Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1437
Peer-review started: October 16, 2023
First decision: January 2, 2024
Revised: January 15, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 16, 2024
Processing time: 147 Days and 14.3 Hours
Our study contributes to the further understanding of the mechanism of foot reflexology. Foot reflexology has been reported to affect hearing recovery, but no physiological evidence has been provided. This lack of evidence hampers the acceptance of the technique in clinical practice.
A girl was taken to North Sichuan Medical University Affiliated Hospital for a hearing screen by her parents. Her parents reported that her hearing level was the same as when she was born. The girl was diagnosed with sensorineural hearing loss (SNHL) by a doctor in the otolaryngology department. After we introduced the foot reflexology project, the parents agreed to participate in the experiment. After 6 months of foot reflexology treatment, the hearing threshold of the girl recovered to a normal level, below 30 dB.
Foot reflexology should be encouraged in clinical practice and for families of infants with SNHL.
Core Tip: Foot reflexology has been found to aid the recovery of hearing ability in infants with sensorineural hearing loss (SNHL). Previous studies have shown that foot reflexology can affect fatigue, sleep, and pain. To our knowledge, this study is the first to report that foot reflexology can improve the hearing ability of infants with SNHL and provide physiological evidence of how foot reflexology affects hearing ability through analysis of functional connectivity of the brain.
- Citation: Lee YJ, Chen MQ, Dong J. Effect of foot reflexology on an infant with sensorineural hearing loss: A case report. World J Clin Cases 2024; 12(8): 1437-1441
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1437.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1437
Previous studies have established the benefits of reflexology in many diseases, such as relieving anxiety in patients who underwent coronary artery bypass surgery[1] and improving auditory processing[2]. However, the physiological mechanism of foot reflexology is unclear. In the present study, we aimed to investigate the neural substrates of foot reflexology in an infant with sensorineural hearing loss (SNHL) via comparison with control (Table 1).
| Sex | Age (month) | Left ear (dB) | Right ear (dB) |
| Male | 11 | 90 | 80 |
| Male | 6 | 80 | 75 |
| Male | 7 | 70 | 90 |
| Male | 6 | 60 | 50 |
| Male | 6 | 95 | 100 |
| Male | 8 | 60 | 80 |
Specifically, alterations in the brain connectivity of auditory and language areas induced by foot reflexology were detected by covariance analysis of resting-state functional magnetic resonance imaging (fMRI) data.
Compared with covariance analysis, traditional task-induced analysis may detect only a subset of a specific neural system and may underestimate the size and number of areas involved in task performance[3,4]. In the present study, we employed resting-state MRI covariance analysis. By calculating the covariance of each voxel in reference to the time course of a selected brain region, it is possible to detect the neurons connected to the selected region.
Links between foot reflex areas and brain areas have been established[3]. Wattanaruangkowit et al[5] used fMRI to examine the effect of foot reflexology on smoking cessation. They observed changes in brain regions correlated with foot stimulation, especially the precentral gyrus of the frontal lobe and the postcentral gyrus of the parietal lobe.
Previous studies have shown that the effects of behavioural treatment on individual subjects can be assessed by fMRI[6-8]. For instance, the study by Zhao et al[8] (2019) assessed the effects of transcutaneous auricular vagus nerve stimulation in treating poststroke insomnia. In this study, our experimental design was based on the paradigm of fMRI case studies.
The language network involves two pathways: the dorsal pathway and the ventral pathway[9]. The two pathways involve different hubs, such as the frontal cortex, temporal cortex, and occipital cortex. The potential therapeutic effects of foot stimulation on patients with SNHL are hypothesized to cause changes in the language network, such as the frontal cortex and temporal cortex.
The patient’s hearing threshold was abnormal.
The girl did not pass the hearing screen test at birth.
The girl failed to pass the first hearing screen test when she was born. At the age of 1- month old, she failed again. When she was 3-month old, our Hearing Screening Center of North Sichuan Medical College Affiliated Hospital for a confirmatory test with auditory brainstem response (ABR). ABR test reported that the girl hearing threshold was still abnormal.
Family members did not suffer hearing loss.
Pure tone audiometry and ABR test.
Before foot reflexology was performed on the girl, fMRI test was performed. Before foot reflexology, the girl received rest-state fMRI of assessment as a baseline (T0). After 6-month stimulation, the girl underwent third assessment (T1). All Images data are acquired on A 3.0 T GE MR750 Discovery with a 32-channel were sedated. Because of big noise in the scanner, foam padding was used to alleviate noise. After the structural images were obtained, the functional images were acquired with an echo-planar imaging sequence. The sequence parameters: 30 contiguous slices with a slice thickness = 4 mm, repetition time = 2000 ms, echo time =30 ms, flip angle = 90°, field of view = 24 cm2 × 24 cm2, data matrix = 64 × 64, and total volumes = 200.
SNHL.
Foot reflexology treatment. The girl received 30 min of foot stimulation each weekday, with pressure applied to the left and right halluxes by the same reflexologist. The girl underwent 24 wk of foot stimulation in total.
The patient’s hearing threshold recovered to a normal level. Now, the girl can speak as well as her peers.
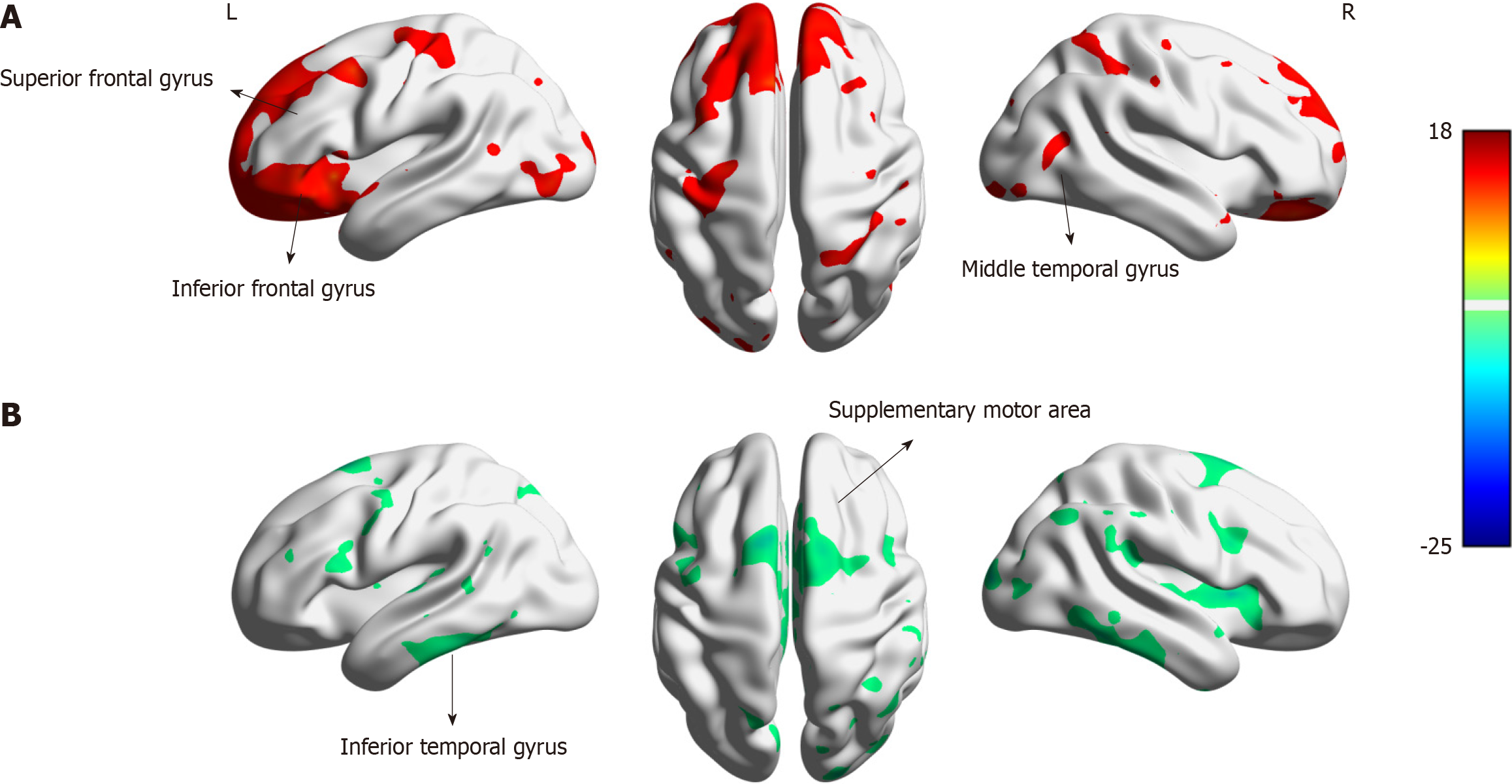
This study aimed to assess the effects of foot reflexology on an infant with SNHL using resting-state fMRI (rs-fMRI). Rs-fMRI is safe and reliable for predicting cognitive phenotypes and measuring foot reflexology responses[3]. Compared with the control group, the treated infant exhibited increased regional homogeneity (ReHo) values in the superior frontal gyrus, inferior frontal gyrus, and right middle temporal cortex (Figure 1).
The inferior frontal gyrus is a major node of the language network. The connection between the inferior frontal gyrus and the temporal cortex supports speech perception[10]. A repetitive transcranial magnetic stimulation study[11] revealed that poststroke aphasia accompanied by left activation of the inferior frontal gyrus is associated with improved language recovery. Applying these results to the present study, increased ReHo in the inferior frontal gyrus indicated improvement in speech perception in the treated infant. This improvement can be attributed to the foot reflexology treatment.
The superior frontal gyrus is a part of the left frontal cortex, which participates in language processing. A study revealed that increased activity in the left frontal cortex is related to speech intelligibility and complex phoneme analysis[12]. In addition, the frontal aslant tract (FAT) connects the superior frontal gyrus with the inferior frontal gyrus, including the Broca area. The FAT is associated with language function[13]. In the present study, the increased ReHo in the superior frontal gyrus after foot reflexology treatment may indicate an improvement in speech processing. The increased ReHo in the right middle temporal cortex may be involved in the improvement in speech prosody.
However, in the present study, we also found decreased ReHo in the left inferior temporal gyrus and the right supplementary motor cortex compared to that in the control group. The left inferior temporal gyrus and the right supplementary motor cortex are important brain regions, and the connection between them is involved in language production[7]. In this study, the subject was young enough that she did not produce language. Therefore, for this infant, the ReHo values of the left inferior temporal gyrus and the right supplementary motor cortex were decreased. In the control group, the left inferior temporal gyrus and the right supplementary motor cortex may have been involved in visual function. Thus, the ReHo values of the left inferior temporal gyrus and the right supplementary motor cortex were increased. Our study confirmed the hypothesis of visual cross-modal reorganization of the brain[14]. A prospective randomized controlled trial comparing foot reflexology with a sham intervention in infants with SNHL is suggested because of the limitations of this case study.
This study showed how foot reflexology affects infants with SNHL. The activation of hubs in the language brain network, such as the superior frontal gyrus, inferior frontal gyrus, and right middle temporal cortex, was enhanced by foot treatment. This finding suggested that foot stimulation can facilitate improvements in prosody processing, analysis of phonemes, and speech processing. To our knowledge, this is the first report to provide physiological evidence on the impact of foot treatment in infants with SNHL.
We thank foot expert Lan-Tu Zhang, who spent a great deal of time and effort treating the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Samadi N, Iran S-Editor: Li L L-Editor: A P-Editor: Zheng XM
| 1. | Abbaszadeh Y, Allahbakhshian A, Seyyedrasooli A, Sarbakhsh P, Goljarian S, Safaei N. Effects of foot reflexology on anxiety and physiological parameters in patients undergoing coronary artery bypass graft surgery: A clinical trial. Complement Ther Clin Pract. 2018;31:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Lee Y, Pan Q, Du Y, Zhang L, Li C, Hu M, Li M, Li B. A Case Study: Effects of Foot Reflexotherapy in an Infant with Sensorineural Hearing Loss. J Acupunct Meridian Stud. 2020;13:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6985] [Cited by in RCA: 7231] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 4. | Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999;8:151-156. [PubMed] [DOI] [Full Text] |
| 5. | Wattanaruangkowit P, Muengtaweepongsa S, Kengganpanich M, Kengganpanich T. The Effects of Foot Reflexology for Smoking Cessation on Brain Activities with Functional Magnetic Resonance Imaging (fMRI): A Pilot Study. Evid Based Complement Alternat Med. 2022;2022:1727479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 6. | Sturm W, Longoni F, Weis S, Specht K, Herzog H, Vohn R, Thimm M, Willmes K. Functional reorganisation in patients with right hemisphere stroke after training of alertness: a longitudinal PET and fMRI study in eight cases. Neuropsychologia. 2004;42:434-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Turkeltaub PE, Flowers DL, Verbalis A, Miranda M, Gareau L, Eden GF. The neural basis of hyperlexic reading: an FMRI case study. Neuron. 2004;41:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Zhao B, Li L, Jiao Y, Luo M, Xu K, Hong Y, Cao JD, Zhang Y, Fang JL, Rong PJ. Transcutaneous auricular vagus nerve stimulation in treating post-stroke insomnia monitored by resting-state fMRI: The first case report. Brain Stimul. 2019;12:824-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol. 2013;23:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | van Straaten HL. Automated auditory brainstem response in neonatal hearing screening. Acta Paediatr Suppl. 1999;88:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Mortensen MV, Mirz F, Gjedde A. Restored speech comprehension linked to activity in left inferior prefrontal and right temporal cortices in postlingual deafness. Neuroimage. 2006;31:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Fujii M, Maesawa S, Motomura K, Futamura M, Hayashi Y, Koba I, Wakabayashi T. Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca's area in the dominant hemisphere of patients with glioma. J Neurosurg. 2015;122:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Strelnikov K, Rouger J, Demonet JF, Lagleyre S, Fraysse B, Deguine O, Barone P. Visual activity predicts auditory recovery from deafness after adult cochlear implantation. Brain. 2013;136:3682-3695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |