Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1544
Peer-review started: December 29, 2023
First decision: January 16, 2024
Revised: February 8, 2024
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: March 16, 2024
Processing time: 74 Days and 3.2 Hours
The clinical manifestations of trisomy 7 mosaicism are diverse and nonspecific, so prenatal diagnosis is very difficult.
Two pregnant women with abnormal prenatal screening results were included. One was a 22-year-old woman (G1P0). At 31st week of gestation, ultrasound revealed that the posterior horn of the left lateral ventricle was 10 mm and the right renal pelvis had a separation of 7 mm. The other pregnant woman was 33 years old (G2P1L1A0), and her fetus was found to have a cardiac malformation at the 24th week of gestation. Copy number variation sequencing, whole-exome sequencing and karyotype analysis were carried out after amniocentesis, and both fetuses were diagnosed with trisomy 7 mosaicism. After parental counseling, one woman continued the pregnancy, and the other woman terminated the pregnancy.
In trisomy 7 mosaicism, the low proportion of trisomy does not lead to abortion, but can result in abnormal fetal development, which can be detected via ultrasound. Therefore, clinicians need to pay more attention to various aspects of fetal growth and development, combining with imaging, cellular, molecular genetics and other methods to perform comprehensive evaluations of fetuses to provide more reliable genetic counseling for pregnant women.
Core Tip: Herein, two fetuses were prenatally diagnosed according to abnormal ultrasound findings, including a widened posterior horn of the left lateral ventricle, renal dysplasia and a cardiac malformation. Copy number variation sequencing, whole-exome sequencing and karyotype analysis were carried out, and both fetuses were confirmed to have trisomy 7 mosaicism.
- Citation: Hou F, Li Y, Jin H. Clinical manifestations and the prenatal diagnosis of trisomy 7 mosaicism: Two case reports. World J Clin Cases 2024; 12(8): 1544-1548
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1544.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1544
Non-mosaic trisomy 7 usually leads to spontaneous abortion in early pregnancy. As a result, the mosaic form of trisomy 7, which is detected by noninvasive prenatal screening (NIPS) or chorionic villus sampling, is more likely to be present, either confined placental mosaicism or fetal mosaicism[1]. Trisomy 7 is the most frequently observed type of rare autosomal trisomy in NIPS[1]. Because it involves imprinting genes, trisomy 7 may lead to uniparental diploidy (UPD) through trisomic rescue. Therefore, it is crucial to provide correct and appropriate prenatal diagnosis and genetic counseling to pregnant women. However, the prenatal diagnosis of trisomy 7 mosaicism is difficult because of its variable and nonspecific clinical features[2]. Here, we report the ultrasound manifestations and prenatal diagnosis of two cases with trisomy 7 mosaicism.
Two pregnant women with abnormal ultrasound results presented to our hospital for prenatal diagnosis.
The prenatal screening results of both pregnant women revealed low risk. However, their ultrasound examination results suggested abnormal fetal development.
Both pregnant women had no history of past illness.
Neither the pregnant women nor their husbands had a significant personal or family history.
The physical examinations of the two pregnant women did not reveal any abnormalities.
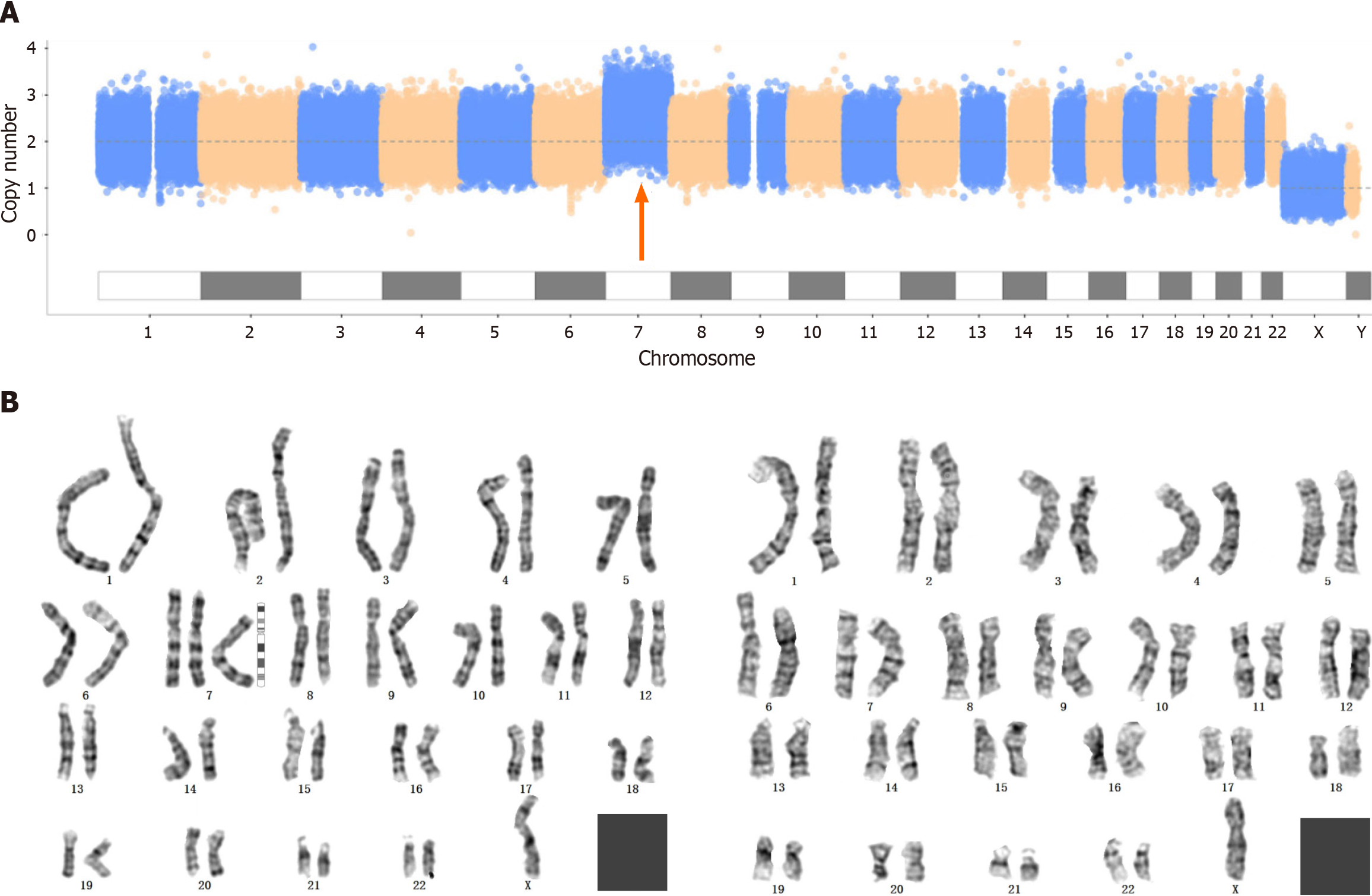
Copy number variation sequencing (CNV-seq), whole-exome sequencing (WES) and karyotype analysis were carried out after amniocentesis. No pathogenic mutations associated with the clinical presentation were detected by WES. The CNV-seq results revealed that the first fetus had trisomy 7 mosaicism with approximately 40% mosaicism, while the karyotype analysis revealed 47,XN,+7[45]/46,XN[34] (Figure 1).
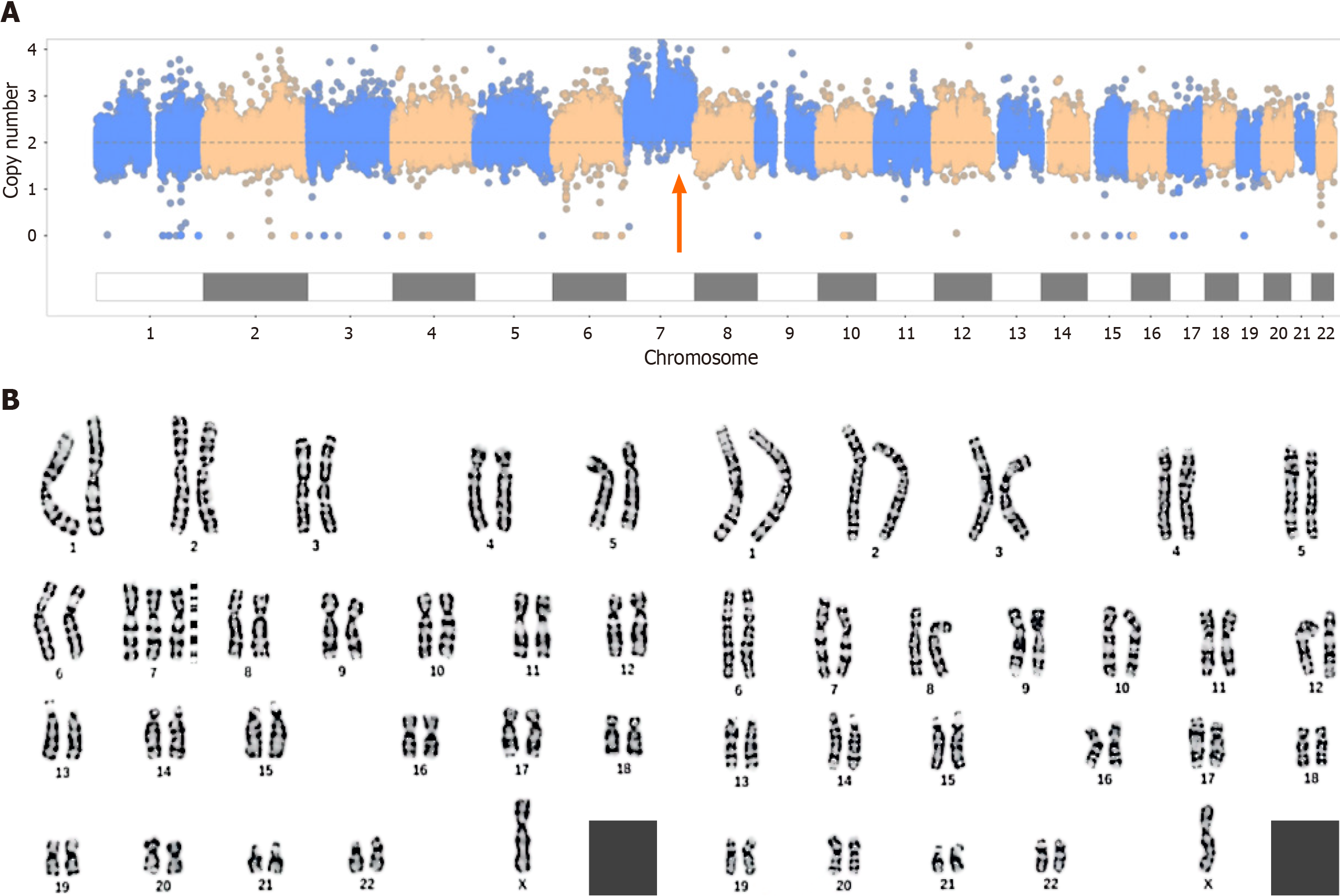
The results for the second fetus were similar to those for the first fetus. CNV-seq revealed 65% trisomy 7, and karyotype analysis revealed 47,XN,+7[65]/46,XN[36] (Figure 2).
In the first pregnant woman, at the 16th week of gestation, ultrasound showed that the fetus had a single umbilical artery and a choroid plexus cyst; at the 21st week of gestation, ultrasound showed an echogenic bowel and mild separation of bilateral renal pelvis; and at the 31st week of gestation, ultrasound showed that the posterior horn of left lateral ventricle was 10 mm and the right renal pelvis had a separation of 7 mm.In the second pregnant woman, at the 24th week of gestation, ultrasound revealed a fetal cardiac malformation, including severe aortic stenosis, mild mitral stenosis with severe regurgitation, left ventricular cord sclerosis and a small foramen ovale.
According to these findings, both fetuses were diagnosed with trisomy 7 mosaicism.
We did not provide treatment.
After parental counseling, the second pregnant woman terminated the pregnancy, and the other pregnant woman delivered a baby at the 39th week of gestation. The baby was followed up for 1 year after birth, at which point no obvious abnormalities were found.
Trisomy 7 usually leads to spontaneous abortion in early pregnancy, and trisomy 7 mosaicism is most commonly reported. Previous studies have shown that 15%-80% of trisomy 7 in the placenta will not lead to stillbirth or miscarriage, and the prognosis is good if UPD or fetal chimerism is not involved[3,4]. Trisomy 7 mosaicism may be associated with Potter syndrome or renal dysplasia, cardiac abnormalities, prenatal and postnatal growth retardation, cleft lip and palate, recurrent ear infections, and epilepsy[5]. Consistent with the findings of previous reports, the two fetuses reported in this study exhibited a certain degree of renal dysplasia or a cardiac malformation due to trisomy 7 mosaicism.
A high proportion of trisomy 7 in the placenta or fetus may lead to intrauterine death[6,7]. The lower the proportion of trisomy is, the lower the effect on the fetus. Hsu et al[7] summarized the outcomes of 8 fetuses prenatally diagnosed with trisomy 7 mosaicism. Among these fetuses, 7 had normal live births, 4 of whom were followed up for 4-54 months and were reported to be developmentally normal. In addition, trisomy 7 mosaicism was confirmed in the foreskin fibroblasts of 2 patients[7]. We found that the proportion of trisomy in the above 4 patients ranged from 5% to 48%. In this study, the first pregnant woman gave birth to a baby with trisomy 7 mosaicism (approximately 40%-45% mosaicism). Like in a previous study, we followed the baby for 1 year after birth and found no significant developmental abnormalities.
Human chromosome 7 is an imprinting chromosome which contains several imprinted genes related to diseases. It has been confirmed that chromosome 7 is closely related to Silver-Russell syndrome (SRS). SRS is characterized by intrauterine growth restriction and postnatal developmental delay and is mostly caused by maternal UPD[2]. The clinical phenotypes of trisomy 7 mosaicism, which have been reported thus far, are quite different. Therefore, for prenatally diagnosed cases, it is difficult to judge risk. The two trisomy 7 mosaicism cases reported in this paper had different prenatal ultrasound findings due to differences in trisomy proportions, resulting in differences in pregnancy outcomes. When conducting genetic counseling, clinicians should comprehensively evaluate fetuses and provide guidance according to specific conditions.
In trisomy 7 mosaicism, the low proportion of trisomy does not lead to abortion, but can result in abnormal fetal development, which can be detected via ultrasound. Therefore, clinicians need to pay more attention to various aspects of fetal growth and development, combining with imaging, cellular, molecular genetics and other methods to perform comprehensive evaluations of fetuses to provide more reliable genetic counseling for pregnant women.
We sincerely thank the participants and their families for their cooperation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Velikova TV, Bulgaria S-Editor: Zhang L L-Editor: A P-Editor: Zhao YQ
| 1. | Zhu X, Lam DYM, Chau MHK, Xue S, Dai P, Zhao G, Cao Y, Cheung SWH, Kwok YKY, Choy KW, Kong X, Leung TY. Clinical Significance of Non-Invasive Prenatal Screening for Trisomy 7: Cohort Study and Literature Review. Genes (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Chen CP, Su YN, Chern SR, Hwu YM, Lin SP, Hsu CH, Tsai FJ, Wang TY, Wu PC, Lee CC, Chen YT, Chen LF, Wang W. Mosaic trisomy 7 at amniocentesis: prenatal diagnosis and molecular genetic analyses. Taiwan J Obstet Gynecol. 2010;49:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Qi Y, Yang J, Hou Y, Guo F, Peng H, Wang D, Du Q, Yin A. The significance of trisomy 7 mosaicism in noninvasive prenatal screening. Hum Genomics. 2019;13:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Ndah BV, Stead JA, Brancazio LR, Hummel M, Wenger SL. Prenatal detection of trisomy for the entire long arm of chromosome 7. J Med Genet. 2000;37:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Kalousek DK, Langlois S, Robinson WP, Telenius A, Bernard L, Barrett IJ, Howard-Peebles PN, Wilson RD. Trisomy 7 CVS mosaicism: pregnancy outcome, placental and DNA analysis in 14 cases. Am J Med Genet. 1996;65:348-352. [PubMed] [DOI] [Full Text] |
| 6. | Bilimoria KY, Rothenberg JM. Prenatal diagnosis of a trisomy 7/maternal uniparental heterodisomy 7 mosaic fetus. Am J Med Genet A. 2003;118A:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Hsu LY, Yu MT, Neu RL, Van Dyke DL, Benn PA, Bradshaw CL, Shaffer LG, Higgins RR, Khodr GS, Morton CC, Wang H, Brothman AR, Chadwick D, Disteche CM, Jenkins LS, Kalousek DK, Pantzar TJ, Wyatt P. Rare trisomy mosaicism diagnosed in amniocytes, involving an autosome other than chromosomes 13, 18, 20, and 21: karyotype/phenotype correlations. Prenat Diagn. 1997;17:201-242. [PubMed] [DOI] [Full Text] |