Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1487
Peer-review started: December 12, 2023
First decision: December 31, 2023
Revised: January 15, 2024
Accepted: February 20, 2024
Article in press: February 20, 2024
Published online: March 16, 2024
Processing time: 90 Days and 24 Hours
High-grade pancreatic intraepithelial neoplasia (PanIN) exhibits no mass and is not detected by any examination modalities. However, it can be diagnosed by pancreatic juice cytology from indirect findings. Most previous cases were diagnosed based on findings of a focal stricture of the main pancreatic duct (MPD) and caudal MPD dilatation and subsequent pancreatic juice cytology using endoscopic retrograde cholangiopancreatography (ERCP). We experienced a case of high-grade PanIN with an unclear MPD over a 20-mm range, but without caudal MPD dilatation on magnetic resonance cholangiopancreatography (MRCP).
A 60-year-old female patient underwent computed tomography for a follow-up of uterine cancer post-excision, which revealed pancreatic cysts. MRCP revealed an unclear MPD of the pancreatic body at a 20-mm length without caudal MPD dilatation. Thus, course observation was performed. After 24 mo, MRCP revealed an increased caudal MPD caliber and a larger pancreatic cyst. We performed ERCP and detected atypical cells suspected of adenocarcinoma by serial pancreatic juice aspiration cytology examination. We performed a distal pancreatectomy and obtained a histopathological diagnosis of high-grade PanIN. Pancreatic parenchyma invasion was not observed, and curative resection was achieved.
High-grade Pan-IN may cause MPD narrowing in a long range without caudal MPD dilatation.
Core Tip: High-grade pancreatic intraepithelial neoplasia (PanIN) is diagnosed using pancreatic juice cytology. Most reasons for performing endoscopic retrograde cholangiopancreatography (ERCP) are focal main pancreatic duct (MPD) stenosis and/or caudal MPD dilatation. Poor MPD depiction without caudal MPD dilatation on magnetic resonance cholangiopancreatography sometimes occurs in normal individuals. Thus, we hesitate to send these patients to undergo ERCP. As such, course observation is necessary to confirm whether or not caudal MPD dilatation and/or cyst formation develop(s). Accordingly, it is better to submit the patient to ERCP to detect high-grade PanIN if caudal MPD dilatation and/or cyst formation occur(s).
- Citation: Furuya N, Yamaguchi A, Kato N, Sugata S, Hamada T, Mizumoto T, Tamaru Y, Kusunoki R, Kuwai T, Kouno H, Kuraoka K, Shibata Y, Tazuma S, Sudo T, Kohno H, Oka S. High-grade pancreatic intraepithelial neoplasia diagnosed based on changes in magnetic resonance cholangiopancreatography findings: A case report. World J Clin Cases 2024; 12(8): 1487-1496
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1487
High-grade pancreatic intraepithelial neoplasia (PanIN) grows in the pancreatic duct with flat or low-papillary progression and is included in pancreatic cancer (PC) stage 0. Thus, high-grade PanIN is considered a curable PC, and attention has been paid to its diagnosis. Unfortunately, PanIN does not form a mass and can only be diagnosed by pancreatic juice cytology from indirect findings [e.g., main pancreatic duct (MPD) stricture and dilatation, pancreatic cyst, and pancreatic atrophy]. Most cases are characterized by limited MPD stenosis following caudal MPD dilatation. Herein, we report a case of high-grade PanIN that demonstrated a poor description of the MPD in a rather long range, with no caudal MPD dilatation.
A 60-year-old female patient underwent a computed tomography scan for a follow-up of uterine cancer post-excision, which revealed pancreatic cysts.
The patient had diabetes mellitus and did not smoke or drink alcohol.
The patient had no family history of PC or chronic pancreatitis.
Physical examination on admission indicated a height of 156 cm, weight of 68.5 kg, and body mass index of 27.4 kg/mm2. Her abdomen was soft and flat with no palpable mass.
The patient’s relevant laboratory data included hemoglobin A1c of 11.8% and CA19-9 of 57 U/mL.
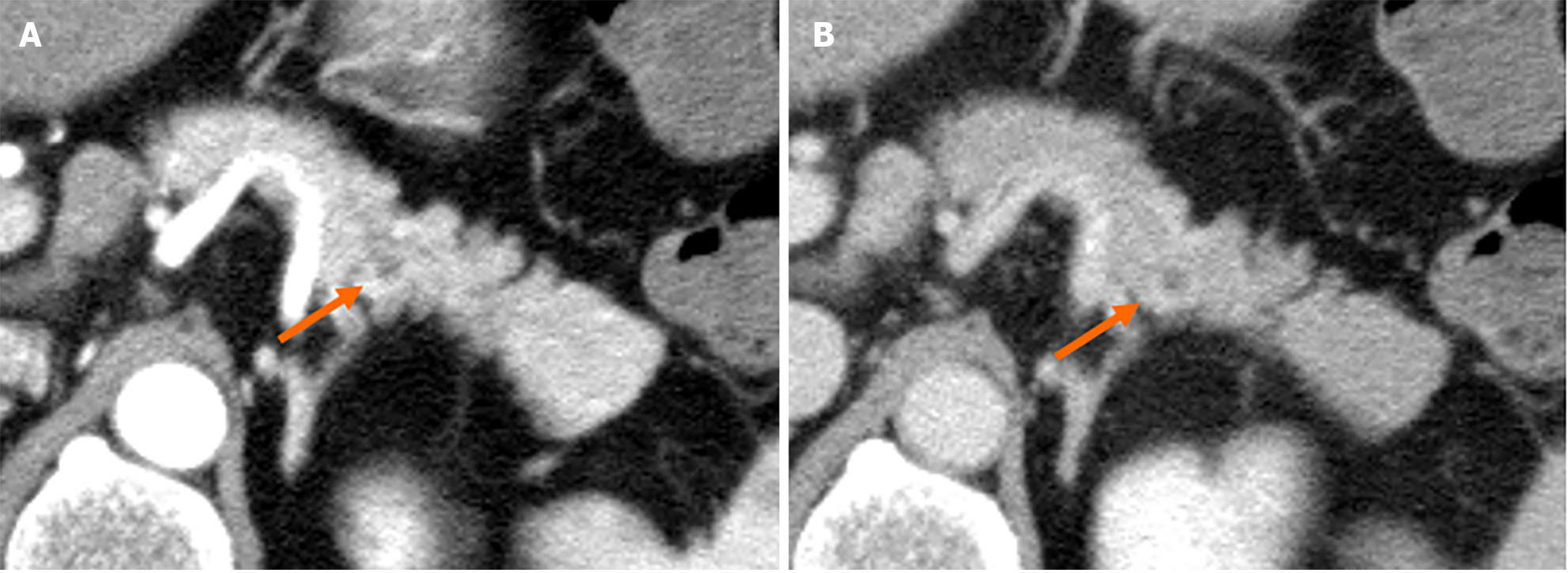
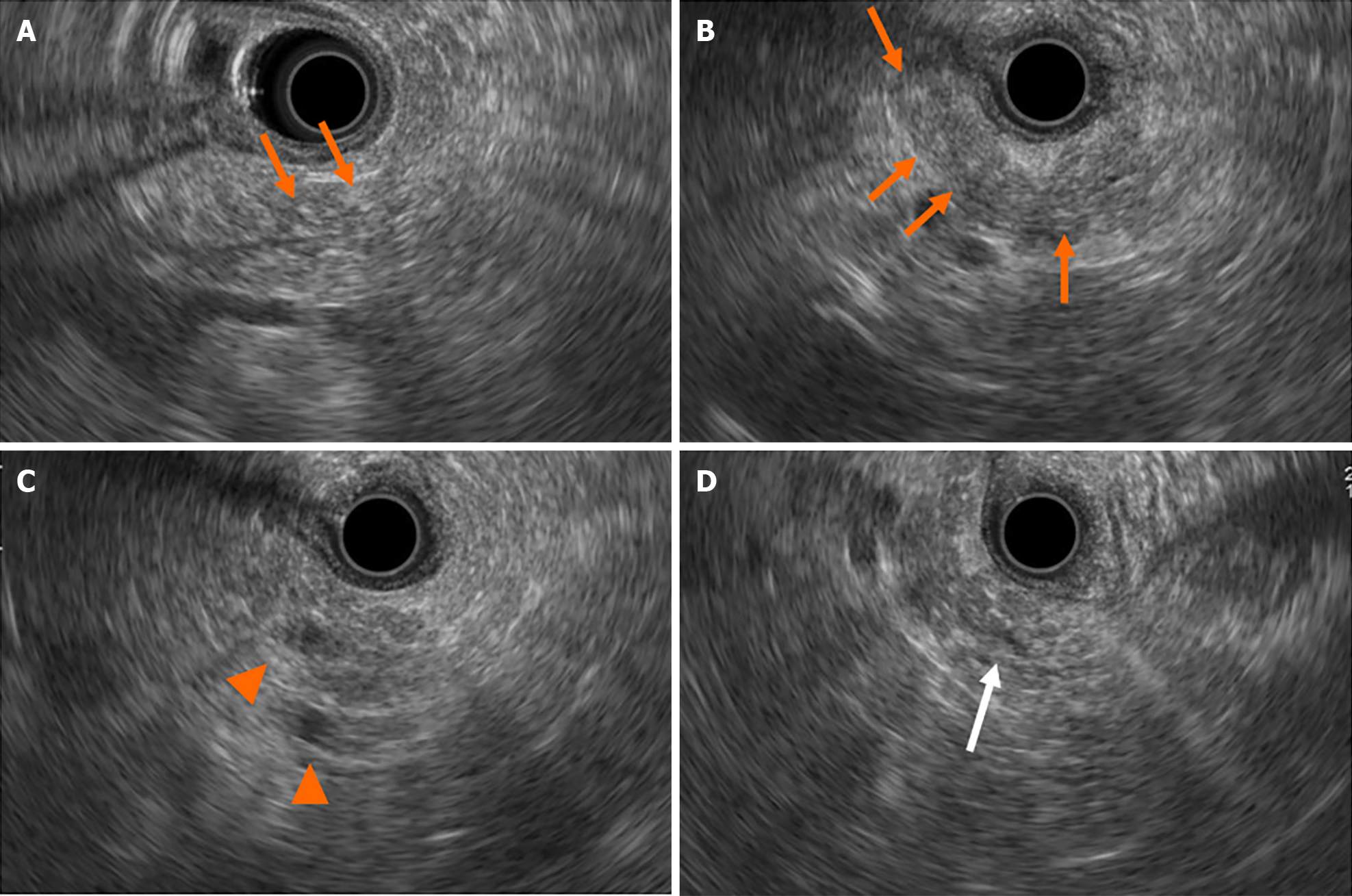
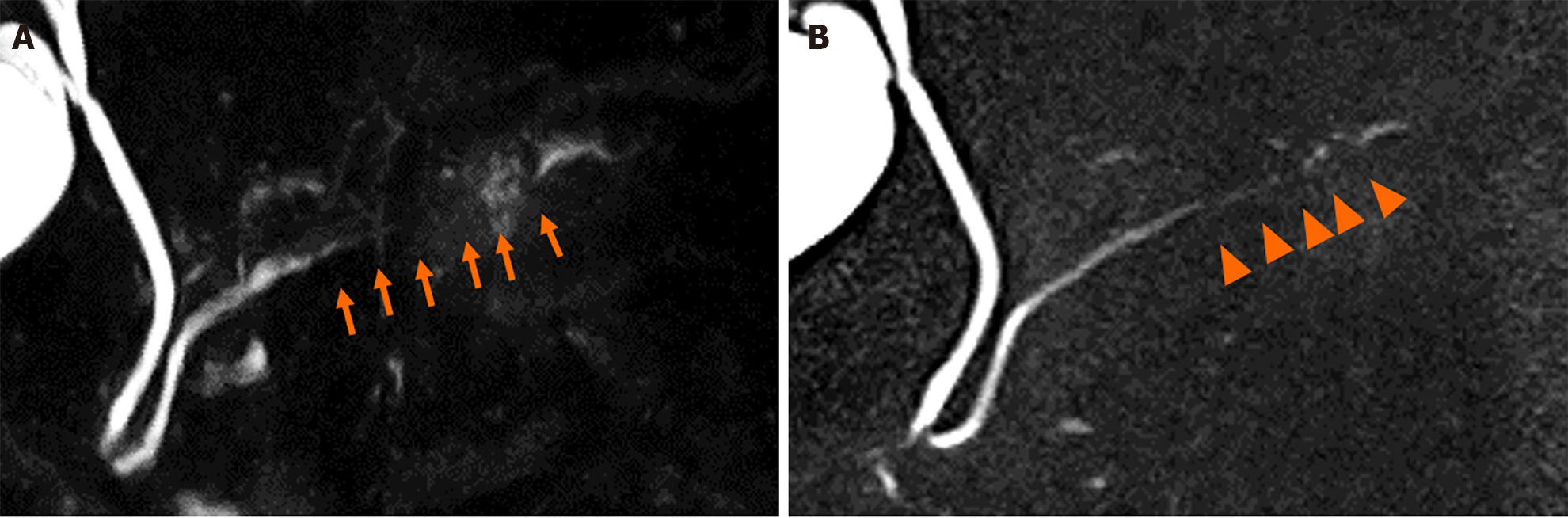
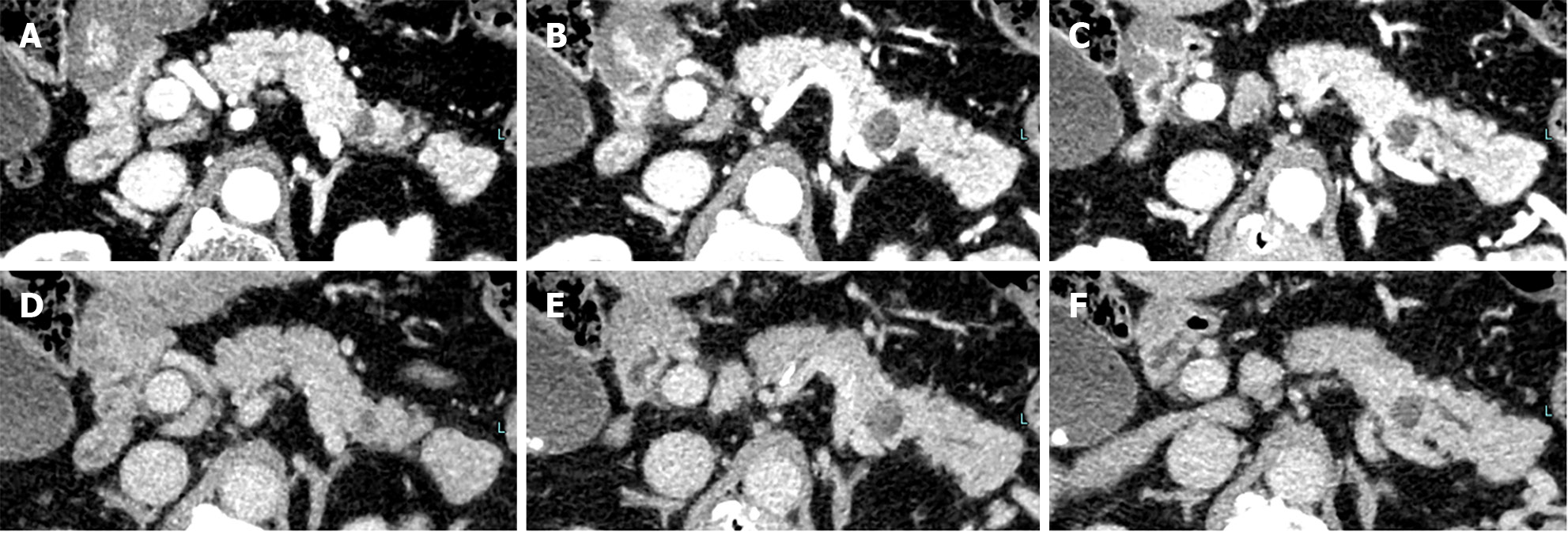
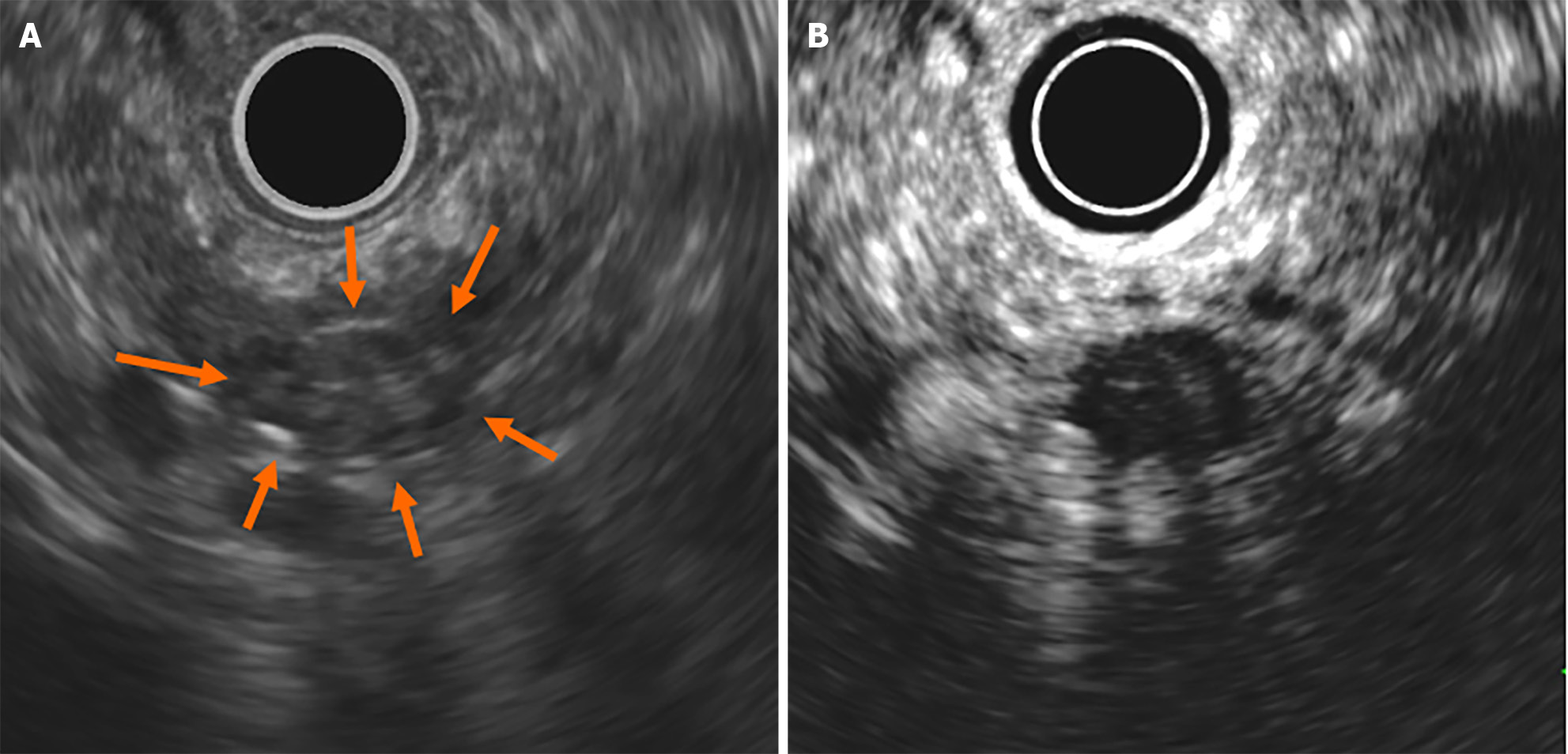
Contrast-enhanced computed tomography (CE-CT) revealed a 5-mm round cyst in the pancreatic tail but with no solid mass, atrophy, or fatty change in the pancreas (Figure 1). Further, endoscopic ultrasonography (EUS) revealed a diffuse high echoic spot in the whole pancreas (Figure 2A and B), indicating early chronic pancreatitis and a small round cyst in the pancreatic tail (Figure 2C), and there was no caudal MPD dilatation (Figure 2D). A solid mass, hypoechoic area around the MPD, or caudal MPD dilation was not detected. Magnetic resonance cholangiopancreatography (MRCP) poorly described the MPD at the 20-mm length of the pancreatic body/tail (Figure 3A), with no caudal MPD dilation. This finding appeared like irregular MPD narrowing in other MRCP sequences (Figure 3B). Imaging examination for pancreatic cysts was followed up twice a year. MPD findings indicated no changes after 6 mo (Figure 4A and B). The range of MPD narrowing did not change over 24 mo, but caudal MPD dilatation was slightly detected and the cyst size had increased (Figure 4C and D).
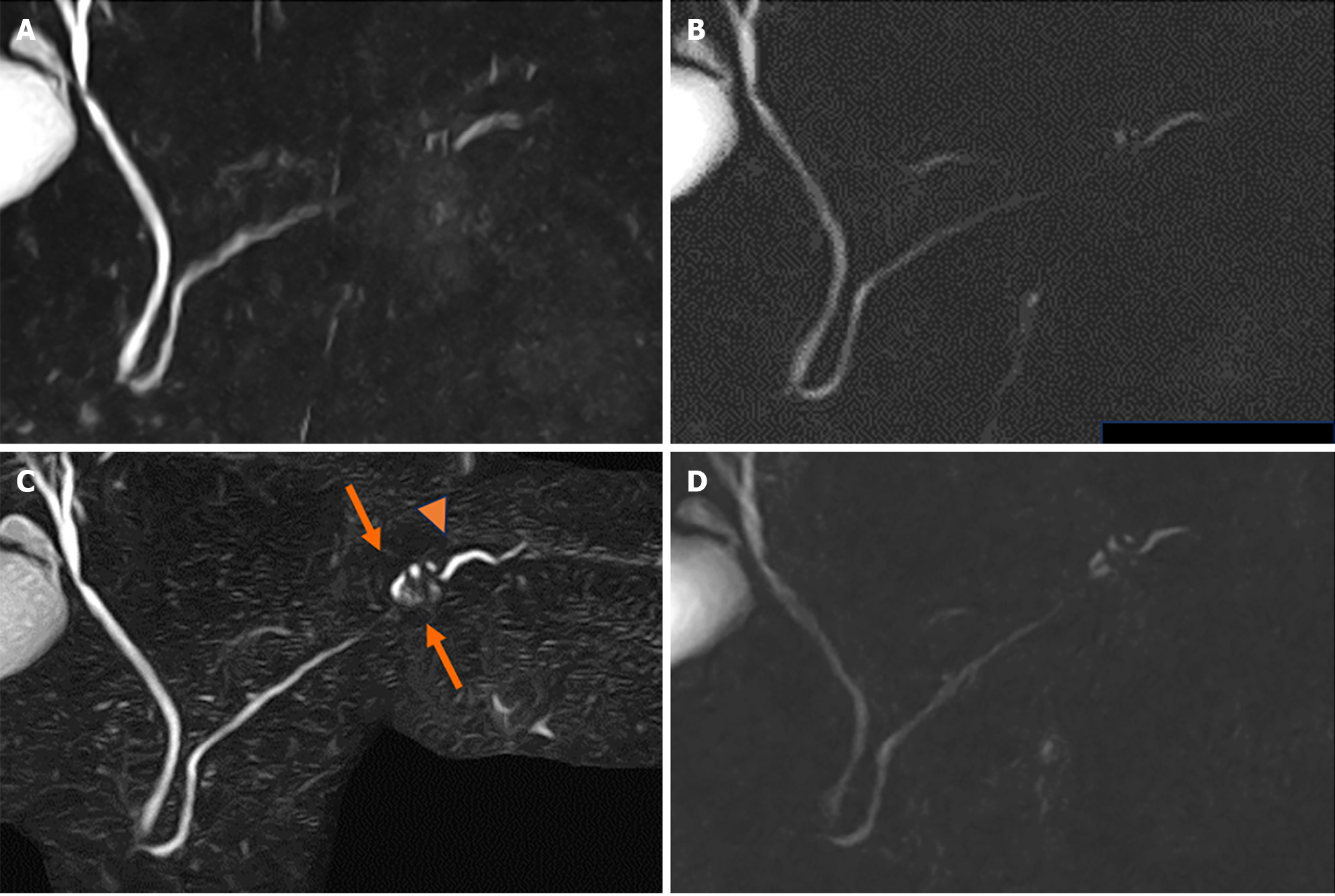
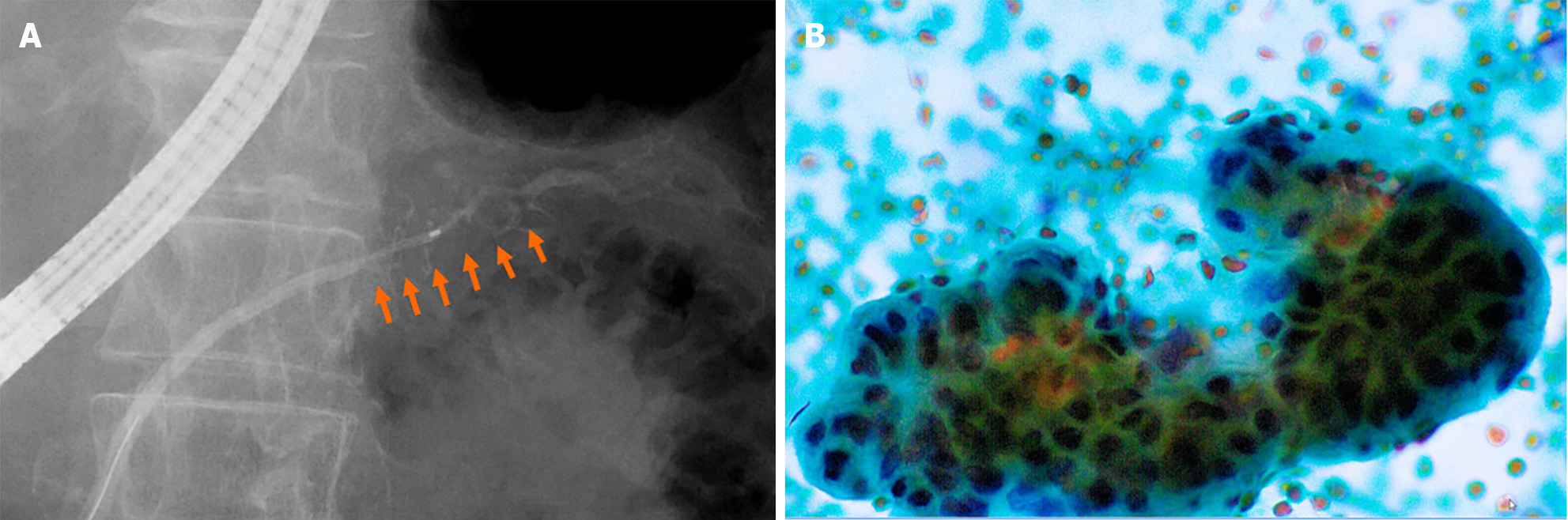
We performed a further examination considering the induction of high-grade PanIN. CE-CT revealed no solid space-occupying lesion, including a delayed enhanced area, but the pancreatic tail cyst grew to 10 mm, and the caudal MPD was slightly dilated (Figures 5). On EUS, the cyst was described as a solid mass (Figure 6A), but contrast-enhanced EUS using Sonazoid showed the mass as a round cystic lesion (Figure 6B). On CT, MRCP, and EUS, we could not find a connection between MPD and the cyst. Endoscopic retrograde cholangiopancreatography (ERCP) revealed that the MPD in the pancreatic tail was irregularly narrowing over a 20-mm range and caudal MPD was slightly dilated (Figure 7A). Serial pancreatic juice aspiration cytology examination (SPACE) revealed adenocarcinomas in three out of four samples (Figure 7B). Whole body CE-CT, positron emission tomography, and magnetic resonance imaging with gadoxetate sodium revealed no PC metastasis.
The patient was diagnosed with high-grade PanIN.
A distal pancreatectomy was performed.
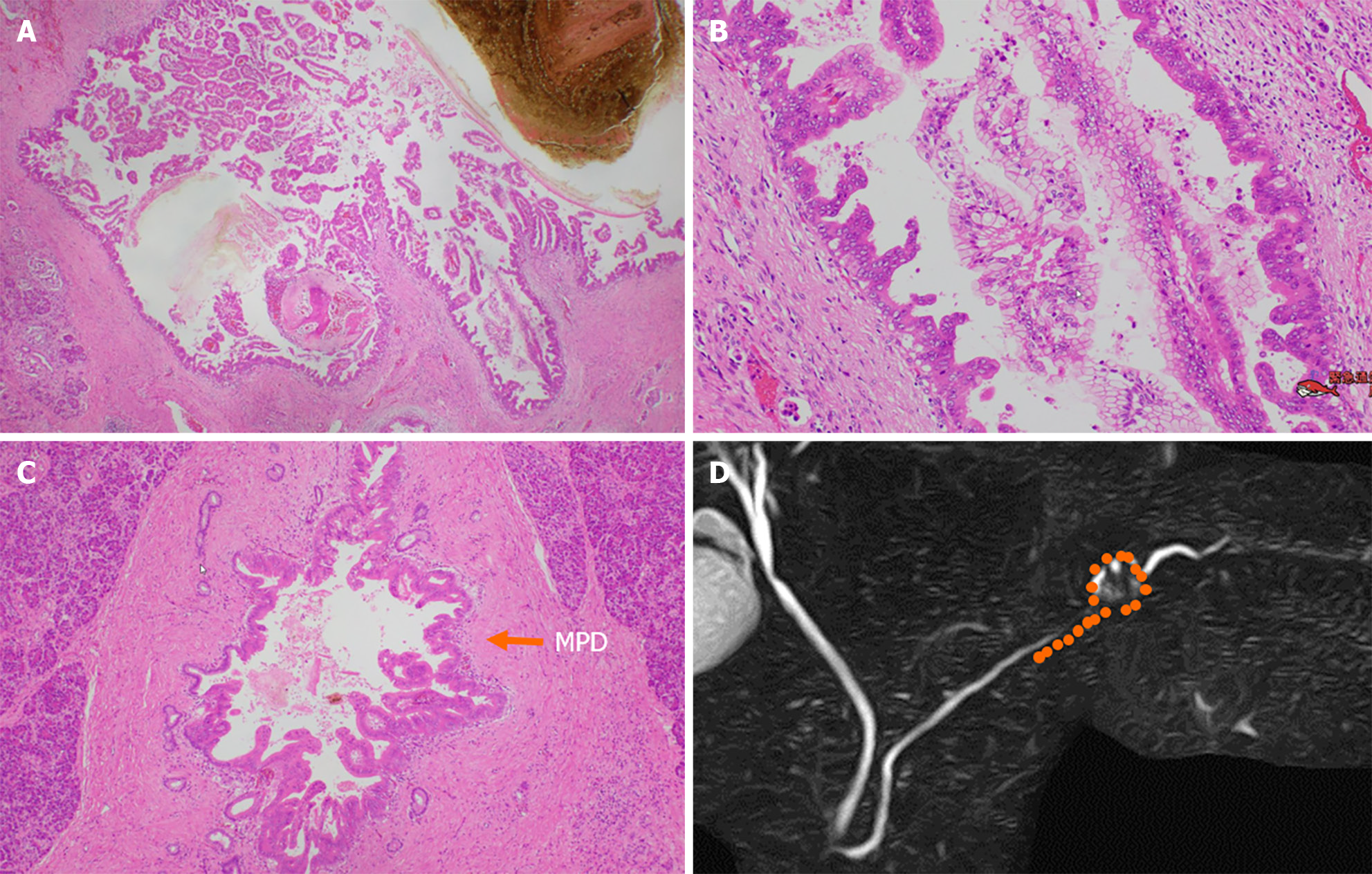
Surgical results included a 10-mm round cyst located in the pancreatic tail and a low-papillary adenocarcinoma (Figure 8A and B) that spread in the cyst wall. Cancer cells had spread to the MPD and branch duct (BD), over a 20-mm range toward the pancreatic body (Figure 8C). Pancreatic parenchyma invasion was not observed, and the final diagnosis of her tumor was high-grade PanIN. The samples demonstrated some low-grade PanINs in the pancreas, with few fibrotic areas around the MPD with high-grade PanIN. Figure 8D shows the distribution of PanIN. The patient was alive without relapse 17 months postoperatively.
We experienced a case of high-grade PanIN, which was effectively diagnosed by course observation with MRCP. Cyst growth and caudal MPD dilatation enabled the SPACE. PC has high malignancy and is frequently detected in progressive situations. Thus, PC is the worst prognostic cancer, with a 5-year survival rate of approximately 7.1% and 10% in Japan and the United States, respectively[1,2]. PanIN is a tumor that develops in a flat or low papillary shape within the pancreatic duct, with highly atypical cases classified as high-grade PanIN and included in PC stage 0. Thus, many physicians have been exerting an effort to detect stage 0 PC. However, the proportion of stages 0 and 1A cases accounted for only 1.7% and 4.1%, respectively[3,4]. High-grade PanIN does not form a mass, and imaging modalities cannot identify the carcinoma; therefore, recently, stage 0 has been diagnosed using pancreatic juice cytology[5,6], focusing on indirect findings, including MPD dilatation and/or stenosis, cyst formation, and focal atrophy of the pancreas[4,7-9]. Fibrosis around the MPD from obstructive pancreatitis by high-grade PanIN or immunological reaction for PanIN was believed to induce MPD stenosis[10].
Clear pancreatic duct stenosis or caudal MPD dilatation detected with any imaging modalities warrants readily recommending a trial of endoscopic retrograde pancreatography (ERP) cytology to the patient. However, the poor delineation of the MPD at relatively long distances by MRCP is a finding observed in normal cases. Pancreatic duct stenosis is frequently detected using MRCP, but which finding should be used to perform ERCP seems to be a problem, which is fraught with the risk of acute pancreatitis.
In recent years, Hanada et al[11] classified high-grade PanIN based on histopathological features. It is categorized into a flat type, in which mainly the flat tumor cells are limited to the BD, and a low papillary type, in which the low papillary tumor cells spread over a long distance from the BD to the MPD. Thus, the reason for MPD stenosis in the flat type is frequently induced by fibrosis around the tumor and the flat type might demonstrate a short length of stenosis and strong caudal MPD dilatation. In comparison to the flat type, the low papillary type induces MPD stenosis by both tumor progression in the MPD and fibrosis around the tumor. Thus, the length of stenosis can be longer than that associated with a flat type due to tumor progression in the MPD. Additionally, the stenosis might be milder in the low papillary type than in the flat type if the low papillary type demonstrates little fibrosis around the tumor. In our case, low papillary progression and little fibrosis around the MPD might have reduced MPD stenosis and caudal MPD dilatation at the first visit.
We collected and performed a review of previous relevant reports. We searched PubMed and Ichushi-web (Japanese) from 2012 to 2022 for cases of high-grade PanIN with detailed information on MRCP and histopathological findings (Table 1). We found 27 cases[8,12-34], including 9 head lesions, 10 body lesions, and 8 tail lesions. Compared to the usual PC, involvement of the body and tail tended to be more frequent. Of the 27 cases, MPD stenosis was observed in 24 cases. Further, all MPD stenosis, caudal MPD dilatation, and caudal cyst findings were present in 13 cases. Notably, not only the caliber of the MPD, but also the caudal cyst, which is a dilated BD, is an important finding in the search for high-grade PanIN. Based on histopathological findings, in 24 cases we found that the cause of MPD stenosis was fibrosis around the MPD. Fibrosis around the stricture and carcinoma in situ proliferation contributed to pancreatic duct stricture in 15 cases, and in four cases it was due to fibrosis alone. Five cases demonstrated almost no fibrosis, and the stenosis may have been caused by the tumor tissue itself. Fibrosis around the pancreatic duct was considered a major factor in stenosis. Among the 21 patients with the low papillary type, 13 demonstrated both fibrosis and carcinoma in situ proliferation in the stenosis area, and four exhibited only fibrosis, indicating the frequent involvement of stenosis. Only three cases were caused by the growth of carcinoma in situ.
| Case number | 27 |
| Sex (M:F) | 17:10 |
| Age (yr), median (range) | 74 (60-83) |
| Localization of tumor | |
| Head:body:tail | 9:10:8 |
| MRCP findings | |
| MPD stenosis | 3 |
| MPD stenosis + caudal MPD dilatation | 5 |
| MPD stenosis + caudal MPD dilatation + caudal cyst formation | 13 |
| Caudal cyst formation | 2 |
| Others | 4 |
| MPD stenosis, yes:no | 24:3 |
| Histological type1 | |
| Flat type:low papillary type | 6:21 |
| Distribution of tumor | |
| MPD:BD:MPD + BD | 10:5:12 |
| Causes of MPD stenosis | |
| Fibrosis around MPD | 4 |
| Tumor itself | 5 |
| Fibrosis around MPD + tumor itself | 15 |
In this case, the cyst expansion over time and the caudal MPD dilatation triggered the discovery of PanIN. Further, the MPD stenosis was thought to be caused by the progression of tumor cells because the tumor tissue was low-papillary and almost no fibrosis was observed in the stenotic part. Papillary hyperplasia may have progressed along the pancreatic duct wall, causing a long and irregular MPD narrowing, which may have taken a long time for the caudal MPD dilation to become clear. Poor MPD visualization by MRCP, as in this case, is a finding that can be observed even in a normal pancreas. However, in this case, PanIN seems to have already existed two years ago. We believe that early diagnosis and treatment will be possible, considering that PanIN caused long and irregular strictures and cases with caudal cysts were numerous.
In this case, there was a challenge to our diagnostic strategy. Recently, fine needle aspiration using EUS (EUS-FNA) has been shown to improve diagnostic accuracy in pancreatic cystic neoplasm (PCN) when differentiating mucinous vs non-mucinous PCN, and malignant vs benign PCN, in cases where the diagnosis by CT or MRI is unclear[35]. We performed ERP, but not EUS-FNA, for the cyst due to concerns of needle tract seeding and peritoneal dissemination by aspiration for a pancreatic tail lesion. In Japan, aspiration for suspicion of cystic neoplasm is typically performed only as clinical research. As such, we need to pay attention to the development of a diagnostic strategy for PCN.
Here, we report a case of high-grade PanIN, which was diagnosed by continuous cytology of pancreatic juice based on changes in MRCP findings over time. This case was considered as very suggestive when considering an early diagnosis system for PC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang JW, China S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, Maguchi H, Yanagisawa A, Tanaka M. A digest of the Pancreatic Cancer Registry Report 2007. Suizo. 2008;23:105-123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15317] [Article Influence: 3063.4] [Reference Citation Analysis (4)] |
| 3. | Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, Maguchi H, Yanagisawa A, Tanaka M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 4. | Kanno A, Masamune A, Hanada K, Maguchi H, Shimizu Y, Ueki T, Hasebe O, Ohtsuka T, Nakamura M, Takenaka M, Kitano M, Kikuyama M, Gabata T, Yoshida K, Sasaki T, Serikawa M, Furukawa T, Yanagisawa A, Shimosegawa T; Japan Study Group on the Early Detection of Pancreatic Cancer (JEDPAC). Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | Iiboshi T, Hanada K, Fukuda T, Yonehara S, Sasaki T, Chayama K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas. 2012;41:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Nakaizumi A, Tatsuta M, Uehara H, Takenaka A, Iishi H, Kitamra T, Ohigashi H, Ishikawa O, Okuda S, Wada A. Effectiveness of the cytologic examination of pure pancreatic juice in the diagnosis of early neoplasia of the pancreas. Cancer. 1995;76:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Hanada K, Okazaki A, Hirano N, Izumi Y, Teraoka Y, Ikemoto J, Kanemitsu K, Hino F, Fukuda T, Yonehara S. Diagnostic strategies for early pancreatic cancer. J Gastroenterol. 2015;50:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Kikuyama M, Hanada K, Ueki T. Pancreatic carcinoma in situ presenting prominent fatty change of the pancreas body on CT: expression from 3 cases. Suizo. 2015;30:626-632. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Nakahodo J, Kikuyama M, Nojiri S, Chiba K, Yoshimoto K, Kamisawa T, Horiguchi SI, Honda G. Focal parenchymal atrophy of pancreas: An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology. 2020;20:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Ohtsubo K, Mouri H, Yamashita K, Makino I, Tajima H, Ohta T, Inoue D, Gabata T, Ikeda H, Zen Y, Watanabe H. [Resection of carcinoma in situ and minimally invasive carcinoma of the pancreas in a patient presenting with stenosis and post-stenotic dilatation of the main pancreatic duct on endoscopic ultrasonography and positive serial pancreatic juice aspiration cytology]. Nihon Shokakibyo Gakkai Zasshi. 2017;114:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Hanada K, Fukuhara M, Minami T, Yano S, Ikemoto J, Shimizu A, Kurihara K, Okuda Y, Ikeda M, Yokode M, Abe T, Yonehara S, Yanagisawa A. Pathological Features and Imaging Findings in Pancreatic Carcinoma In Situ. Pancreas. 2021;50:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Yuge R, Hanada K, Iiboshi T, Sagami S, Fukumoto A, Onogawa S, Hirano N, Amano H, Hino F, Obayashi M, Chayama K, Fukuda T, Yonehara S, Sasaki T. A case of ductal carcinoma in situ of the pancreas initially suggested by the slightly dilatation of the main pancreatic duct. Hepato-Biliary-Pancreatic Imaging. 2012;14:72-77. [DOI] [Full Text] |
| 13. | Shindo H, Fukasawa M, Takano S, Kadokura M, Takahashi Ei, Yokota Y, Hirose S, Sato T, Kawaida H, Itakura J, Fujii H, Ohishi N, Enomoto N. A case of carcinoma in situ of the pancreas concomitant with branch duct intraductal papillary mucinous neoplasm. Suizo. 2014;29:742-748. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kato S, Zakimi M, Yamada K, Chinen K, Kubota T, Arashiro M, Kikuchi K, Murakami T, Kunishima F. Efficacy of repeated cytology of pancreatic juice obtained by endoscopic nasopancreatic drainage tube for early diagnosis of pancreatic cancer: a case series including a case of carcinoma in situ. Clin J Gastroenterol. 2015;8:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Takano Y, Nagahama M, Yamamura E, Maruoka N, Yokomizo K, Mizukami H, Tanaka J, Ohike N. A case of concurrent pancreatic intraepithelial neoplasia and type 1 autoimmune pancreatitis with marked pancreatic duct dilatation. Clin J Gastroenterol. 2016;9:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Ito T, Usui M, Iizawa Y, Kato H, Murata Y, Tanemura A, Kuriyama N, Azumi Y, Kishiwada M, Mizuno S, Sakurai H, Isaji S. A case of carcinoma in situ of pancreas detected by acute pancreatitis and atrophy of pancreas body. Kan Tan Sui Chiryo Kenkyukaisi. 2016;14:56-62. |
| 17. | Kakizaki Y, Makino N, Ando Y, Matsuda A, Ishizawa T, Saito Y, Suto A, Yamakawa M, Kimura W, Ueno Y. [A case of pancreatic intraepithelial neoplasia-3 with autoimmune pancreatitis]. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Kaneko J, Matsushita M, Tanaka Y, Nagasawa M, Ishibashi K, Watanabe S, Kaneyama H, Morishita S, Ashizawa N, Kamimura K, Tachibana M. Evaluation of EUS and serial pancreatic juice aspiration cytological examination in a case of pancreatic carcinoma in situ. Suizo. 2017;32:821-828. [DOI] [Full Text] |
| 19. | Murabayashi T, Koshita S, Masu K, Kanno Y, Ogawa T, Okada T, Oikawa M, Noda Y, Sawai T, Ito K. [Detection of small pancreatic cancer during surveillance of the main pancreatic duct stenosis]. Nihon Shokakibyo Gakkai Zasshi. 2019;116:99-108. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Inomata N, Kobayashi T, Masuda A, Masuda S, Ashina S, Gonda M, Abe S, Yamakawa K, Tsujimae M, Tanaka T, Yamada Y, Tanaka S, Kakihara M, Nakano R, Ikegawa T, Sakai A, Shiomi H, Kannzawa M, Toyama H, Itoh T, Fukumoto T, Kodama Y. A case of high-grade pancreatic intraepithelial neoplasia diagnosed based on focal pancreatic parenchymal atrophy after acute pancreatitis. Clin J Gastroenterol. 2020;13:1338-1342. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Kuruma S, Kikuyama M, Chiba K, Yoshimoto K, Kamisawa T, Honda G, Horiguchi S, Nakahodo J. Carcinoma in situ of the pancreas with pancreatic duct stricture persistent for 4 years diagnosed by serial pancreatic juice aspiration cytologic examination (SPACE). Clin J Gastroenterol. 2020;13:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kikuyama M, Nakahodo J, Chiba K, Tabata H, Kamisawa T, Horiguchi S, Honda G, Oome Y, Kawamoto Y, Yoshida N. Focal Pancreatic Parenchymal Atrophy. Tan to Sui. 2020;41:1387-1397. |
| 23. | Kawaguchi S, Toda T, Manabe A, Ishiguro T, Matsuda M, Terada S, Endo S, Shirane N, Muramatsu A, Kanemoto H. High-Grade Pancreatic Intraepithelial Neoplasia with Pancreas Divisum Diagnosed Using Serial Pancreatic-Juice Aspiration Cytologic Examinatioii Through the Minor Papilla. Tan to Sui. 2021;42:255-258. |
| 24. | Tabata H, Kikuyama M, Nakahodo J, Watanabe K, Chiba K, Kamisawa T, Suzuki M, Inui T, Horiguchi S. Intradisciplinary Approach to Detect Early Pancreatic Cancer. Tan to Sui. 2021;42:401-407. |
| 25. | Toyonaga H, Nakamura R, Ando R, Shimizu T, Ishii T, Kin T, Hayashi T, Takahashi K, Katanuma A. Today’s conference: Early diagnosis of biliary and pancreatic cancer. Shokaki Clinical Update. 2021;3:82-93. |
| 26. | Kimura K. Early Detection of Pancreatic Cancer:Specific Imaging Features and Pathological Findings of Pancreatic Intraepithelial Neoplasms. IRYO. 2022;76:240-244. |
| 27. | Ito H, Kawaguchi Y, Kawashima Y, Maruno A, Ogawa M, Hirabayashi K, Mine T. A case of pancreatic intraepithelial neoplasia that was difficult to diagnose preoperatively. Case Rep Oncol. 2015;8:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Kawanishi A, Hirabayashi K, Kono H, Takanashi Y, Hadano A, Kawashima Y, Ogawa M, Kawaguchi Y, Yamada M, Nakagohri T, Nakamura N, Mine T. A Serous Cystic Neoplasm of the Pancreas Coexisting with High-Grade Pancreatic Intraepithelial Neoplasia Mimicking an Intraepithelial Papillary Mucinous Neoplasm: A Case Report. Case Rep Oncol. 2017;10:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Yamao K, Takenaka M, Nakai A, Omoto S, Kamata K, Minaga K, Miyata T, Imai H, Sakurai T, Watanabe T, Nishida N, Matsumoto I, Takeyama Y, Chikugo T, Kudo M. Detection of High-Grade Pancreatic Intraepithelial Neoplasia without Morphological Changes of the Main Pancreatic Duct over a Long Period: Importance for Close Follow-Up for Confirmation. Oncology. 2017;93 Suppl 1:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Mori T, Yamaguchi A, Kuwai T, Kouno H, Matsuura N, Toyota N, Nakahira S, Kuraoka K, Kohno H. Carcinoma in situ of the pancreas with fibrosis area around the carcinoma: A case report. Medicine (Baltimore). 2020;99:e22645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Inoue M, Hakoda K, Sawada H, Hotta R, Ohmori I, Miyamoto K, Toyota K, Sadamoto S, Takahashi T. Synchronous gastric cancer and carcinoma in situ of the pancreas. Clin Case Rep. 2021;9:e04892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Miyata T, Takenaka M, Omoto S, Kamata K, Minaga K, Yamao K, Imai H, Kudo M. A Case of Pancreatic Carcinoma in situ Diagnosed by Repeated Pancreatic Juice Cytology. Oncology. 2017;93 Suppl 1:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Yoshizumi A, Hatori T, Miyazaki M, Itano O, Kato A, Imai S, Higuchi H, Aida S. High-grade pancreatic intraepithelial neoplasm with a mass and a high level of serum elastase 1: a case report. Suizo. 2020;35:568-574. [DOI] [Full Text] |
| 34. | Tanaka S, Miyamoto K, Sugimoto A, Nishiyama H, Nakagawa T, Hara A, Yoshino C, Takakura R, Uehara H. A Case of Stage 0 Pancreatic Adenocarcinoma (High-grade PanIN) Incidentally Detected on Routine Screening Ultrasonography. Ningen Dock International. 2021;8:59-63. [DOI] [Full Text] |
| 35. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |