Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1467
Peer-review started: November 24, 2023
First decision: January 9, 2024
Revised: January 16, 2024
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 16, 2024
Processing time: 109 Days and 6 Hours
Malignant triton tumors (MTTs) comprise a subgroup of malignant peripheral nerve sheath tumors (MPNSTs) that exhibits rhabdomyosarcomatous differentiation and follow an aggressive course. MTTs are primarily located along peripheral nerves. Cases of MTTs in the abdominal wall have not been reported. MTT has a poorer prognosis than classic MPNSTs, and accurate diagnosis necessitates a keen understanding of the clinical history and knowledge of its differential diagnosis intricacies. Treatment for MTTs mirrors that for MPNSTs and is predominantly surgical.
A 49-year-old woman presented with a subcutaneous mass in her lower abdo
An abdominal MTT was efficaciously treated with extensive excision and abdominal wall reconstruction, eliminating the need for postoperative radiotherapy.
Core Tip: Malignant triton tumor is an uncommon condition characterized by a poor prognosis. Cases emerging in the abdominal wall are especially rare. Swift differential diagnosis and comprehensive surgical removal play a pivotal role in management. In this instance, we managed to treat the patient without the necessity for postoperative radiotherapy, thanks to a wide excision complemented by suitable reconstruction.
- Citation: Yang HJ, Kim D, Lee WS, Oh SH. Malignant triton tumor in the abdominal wall: A case report. World J Clin Cases 2024; 12(8): 1467-1473
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1467.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1467
Malignant peripheral nerve sheath tumors (MPNSTs) constitute approximately 5%-10% of soft tissue sarcomas and are believed to originate from Schwann cells or adjacent cells with perineural differentiation[1]. Malignant triton tumor (MTT), a subtype of MPNST displaying rhabdomyoblastic differentiation, presents in two primary forms: sporadic, and neurofibromatosis type 1 (NF-1)-associated forms. MTT represents approximately 5% of all MPNSTs, and its nomen
MTTs are predominantly located along peripheral nerves, frequently near the spine, in the head and neck region, or within the upper and lower extremities. Other uncommon sites include the buttocks, viscera, retroperitoneum, mediastinum, and intracranial locations such as the parieto-occipital lobe, lateral ventricle, and cerebellopontine angle[2-6]. The extreme rarity of this tumor in the abdominal wall motivated this case report.
The presentation, progression, and response to treatment of MTTs remain largely undefined, as published data predominantly consist of case reports and limited patient series. MTT displays aggressive biological behavior, with various factors like location, size, and tumor stage potentially impacting patient prognosis and survival. Non-extremity MTTs often result in a poor prognosis. The metastasis rate for MTT stands at 31.4% and correlates with reduced survival in its sporadic form[7]. Adjuvant radiotherapy plays a crucial role in treatment, with complete surgical excision of MTT providing a survival advantage[8]. However, no studies have explored the relationship between the quality of surgical margins and treatment outcomes.
To shed light on the progression and prognosis of this infrequent neoplasm in the abdominal wall, we detail a case of sporadic MTT originating in the abdominal wall with a distinct prognosis.
A 49-year-old woman presented with a subcutaneous mass in her lower abdominal wall alongside a previous surgical scar.
A subcutaneous mass had enlarged over the 3-4 months prior to consultation.
Approximately 5 years before this consultation, the patient had undergone a radical hysterectomy and concurrent chemo-radiotherapy (135 Gy) for cervical cancer.
The patient did not display café-au-lait spots or cutaneous neurofibromas. Both her medical and family histories related to NF-1 were unremarkable.
A firm, painless subcutaneous mass measuring approximately 1 cm was identified. No inguinal lymphadenopathy was detected upon palpation.
There were no specific findings.
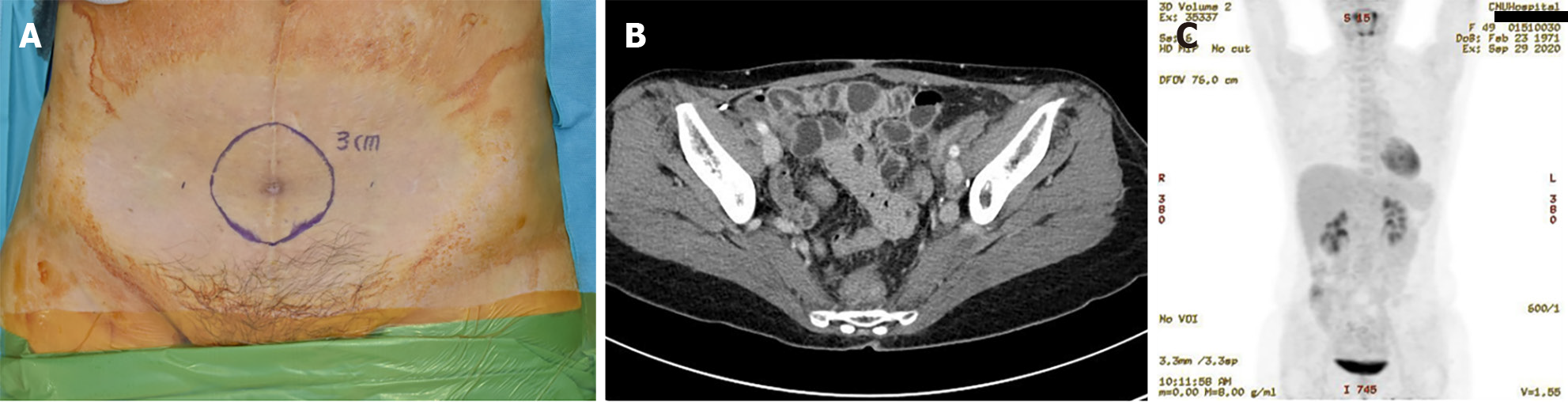
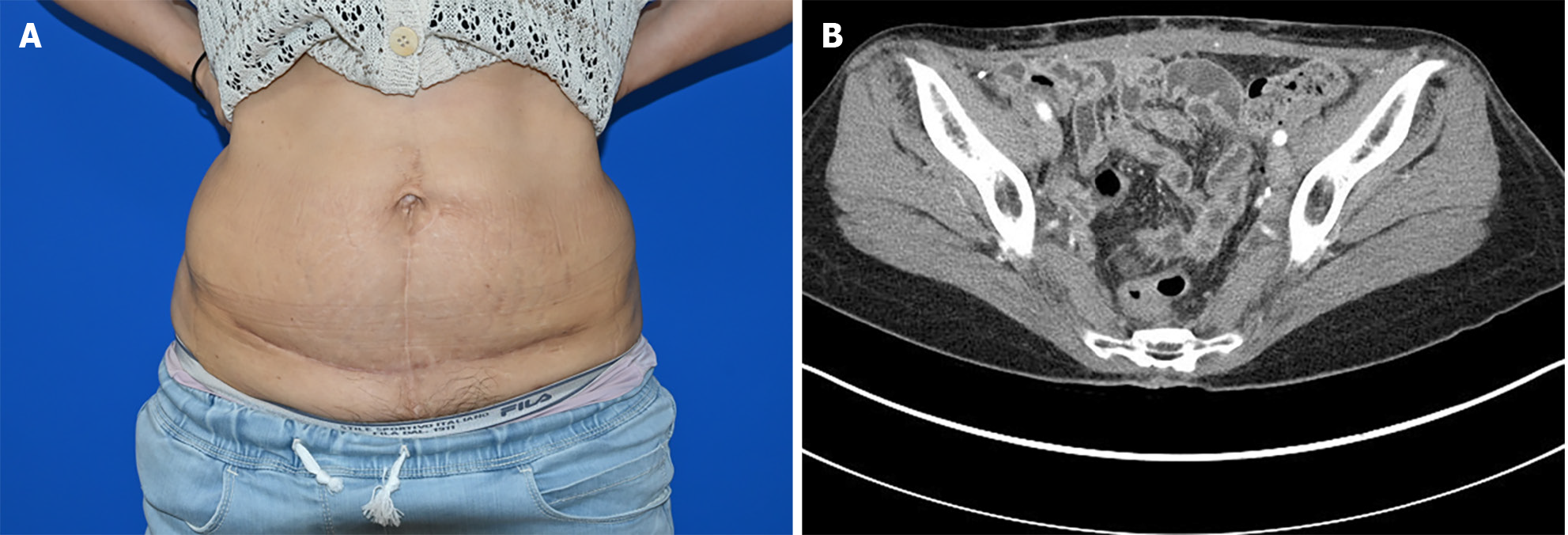
Abdominal computed tomography (CT) showed a 1.3 cm midline mass in the lower abdomen with infiltration into the rectus abdominis muscle. Comprehensive CT scans (encompassing the chest, abdomen, and pelvis), combined with positron emission tomography/CT and colonoscopy, found no signs of metastasis (T1N0M0) (Figure 1).
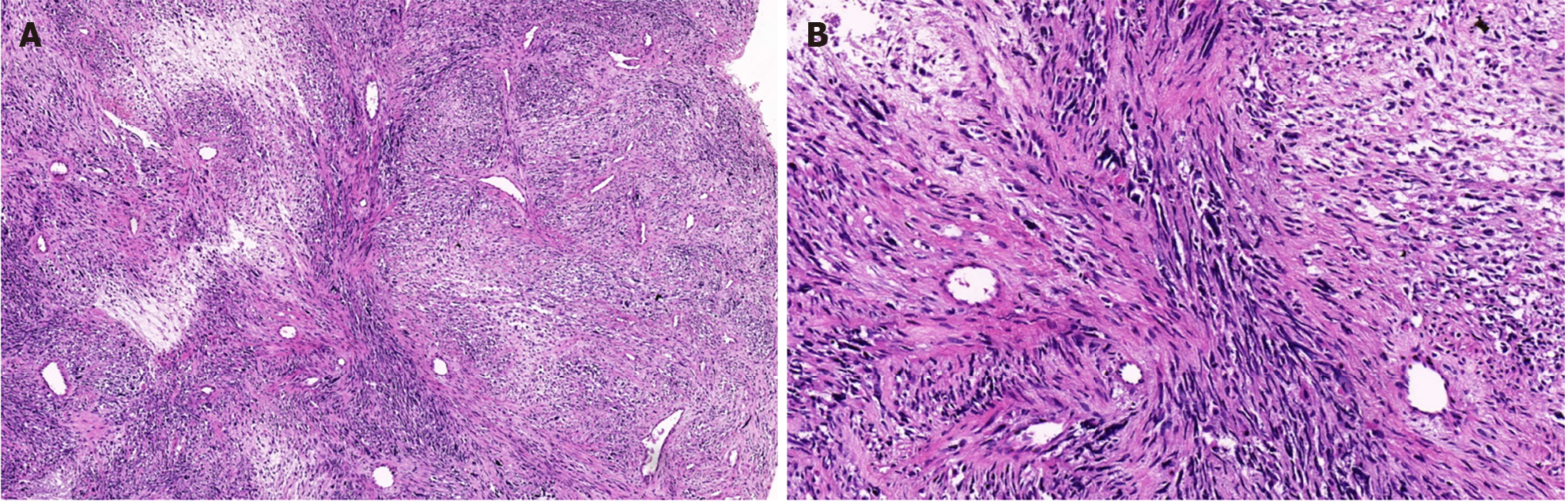
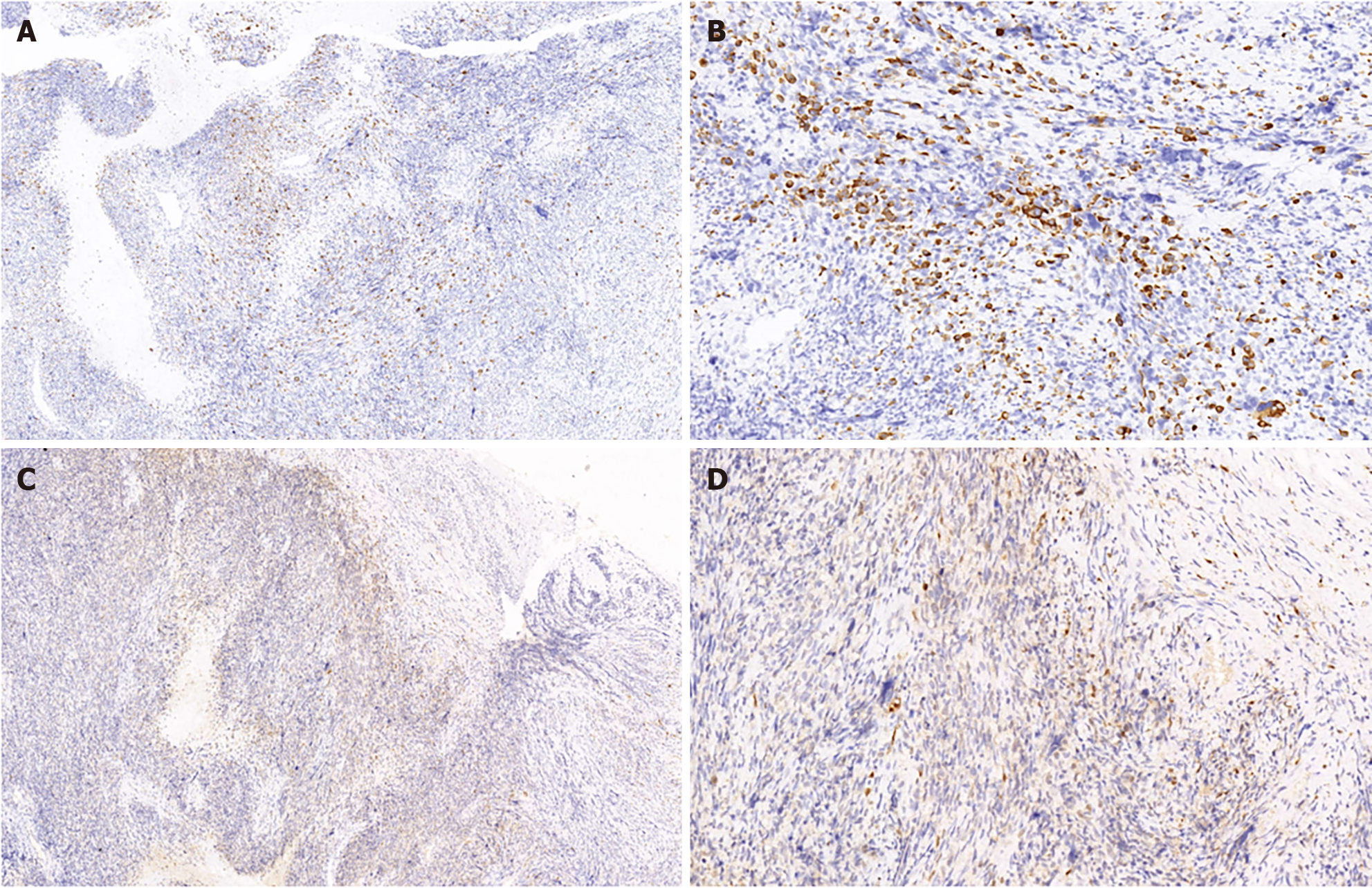
An incisional biopsy identified sporadic MTT in the lower abdomen. Immuno-histochemical analysis revealed that the wavy spindle cells were diffusely and intensely positive for S-100 protein, confirming their neurogenic lineage. The large pleomorphic cells displayed myogenic differentiation, as evidenced by strong positivity for desmin and moderate positivity for myoglobin. These microscopic findings coupled with the expression of the mentioned markers solidified the MTT diagnosis (Figures 2 and 3).
Combined with the patient’s medical history, the final diagnosis was MTT in the lower abdomen.
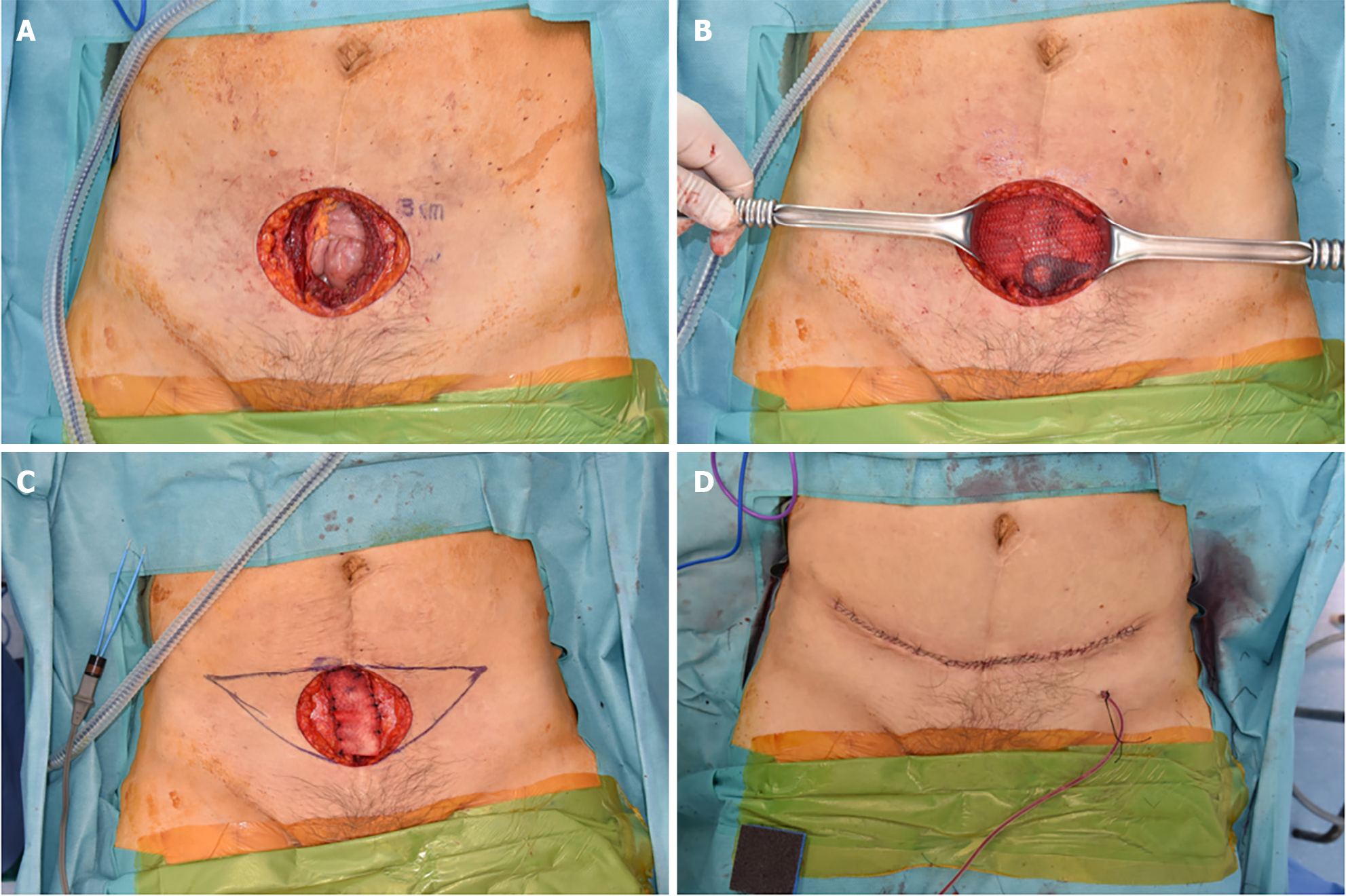
Under general anesthesia, a wide surgical excision of the mass ensuring a 3 cm margin that included the peritoneum was performed. The resected margins were found to be clear. Following this, the general surgeon employed a technique akin to the open peritoneal onlay mesh method to prevent herniation of abdominal wall defects. For reinforcement, a composite mesh (Parietene composite mesh; COVIDIEN, Dublin, Ireland) was introduced and anchored to both pelvic symphyses. The peritoneum was subsequently advanced and repaired on both sides. The anterior fascia's deficit was addressed using an acellular dermal matrix (SureDerm; Hans BioMed, Seoul, South Korea) with further reinforcement and rectification. For minimal effects of scarring, the skin defect was excised in the pattern of a mini-abdominoplasty and was sutured in layers (Figure 4).
The post-operative period was uneventful, and annual follow-up CT scans showed no recurrence or metastasis. Three years post-diagnosis, the patient remains alive and shows no sign of recurrence (Figure 5).
MTT presents in two principal forms: sporadic and associated with NF-1. The sporadic form must be distinguished from other spindle cell sarcomas, such as fibrosarcoma, malignant fibrous histiocytoma, and rhabdomyosarcoma, during differential diagnosis[2]. The most widely accepted view suggests that MTT originates from Schwann or ectodermal cells of the neural crest. More than 50% of patients with MTT develop NF-1, with the remainder being disseminated[5]. MTT associated with NF-1 is notably more prevalent in men, particularly in younger age groups (28-36-years-old). The sporadic form tends to appear in older women (40-44-years-old). Divergent differentiation strongly suggests NF-1, and 57% of patients with MTT, which manifests after a prolonged latent period of 10-20 years, have NF-1[9,10]. As many as 6% of malignant or atypical peripheral nerve tumors inclusive of MTT are associated with prior radiotherapy[11], a crucial factor highlighted in our case report. In this instance, the tumor emerged sporadically in a 49-year-old woman and was linked to prior radiotherapy.
The MTT diagnosis chiefly rests on the histopathological and immune-histochemical attributes of the tumor. There is consensus that an MPNST diagnosis can be rooted in morphological features, bolstered by S-100 protein expression. Morphological characteristics include alternating hypocellular and hypercellular regions, the presence of thin wavy comma-shaped or bullet-shaped nuclei in hypocellular areas, nuclear palisading, nerve whorls or tactoid bodies resembling Wagner-Meissner corpuscles, prominent thick-walled vasculature, and heterologous elements like rhabdomyoblasts, cartilage, and bone. Such tumors exhibit focal positivity for the S-100 protein in 50%-90% of cases, pointing to a nerve sheath derivation. Rhabdomyoblasts test positive for desmin, myogenin, and myo-D1[2].
MTTs typically have an unfavorable prognosis depending on the tumor location, grade, and the completeness of surgical margins. Cases located in the head, neck, and extremities are less intricate than those found in other sites, like the buttocks. Past research indicates that in conjunction with NF-1, MTT has a worse prognosis relative to its sporadic counterparts[9,12]. There was no discernible difference between MTT and MPNST groups regarding rates of local recurrence or metastasis. Nonetheless, the 5-year survival rate for MTTs stands at a mere 5%-15% compared to 50%-60% for MPNSTs. Several factors underlie these disparate survival rates. MTT proliferates swiftly and, in its nascent stages, is susceptible to local recurrence and hematogenous metastases. In comparison to MPNST, MTT often arises in more advanced-aged patients, predominantly manifests in the trunk, and results in larger tumors. Furthermore, patients diagnosed with MTT experience a reduced metastasis-free interval and diminished overall survival. Thus, MTT's aggressive nature sets it apart from MPNST, underscoring the significance of differential diagnosis[13]. Although the association of genomic alteration and prognosis of MTT has not been fully investigated, there have been a few studies that evaluated such a relationship; worse survival was associated with tumors having gains and loss of particular genes, suggesting promise for understanding this disease[14-16].
The treatment approach for MTT mirrors that of MPNST and is predominantly surgical. The Oncology Consensus Group endorses postoperative radiotherapy as a standard treatment protocol for MTT. Typically, radical excision followed by high-dose radiotherapy is employed, and recent studies hint at the potential of neoadjuvant therapy and adjuvant chemotherapy in eliminating micro-metastases. However, this treatment remains controversial. A gluteal MTT case underwent full resection with postoperative chemoradiotherapy and there were no recurrence symptoms for 4 years after treatment[17]. In contrast, another case adopted the same treatment for a patient with rectal MTT, who passed away under palliative therapy and survived only 9 mo[4]. Therefore, it is necessary to seek effective methods for MTT treatment[6]. In the discussed case, the MTT developed post-radiotherapy, a modality that can induce significant side effects when aimed at the abdomen[18]. Given the comprehensive nature of the surgical excision of the abdominal wall tumor, postoperative radiotherapy was deemed unnecessary. While no recurrence or metastasis has been observed, consistent follow-up is imperative, especially since the post-surgery window is under 5 years.
MTT is a rare condition with an unfavorable prognosis. Prompt differential diagnosis and thorough surgical removal are crucial. However, in the presented case, extensive surgical removal was deemed sufficient without the need for postoperative radiotherapy.
MTT is an uncommon condition characterized by a poor prognosis. Cases emerging in the abdominal wall are especially rare. Swift differential diagnosis and comprehensive surgical removal play a pivotal role in management. In this instance, we managed to treat the patient without the necessity for postoperative radiotherapy, thanks to a wide excision complemented by suitable reconstruction.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mei LC, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yu HG
| 1. | Woodruff JM, Chernik NL, Smith MC, Millett WB, Foote FW Jr. Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant "Triton" tumors). Cancer. 1973;32:426-439. [PubMed] |
| 2. | Tripathy K, Mallik R, Mishra A, Misra D, Rout N, Nayak P, Samantray S, Rath J. A Rare Malignant Triton Tumor. Case Rep Neurol. 2010;2:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Kabir S, Kapetanakis EI, Shabbo F. Intracardiac malignant Triton tumor: a first presentation. Ann Thorac Surg. 2010;89:968-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Bins RB, Pinzon CE, da Silva Pereira LD, Bertuol M, Isolan PMBS, Takamatu EE. Malignant triton tumor of the kidney in a child: A case report. Int J Surg Case Rep. 2021;85:106252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Liu M, Bian J. Malignant Triton tumor of the retroperitoneum in a male unaffected by neurofibromatosis 1: A case report and literature review. Asian J Surg. 2022;45:2766-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Jiang TL, Liu Y, Ji B, Sheng DH, He QC, Song JC, Wang G, Wang K. Malignant triton tumor of uterus: A case report and literature review. J Clin Ultrasound. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Omar T, Raslan H, El Sheikh S, Rizq M, Draz A. Low-grade malignant triton tumor of the neck: a case report and review of the literature. Case Rep Pathol. 2014;2014:674094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Tsagozis P, Gaston CL, Haglund F, Balko J, Sumathi V, Grimer R, Parry M. The importance of surgical margins in malignant Triton tumour of the trunk and extremities. Oncol Lett. 2021;21:120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Brooks JS, Freeman M, Enterline HT. Malignant "Triton" tumors. Natural history and immunohistochemistry of nine new cases with literature review. Cancer. 1985;55:2543-2549. [PubMed] [DOI] [Full Text] |
| 10. | Stasik CJ, Tawfik O. Malignant peripheral nerve sheath tumor with rhabdomyosarcomatous differentiation (malignant triton tumor). Arch Pathol Lab Med. 2006;130:1878-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Victoria L, McCulloch TM, Callaghan EJ, Bauman NM. Malignant triton tumor of the head and neck: A case report and review of the literature. Head Neck. 1999;21:663-670. [PubMed] [DOI] [Full Text] |
| 12. | Kudo M, Matsumoto M, Terao H. Malignant nerve sheath tumor of acoustic nerve. Arch Pathol Lab Med. 1983;107:293-297. [PubMed] |
| 13. | Mae K, Kato Y, Usui K, Abe N, Tsuboi R. A case of malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation: malignant triton tumor. Case Rep Dermatol. 2013;5:373-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573-8578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 439] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Yang J, Ylipää A, Sun Y, Zheng H, Chen K, Nykter M, Trent J, Ratner N, Lev DC, Zhang W. Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin Cancer Res. 2011;17:7563-7573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Ghafouri RS, Hakim NM, Konstantinidis IT, Kafchinski L, Philipovskiy A. A Rare Case of Progressive Malignant Triton Tumor With Rare Somatic Mutation in TSC2 Gene. Anticancer Res. 2021;41:3029-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Merter A, Başarır K, Yıldız Y, Sağlık Y. Malignant triton tumor of the gluteal region in a patient unaffected by neurofibromatosis: A case report. Acta Orthop Traumatol Turc. 2018;52:236-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Shadad AK, Sullivan FJ, Martin JD, Egan LJ. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |