.06 | 5.38 | 17.368 | 5 | 0.004 |
| Postoperative 1-wk | 4 | 19.75 | 6.02 | 5.00 | ||||
| Postoperative 1-month | 4 | 19.50 | 6.35 | 4.25 | ||||
| Postoperative 2-month | 4 | 18.25 | 5.74 | 2.63 | ||||
| Postoperative 4-month | 4 | 17.75 | 5.25 | 1.88 | ||||
| Postoperative 1-year | 4 | 17.75 | 5.25 | 1.88 | ||||
Figure 3B presents our patients’ GMFCS and MACS scores at different times. Accordingly, there were some statistically significant changes in these scores between preoperative and postoperative measurements. To determine the cut-off times for significant improvement, we compared the preoperative results with each follow-up period individually. For GMFCS, preoperative and postoperative first week scores were the same for all cases. While GMFCS scores showed some decrease after the first week, these changes were not statistically significant compared to the baseline measurements (one month: χ2 = 2000, P = 0.157; two months: χ2 = 3000, P = 0.083; four months: χ2 = 3000, P = 0.083). However, GMFCS scores at 12 months were significantly lower than baseline (χ2 = 4000, P = 0.046). In other words, the GMFCS scores of our cases continued to decrease after the first week of intervention, but this improvement was only statistically significant at 12 months. MACS scores followed a similar trend. There was no difference between preoperative and postoperative first week measurements. Then, there were insignificant improvements up to the fourth month (one month: χ2 = 2000, P = 0.157; two months: χ2 = 3000, P = 0.083; four months: χ2 = 3000, P = 0.083). Again, MACS scores at 12 months were significantly lower than baseline (χ2 = 4000, P = 0.046). Thus, the MACS scores of our cases continued to go down after the first week of treatment, but this change was only statistically significant at the one-year mark after the intervention.
CP is a group of disorders affecting movement, balance, and posture. UC-MSCs have been researched for CP treatment, albeit with limited or inconclusive clinical evidence regarding their benefits. One randomized study investigated the safety and efficacy of UC-MSC transplantation in CP patients and observed improvement in gross motor and functions after treatment combined with rehabilitation[14]. Other researchers have emphasized that the recovery of cerebral metabolic activity may be crucial for the development of brain functions in CP patients and the therapeutic window, transfusion route, and dosage are key for reference in clinical practice[29]. We believe that our findings can constitute a similar reference.
One trial focused on motor functions after treatment with allogeneic umbilical cord blood (AlloCB) in 91 children diagnosed with CP due to hypoxic-ischemic encephalopathy, stroke, or periventricular leukomalacia[30]. The authors used the Gross Motor Function Measure-66 and Peabody Developmental Motor Scale, Second Edition to measure motor functions. Accordingly, treatment with AlloCB and UC-MSC proved safe. They observed no significant change in motor functions at six months after treatment and AlloCB was associated with greater increases in Gross Motor Function Measure-66 scores at the one year mark[30].
We performed neurological and functional evaluations using GMFSC, MAS, and WeeFIM at different times up to 12 months after treatment. Based on WeeFIM scores, we observed a significant improvement in spasticity (P = 0.046) but no significant difference in motor functions (P > 0.05). GMFSC scores revealed improvement (P = 0.046), but there was no significant change in cognitive functions (P > 0.05).
UC-MSCs are a new and promising treatment modality for CP[8]. We observed no complications in any of our cases during one year of monitoring after six applications. Similarly, previous research reported no serious side effects[16-18]. Symptoms such as lower back pain and a mild increase in temperature were observed as minor side effects due to IT applications[31-33]. UC-MSCs release neurotrophic factors and increase muscle mass, displaying neuroprotective and neuro-regenerative activities on cognitive functions[8,34,35]. UC-MSCs have facilitated neuron regeneration in Par
According to a systematic review that listed all findings after cell therapy in CP and the measurement tools used, 2066 participants have undergone various cell therapy interventions in 54 trials[43]. Movement and posture were the most frequently reported outcome categories, followed by safety, although quality of life and various common comorbidities and complications associated with CP have rarely been reported[43].
We observed significant improvements in spasticity and motor functions in our sample, which is compatible with some studies in the relevant literature[44-46] and conflicting with others[8]. Our patients displayed no difference in cognitive functions after therapy, but Vaquero et al[44] reported a significant improvement in the cognitive skills of patients with spinal cord injury after treatment. We associate this conflicting result with the low number of cases in our sample[44].
One of the limitations in the current study was the small group of subjects of the same race and of a wide age range. Also, we did not include a control group of volunteer CP patients who did not receive UC-MSC therapy. However, we believe that our data can make notable contributions to the existing literature.
In conclusion, UC-MSC transplantation yielded improvements in spasticity and motor functions at 12 months after treatment in CP patients. No clear improvement was gained in cognitive skills. Future randomized controlled trials with larger samples can illuminate the advantages and limitations of UC-MSC therapy.
Cerebral palsy (CP) describes a group of nonprogressive disorders affecting movement, posture, and motor functions. It occurs in early childhood and persists until the end of life. Currently, the treatment of CP involves numerous modalities, such as various surgical treatments (selective dorsal rhizotomy, selective peripheral neurotomy, etc.), pharmacotherapies for generalized spasticity, Botox A for focal spasticity, and antiepileptics for epilepsy.
Current modalities can only provide partial symptom relief, which warrants research into new methods. One novel option for treating CP is mesenchymal stem cell therapy.
We aimed to investigate the efficacy and safety of mesenchymal stem cell therapy in CP cases.
Our sample consisted of four patients who were unable to stand or walk without external support. All cases received allogeneic mesenchymal stem cell therapy six times as 1 × 106/kg intrathecally, intravenously, and intramuscularly using umbilical cord-derived mesenchymal stem cells. We monitored the patients before and after the treatment using the Wee Functional Independence Measure, the Gross Motor Function Classification System, the Manual Ability Classification Scale, and the Modified Ashworth Scale.
Spasticity measures showed significant improvement in both sides after the intervention. There was no significant change in motor functions or cognitive functions. Gross motor function and manual ability measures differed statistically significantly at 12 months after treatment compared to baseline values.
In light of our findings, umbilical cord-derived mesenchymal stem cell therapy shows positive effects on spasticity and partial improvement in motor functions.
In this study, we demonstrated that allogeneic mesenchymal stem cell application via intrathecal, intramuscular and intravenous routes is safe and effective in cerebral palsy patients. The effectiveness of this treatment protocol should be evaluated at a higher level of evidence by conducting randomized, double-blind, case-control studies with a high number of participants in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cooper KM, United States; Isac S, Romania; Zhou X, China S-Editor: Li L L-Editor: Webster JR P-Editor: Xu ZH
| 1. | Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr Dis Treat. 2020;16:1505-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 2. | Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D; Executive Committee for the Definition of Cerebral Palsy. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1529] [Cited by in RCA: 1375] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 3. | Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol. 2000;42:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 696] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol. 2006;33:251-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N. The complex aetiology of cerebral palsy. Nat Rev Neurol. 2018;14:528-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Christine C, Dolk H, Platt MJ, Colver A, Prasauskiene A, Krägeloh-Mann I; SCPE Collaborative Group. Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Dev Med Child Neurol Suppl. 2007;109:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Okur SÇ, Erdoğan S, Demir CS, Günel G, Karaöz E. The Effect of Umbilical Cord-derived Mesenchymal Stem Cell Transplantation in a Patient with Cerebral Palsy: A Case Report. Int J Stem Cells. 2018;11:141-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Jiao Y, Li XY, Liu J. A New Approach to Cerebral Palsy Treatment: Discussion of the Effective Components of Umbilical Cord Blood and its Mechanisms of Action. Cell Transplant. 2019;28:497-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Fan HC, Ho LI, Chi CS, Cheng SN, Juan CJ, Chiang KL, Lin SZ, Harn HJ. Current proceedings of cerebral palsy. Cell Transplant. 2015;24:471-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Jantzie LL, Scafidi J, Robinson S. Stem cells and cell-based therapies for cerebral palsy: a call for rigor. Pediatr Res. 2018;83:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Zhuang WZ, Lin YH, Su LJ, Wu MS, Jeng HY, Chang HC, Huang YH, Ling TY. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 14. | Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 15. | Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 432] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Huang P, Gebhart N, Richelson E, Brott TG, Meschia JF, Zubair AC. Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy. 2014;16:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 762] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 19. | Jung JE, Kim GS, Chan PH. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke. 2011;42:3574-3579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Martínez-Morales PL, Revilla A, Ocaña I, González C, Sainz P, McGuire D, Liste I. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev Rep. 2013;9:685-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Sherman LS, Romagano MP, Williams SF, Rameshwar P. Mesenchymal stem cell therapies in brain disease. Semin Cell Dev Biol. 2019;95:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, Bae JS. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 23. | Kim HJ, Seo SW, Chang JW, Lee JI, Kim CH, Chin J, Choi SJ, Kwon H, Yun HJ, Lee JM, Kim ST, Choe YS, Lee KH, Na DL. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: A phase 1 clinical trial. Alzheimers Dement (NY). 2015;1:95-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1053] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 25. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 848] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 27. | Kabatas S, Civelek E, Savrunlu EC, Kaplan N, Boyalı O, Diren F, Can H, Genç A, Akkoç T, Karaöz E. Feasibility of allogeneic mesenchymal stem cells in pediatric hypoxic-ischemic encephalopathy: Phase I study. World J Stem Cells. 2021;13:470-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 28. | Xie J, Jiang L, Li Y, Chen B, Li F, Jiang Y, Gao D, Deng L, Lv X, Ma X, Yin G, Yao D, Xu P. Rehabilitation of motor function in children with cerebral palsy based on motor imagery. Cogn Neurodyn. 2021;15:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Gu J, Huang L, Zhang C, Wang Y, Zhang R, Tu Z, Wang H, Zhou X, Xiao Z, Liu Z, Hu X, Ke Z, Wang D, Liu L. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res Ther. 2020;11:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (2)] |
| 30. | Sun JM, Case LE, McLaughlin C, Burgess A, Skergan N, Crane S, Jasien JM, Mikati MA, Troy J, Kurtzberg J. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev Med Child Neurol. 2022;64:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Wang L, Ji H, Zhou J, Xie J, Zhong Z, Li M, Bai W, Li N, Zhang Z, Wang X, Zhu D, Liu Y, Wu M. Therapeutic potential of umbilical cord mesenchymal stromal cells transplantation for cerebral palsy: a case report. Case Rep Transplant. 2013;2013:146347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Mehta T, Feroz A, Thakkar U, Vanikar A, Shah V, Trivedi H. Subarachnoid placement of stem cells in neurological disorders. Transplant Proc. 2008;40:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Rengasamy M, Gupta PK, Kolkundkar U, Singh G, Balasubramanian S, SundarRaj S, Chullikana A, Majumdar AS. Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells. Indian J Med Res. 2016;144:852-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Cruz-Martinez P, Pastor D, Estirado A, Pacheco-Torres J, Martinez S, Jones J. Stem cell injection in the hindlimb skeletal muscle enhances neurorepair in mice with spinal cord injury. Regen Med. 2014;9:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 2014;23:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94:11663-11668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 330] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Lv ZY, Li Y, Liu J. Progress in clinical trials of stem cell therapy for cerebral palsy. Neural Regen Res. 2021;16:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Nguyen LT, Nguyen AT, Vu CD, Ngo DV, Bui AV. Outcomes of autologous bone marrow mononuclear cells for cerebral palsy: an open label uncontrolled clinical trial. BMC Pediatr. 2017;17:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Nguyen TL, Nguyen HP, Nguyen TK. The effects of bone marrow mononuclear cell transplantation on the quality of life of children with cerebral palsy. Health Qual Life Outcomes. 2018;16:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Sharma A, Sane H, Gokulchandran N, Kulkarni P, Gandhi S, Sundaram J, Paranjape A, Shetty A, Bhagwanani K, Biju H, Badhe P. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: a new frontier. Stem Cells Int. 2015;2015:905874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Luan Z, Liu W, Qu S, Du K, He S, Wang Z, Yang Y, Wang C, Gong X. Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant. 2012;21:S91-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Finch-Edmondson M, Paton MCB, Honan I, Karlsson P, Stephenson C, Chiu D, Reedman S, Griffin AR, Morgan C, Novak I. Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernández C, Tapiador N, Sevilla M, Morejón C, Montilla J, Martínez F, Marín E, Bustamante S, Vázquez D, Carballido J, Rodríguez A, Martínez P, García C, Ovejero M, Fernández MV; Neurological Cell Therapy Group. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 45. | Broughton BR, Lim R, Arumugam TV, Drummond GR, Wallace EM, Sobey CG. Post-stroke inflammation and the potential efficacy of novel stem cell therapies: focus on amnion epithelial cells. Front Cell Neurosci. 2012;6:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Lindvall O, Kokaia Z. Recovery and rehabilitation in stroke: stem cells. Stroke. 2004;35:2691-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |