Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1422
Peer-review started: November 22, 2023
First decision: December 27, 2023
Revised: January 8, 2024
Accepted: February 8, 2024
Article in press: February 8, 2024
Published online: March 16, 2024
Processing time: 110 Days and 14.5 Hours
Pulmonary mucoepidermoid carcinoma (PMEC) is a rare malignancy that arises from minor salivary glands within the tracheobronchial tree. The clear cell variant of PMEC is exceptionally uncommon and presents notable diagnostic challenges, primarily attributable to its morphological similarity to other tumors containing clear cells.
A 22-year-old male, formerly in good health, came in with a two-month duration of persistent cough and production of sputum. Subsequent imaging and bronchoscopy examinations revealed a 2 cm tumor in the distal left main bronchus, which resulted in complete atelectasis of the left lung. Further assessment via positron emission tomography/computed tomography scans and endoscopic biopsy confirmed the primary malignant nature of the tumor, characterized by clear cell morphology in most of the tumor cells. The patient underwent a left lower lobe sleeve resection accompanied by systematic mediastinal lymph node dissection. Molecular pathology analysis subsequently revealed a CRTC3-MAML2 gene fusion, leading to a definitive pathological diagnosis of the clear cell variant of PMEC, staged as T2N0M0. After surgery, the patient experienced a smooth recovery and exhibited no signs of recurrence during the one-and-a-half-year follow-up period.
This article describes an unusual case of a clear cell variant of PMEC characterized by the presence of a CRTC3-MAML2 gene fusion in a 22-year-old male. The patient underwent successful left lower lobe sleeve resection. This case underscores the distinctive challenges associated with diagnosing and treating this uncommon malignancy, underscoring the importance of precise diagnosis and personalized treatment strategies.
Core Tip: Pulmonary mucoepidermoid carcinoma (PMEC) is an uncommon malignancy originating from the minor salivary glands of the tracheobronchial tree. Its clear cell variant, even rarer, presents distinctive diagnostic challenges due to its morphological similarity to other clear cell tumors. This report describes the case of a 22-year-old male diagnosed with the clear cell variant of PMEC characterized by the CRTC3-MAML2 gene fusion. The patient successfully underwent a left lower lobe sleeve resection without the need for adjuvant therapy. At the one-and-a-half-year postoperative follow-up assessment, he exhibited no signs of recurrence.
- Citation: Yu XH, Wang WX, Yang DS, Gong LH. Left lower lobe sleeve resection for the clear cell variant of pulmonary mucoepidermoid carcinoma: A case report. World J Clin Cases 2024; 12(8): 1422-1429
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1422.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1422
Pulmonary mucoepidermoid carcinoma (MEC) is an uncommon malignant neoplasm that accounts for only 0.1%-0.2% of all lung malignancies[1]. Originating from minor salivary glands within the tracheobronchial tree, Pulmonary MEC (PMEC) is histologically characterized by mucous-producing, epidermoid, and intermediate cells[2]. The clear cell variant of PMEC, distinguished by prominent cytoplasmic clearing, poses diagnostic challenges due to its similarity to other clear cell-containing tumors[3]. Recent genetic advancements have identified MAML2 as a critical driver gene in MEC, establishing the CRTC1/3-MAML2 gene fusion as a valuable diagnostic biomarker[4]. Despite these genetic insights, there is currently no standardized therapeutic strategy for PMEC; however, complete surgical resection remains the primary treatment approach[5]. This case report discusses the case of a patient who was diagnosed with the clear cell variant of PMEC harboring the CRTC3-MAML2 gene fusion and underwent left lower lobe sleeve resection. The objective is to highlight the distinctive challenges associated with diagnosing and managing this rare malignancy, emphasizing the critical importance of accurate diagnosis and personalized treatment strategies.
A 22-year-old Chinese male sought medical attention, presenting at the hospital with a persistent 2-mo history of coughing coupled with the production of sputum.
A 22-year-old male presented with symptoms that started approximately two months earlier without identifiable precipitating events or triggers. Initial complaints included a persistent cough and the production of white, mucoid sputum, occasionally encompassing streaks of blood. He sought medical attention at a local facility after experiencing these symptoms for a month, and the initial assessment suggested bronchopneumonia, which led to treatment with cephalosporin antibiotics. However, over subsequent days, symptoms not only persisted but also worsened. The cough intensified and was accompanied by dyspnea and intermittent left-sided chest pain. Due to the lack of improvement, additional diagnostic investigations were initiated. High-resolution chest computed tomography (CT) and bronchoscopy revealed a 2 cm contrast-enhanced nodule in the left main bronchus, causing complete atelectasis of the left lung and a mediastinal shift to the left. Biopsy was challenging due to the necrotic surface of the lesion. In response, the hospital's respiratory team implemented an interventional approach, which included a high-frequency snare technique combined with cryotherapy to excise a section of the lesion. Subsequent histopathological examination revealed that the tumor was a primary lung carcinoma with clear cell morphology, although the exact pathological classification was indeterminate. Following the intervention, the patient reported significant alleviation of respiratory symptoms. However, a subsequent CT scan indicated left lower bronchus obstruction despite re-expansion of the upper left lung. Given the malignancy, comprehensive positron emission tomography (PET)/CT revealed metabolic activity confined to the known mass in the left main bronchus, with no distant metastases, classifying the clinical stage as cT2N0M0. In addition to respiratory complaints, the patient denied fever, night sweats, hoarseness, dizziness, headache, or pain in his spine or extremities since symptom onset. Additionally, he maintained a consistent mental state, appetite, sleep pattern, urinary and bowel habits, and weight. Given his diagnosis and the need for specialized treatment, he was referred to our institution for potential surgical management and further evaluation.
The patient reported no history of surgery, trauma, significant infections, or other notable medical conditions. Furthermore, he confirmed no prior encounters with coronavirus disease 2019 infection.
The patient reported no significant family history of related illnesses. When employed as an automotive welder for four years following high school graduation, he suggested possible occupational exposure to dust during this period.
The patient's vital signs were recorded: Body temperature, 36.5 ˚C; height, 175 cm; weight, 64 kg; respiratory rate, 20 breaths/min; heart rate, 89 beats/min; and blood pressure, 122/78 mmHg. No palpable superficial lymph nodes were observed in the supraclavicular or neck regions. Symmetry characterized the chest wall, devoid of any deformities. Although breath sounds were reduced in the left lung, the patient maintained a regular breathing pattern. No other notable abnormalities were evident.
The laboratory findings were as follows: A white blood cell count of 7.8 × 109/L, a neutrophil percentage of 58%; a hemoglobin level of 149 g/L; a hematocrit of 47.3%; a fasting blood glucose level of 4.3 mmol/L; a total protein level of 81 g/L; and a globulin level of 34.2 g/L. A comprehensive panel of 12 tumor markers, including alpha-fetoprotein, carcinoembryonic antigen, neuron-specific enolase, cancer antigen (CA) 125, CA 15-3, CA 242, CA 19-9, prostate-specific antigen (PSA), free PSA, ferritin, and beta-human chorionic gonadotropin, was assessed via protein biochip detection in serum, with all the values falling within normal ranges. Pulmonary function tests revealed a forced vital capacity of 2.7 L (53.4% predicted) and a forced expiratory volume in one second of 2.5 L (57.7% predicted). Additional routine laboratory examinations, encompassing analyses of urine and stool, liver and kidney functions, and electrolyte levels, were within normal limits. Furthermore, screenings for infectious diseases such as hepatitis B, hepatitis C, syphilis, and human immunodeficiency virus yielded no abnormalities.
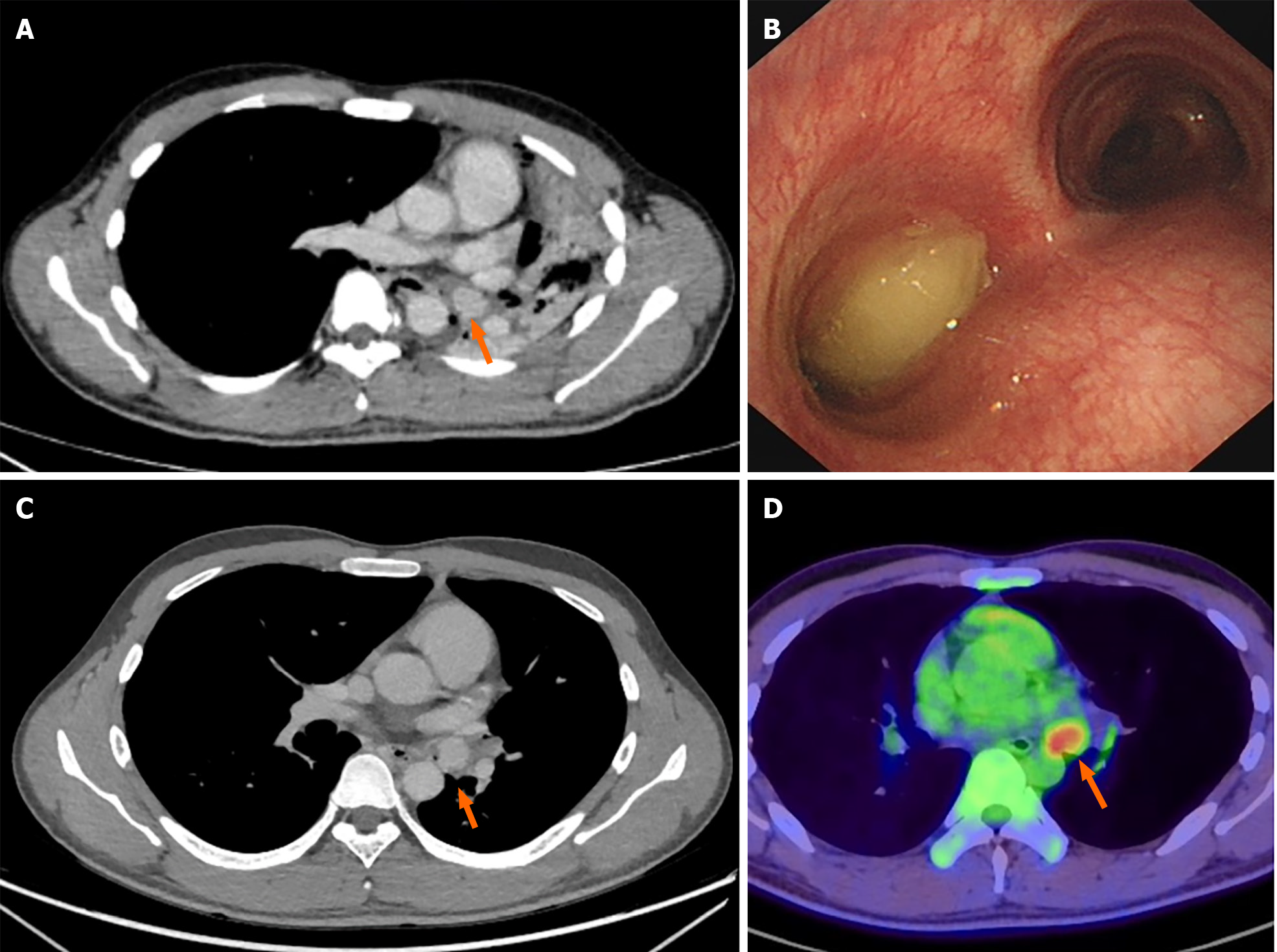
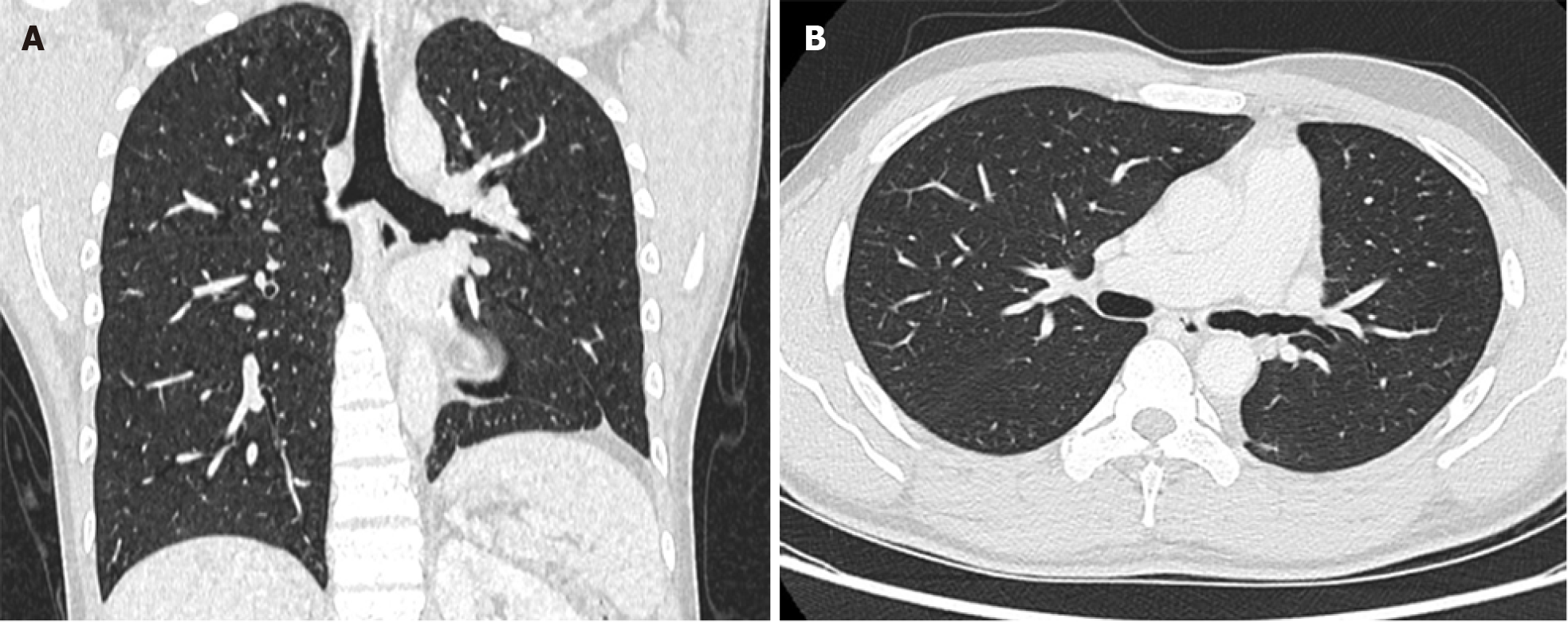
A chest CT conducted at a local hospital revealed a 2 cm contrast-enhanced nodule of soft tissue density within the left main bronchus. This nodule caused complete obstruction, resulting in total atelectasis of the left lung and a leftward mediastinal shift (Figure 1A). Consistent with the CT findings, the results of electronic bronchoscopy revealed a polypoid tumor in the left main bronchus. The tumor surface exhibited gray-yellow necrotic material, preventing further biopsy via bronchoscopic forceps (Figure 1B). In the local hospital’s respiratory department, a segment of the tumor was excised using a high-frequency snare combined with a cryotherapy approach and was subsequently subjected to histopathological evaluation. Following endoscopic intervention and airway secretion clearance, a noticeable improvement in the patient’s respiratory symptoms was observed. An examination revealed a patent left upper lobe bronchus with a healthy mucosal appearance, while the left lower lobe bronchus remained obstructed. Subsequent CT scanning revealed re-expansion of the left upper lung postintervention, but the mass in the left main bronchus persisted, continuing to obstruct the left lower lobe (Figure 1C). The patient subsequently underwent whole-body PET/CT at the same local hospital, at which the mass in the left main bronchus was identified and considered a metabolically active lesion, suggesting malignancy. Importantly, no abnormal metabolic activity was detected elsewhere, providing no evidence of metastatic spread (Figure 1D).
A multidisciplinary team (MDT) comprising thoracic surgeons, oncologists, anesthetists, radiologists, and pathologists was assembled to assess the conditions of the patients. Imaging and bronchoscopy examinations revealed a tumor in the distal left main bronchus. Comprehensive evidence from whole-body PET/CT and endoscopic biopsy confirmed that the tumor was primary malignant and predominantly exhibited clear cell morphology. While the exact pathological type has yet to be determined, consideration should be given to the potential diagnosis of a primary pulmonary salivary gland tumor. Clinical staging revealed no signs of local or distant metastasis, and the tumor was categorized as cT2N0M0. Routine blood test results and tumor marker levels were within normal limits. Pulmonary function tests demonstrated moderate restrictive ventilation impairment. Based on these findings, the MDT recommended a timely curative surgical approach, specifically left lower lobe sleeve resection. The tissue obtained from this procedure will further inform the diagnosis and subsequent therapeutic decisions.
Pathological examination of the specimen retrieved via bronchoscopy biopsy revealed tumor cells arranged in nest-like and papillary patterns. These cells exhibited clear, abundant cytoplasm with pronounced, deeply basophilic nuclei, indicative of malignancy. Immunohistochemically, the tumor tissue tested positive for cytokeratin, cytokeratin 7 (CK7), and vimentin; focal regions also exhibited P63 expression. Conversely, the cells tested negative for thyroid transcription factor-1 (TTF-1), Napsin A, calponin, paired box gene 2, paired box gene 8 (PAX-8), carbonic anhydrase IX, cluster of differentiation 117 (CD117), cluster of differentiation 10 (CD10), human melanoma black-45, melanoma antigen-A, inhibin A, cytokeratin 20, Villin, caudal type homeobox transcription factor 2, cytokeratin 5/6 (CK5/6), and P40. Antigen Kiel 67 (Ki-67) staining revealed a proliferation index of approximately 40%. Based on these findings, the primary diagnosis was a bronchogenic malignancy with a clear cell phenotype. A comprehensive assessment incorporating both immunohistochemical data and PET/CT scan results indicated that metastasis from renal, gastrointestinal, or other clear cell carcinomas was unlikely. Key differential diagnoses under consideration included pulmonary salivary gland tumor, pulmonary squamous cell carcinoma with clear cell differentiation, and hyalinizing clear cell carcinoma.
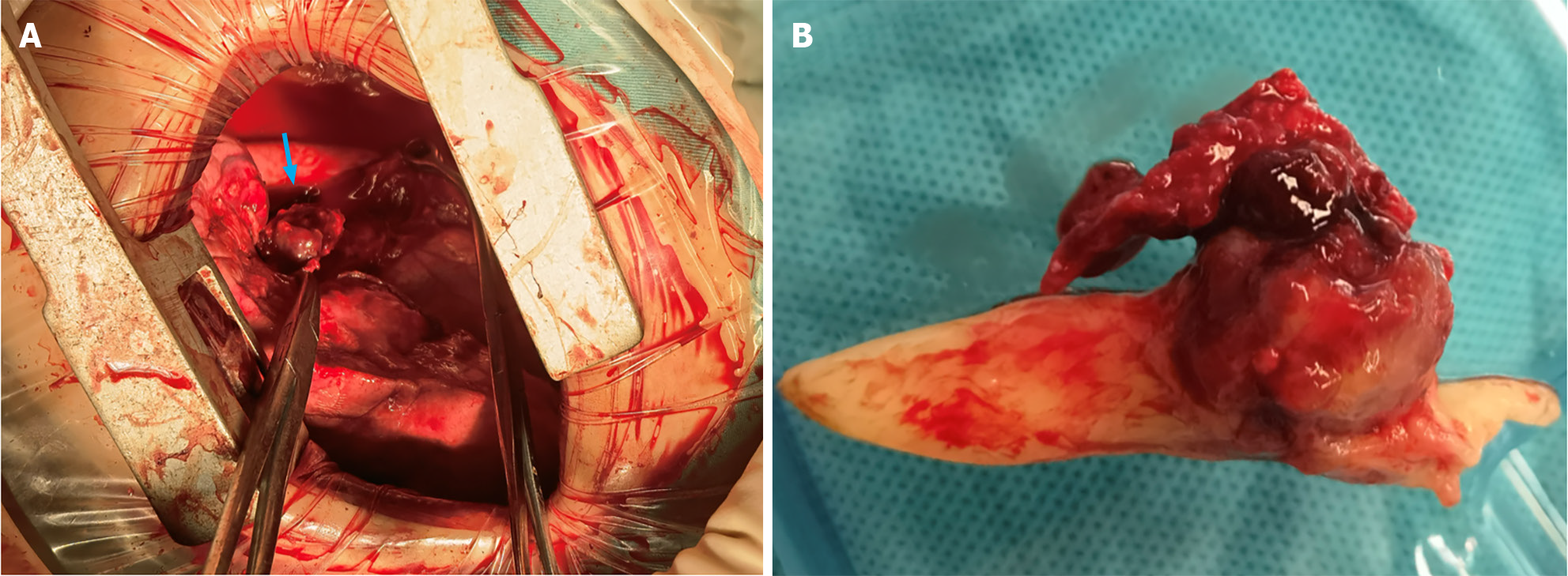
At our institution, the patient underwent both left lower lobe sleeve resection and systematic mediastinal lymph node dissection. An anterolateral incision measuring 12 cm was made in the fourth intercostal space. Intraoperatively, observations revealed smooth visceral and parietal pleura with no nodular presence. Mild adhesions were observed within the thoracic cavity, and the oblique fissure was prominent. While the left upper lobe was hyperinflated, the left lower lobe was entirely obstructed and consolidated. After adhesions were appropriately resolved, the pulmonary hilum and mediastinal lymph nodes were effectively cleared. The left lower pulmonary vein and artery were sequentially exposed, sealed, and sectioned using an endoscopic linear cutting stapler. A subsequent incision in the left lower lobe bronchus exposed a 2 cm dark-red spherical tumor blocking the bronchial orifice and permeating its wall. Attached to the tumor, a white, tongue-shaped coagulum extended into the left upper bronchus, significantly occluding its diameter (Figure 2). After removal of the left lower lobe, the left main and left upper lobe bronchi were trimmed to ensure tumor-free margins, as validated by rapid intraoperative pathologic assessment. To reconstruct the left upper lobe bronchus, an end-to-end anastomosis was created using 3/0 Prolene sutures. The surgical procedure lasted 2.5 h, with a recorded blood loss of 100 mL and no intraoperative complications. The patient's postoperative recovery was uneventful; the drainage tube was removed on the 4th day after surgery, and the patient was subsequently discharged the following day.
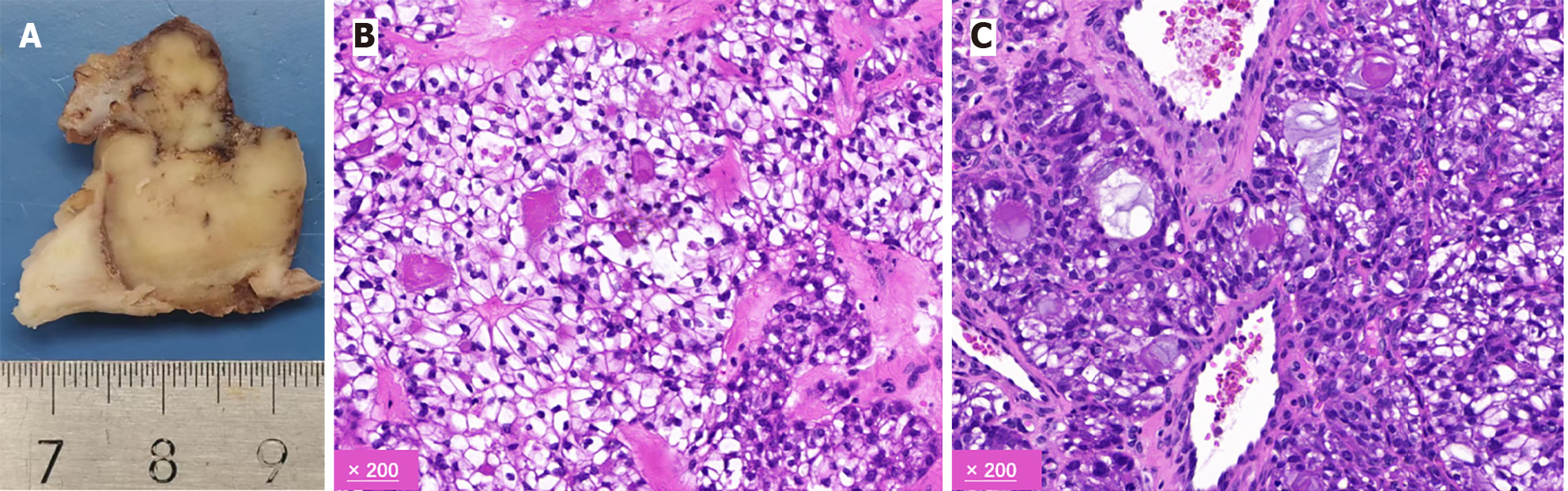
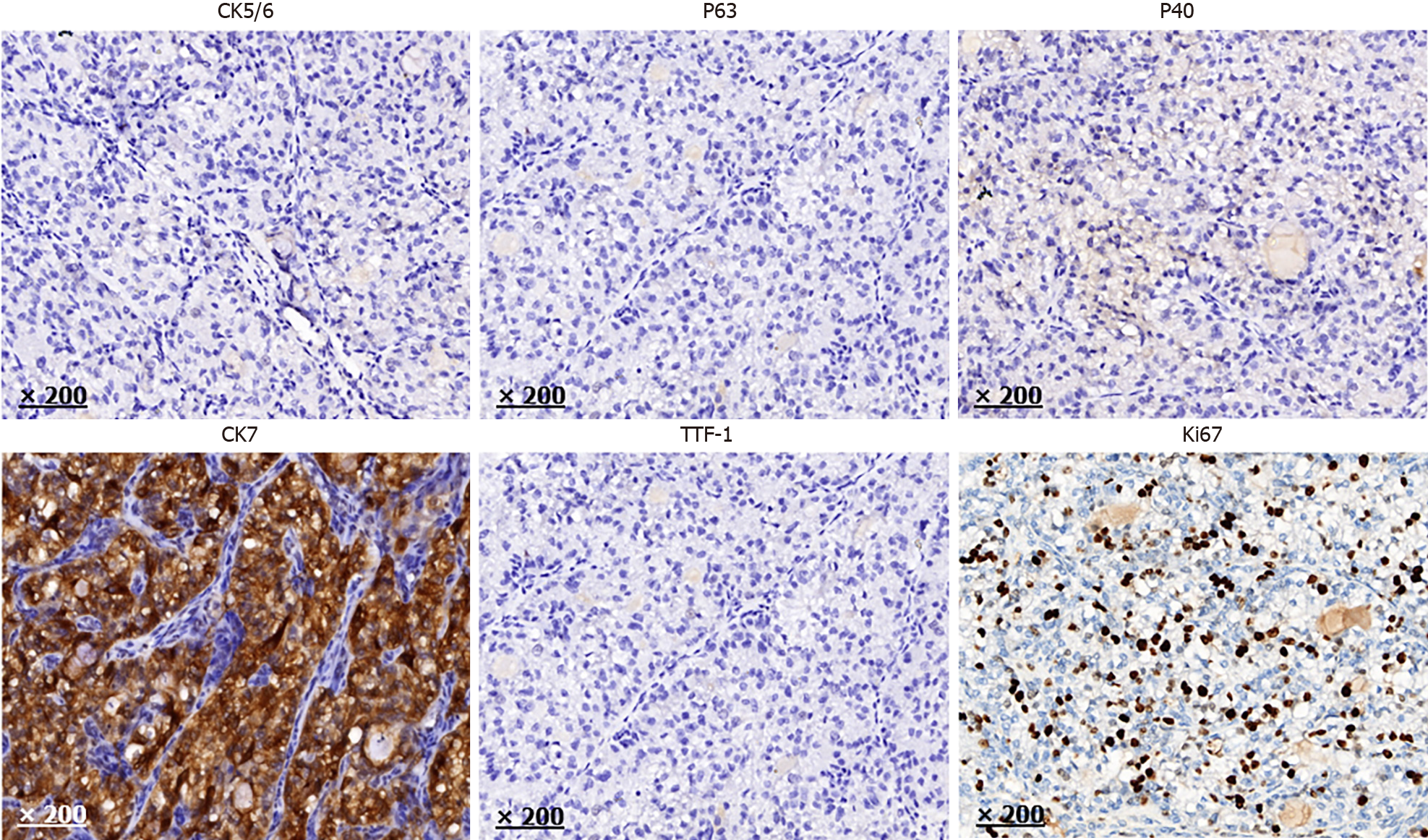
The results of the postoperative pathological analysis were consistent with those of the preoperative bronchial biopsy, indicating a malignant invasive tumor in the left main bronchus with clear cell morphology. The tumor, measuring approximately 2.5 cm, had breached the bronchial wall. Lymph nodes from the hilar and mediastinal areas tested negative for malignancy. Immunohistochemical staining was positive for CK7, pan-cytokeratin, epithelial membrane antigen, and Ki-67 (approximately 30%). Conversely, markers such as carbonic anhydrase 9, calponin, CD10, CD117, napsin A, P40, P63, PAX-8, PSA, S-100, synaptophysin, TTF-1, CK5/6, smooth muscle actin, lysozyme, α-methylacyl-CoA racemase, SRY-Box transcription factor 10, and GATA binding protein 3 were not detected (Figures 3 and 4).
Diagnosing clear cell malignancy in the left main bronchus is challenging due to its uncommon presentation. Traditional pathological and immunohistochemical evaluations are often limited in providing a definitive diagnosis. To overcome this limitation, we utilized molecular pathology and next-generation sequencing of the tumor tissue. While DNA sequencing did not reveal any prevalent driver mutations, RNA sequencing revealed a gene fusion between CRTC3 (NM_022769.4: exon 1) and MAML2 (NM_032427.1: exon 2). Reports highlight the pivotal role of MAML2 in MEC, in which the CRTC1/3-MAML2 gene fusion is considered a significant diagnostic marker. As a result, our conclusive pathological evaluation identified PMEC in the left main bronchus, specifically the clear cell variant, and it was staged as T2N0M0. Based on these insights and the current therapeutic literature, we do not recommend postoperative adjuvant therapy for these patients. At the 1.5-year follow-up assessment, there were no signs of recurrence (Figure 5).
Primary salivary gland-type tumors of the lung (PSGTTL) represent a rare subset of lung cancers, constituting less than 1% of all primary lung tumors[6]. The most common PSGTTL subtype is PMEC. While salivary gland tumors typically occur in the head and neck, PMEC is very rare in the lungs and is currently thought to have its origin in the salivary glands of the tracheobronchial tree, specifically within the proximal bronchi[1].
The clinical manifestations of PMEC vary, and patients commonly display symptoms related to airway obstruction or irritation, such as cough, dyspnea, and shortness of breath[7]. The diagnosis of PMEC heavily relies on high-resolution CT imaging, which detects nodules or masses with well-defined borders, appearing round, oval, or lobulated. These lesions generally present densities similar to muscle tissue, and enhanced scans commonly reveal visible enhancement[8].
As a result of the infrequency of PMEC and the nonspecific nature of clinical and imaging diagnostic approaches, the primary diagnostic method remains histological examination. It is imperative to distinguish PMEC from other malignancies, particularly when dealing with small biopsy specimens acquired through fiber optic bronchoscopy or lung puncture[1]. A conclusive PMEC diagnosis necessitates the identification of mucoepidermoid, epidermoid, and intermediate cells. Additional diagnostic techniques, such as immunohistochemistry, cytochemical staining, and analysis of MAML2 gene translocation, can further contribute to the diagnostic process[9].
PMEC is primarily managed with surgical intervention, and complete resection and lymph node dissection is necessary for optimal long-term survival. Postoperative chemotherapy and/or radiation therapy may be deemed necessary for patients exhibiting positive lymph node metastases, involved tumor margins, or a high tumor grade[10]. Thorough surgical resection is paramount for an enhanced prognosis, and techniques such as bronchoplasty and extended resection are considered valuable in the surgical management of this disease[10]. Additionally, preoperative endobronchial interventions are considered potentially useful in cases of obstructive pneumonia to mitigate complications preceding surgery[11].
This case report highlights the unique challenges associated with diagnosing and managing this clear cell variant of PMEC. The clear cell variant can be particularly challenging to diagnose due to its resemblance to other clear cell-containing tumors. In this case, RNA sequencing plays a pivotal role in identifying the CRTC3-MAML2 gene fusion, thereby enabling a definitive diagnosis of the clear cell variant of PMEC. These findings underscore the importance of molecular pathological analysis for accurately diagnosing rare malignancies.
Regarding treatment outcomes, the 5-year overall survival rate for PMEC patients who undergo lobectomy or sleeve resection is approximately 85%, with a more favorable prognosis associated with complete resection, younger age, female sex, early stage, smaller tumor size, lower histological grade, and the presence of the MAML2 fusion gene[11,12]. In this case, a 22-year-old male patient with the CRTC3-MAML2 fusion gene underwent left lower lobe sleeve resection and exhibited no lymph node metastasis, suggesting a positive prognosis.
In conclusion, this case report underscores the importance of an accurate diagnosis, primarily through molecular pathological analysis, in guiding personalized treatment strategies for the clear cell variant of PMEC. Surgical intervention remains the primary treatment modality, and while the prognosis is generally favorable for PMEC patients, individual characteristics such as gene fusion status, age, sex, tumor stage, and histological grade play crucial roles in determining long-term outcomes.
This article presents an uncommon case of a clear cell variant of PMEC featuring a CRTC3-MAML2 gene fusion in a 22-year-old male. The patient underwent successful left lower lobe sleeve resection, and the postoperative outcome was notably favorable. This outcome reiterates the significance of surgery as the primary therapeutic approach for early-stage PMEC. This case highlights the unique challenges in diagnosing and treating this uncommon malignancy, thus emphasizing the critical role of accurate diagnosis and personalized treatment strategies.
The authors would like to express their gratitude to the patient for his participation and cooperation in this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nambi G, Saudi Arabia S-Editor: Liu H L-Editor: Filipodia P-Editor: Yu HG
| 1. | Shen W, Yang T, Fan Y, Li X, Ai C, Wang X, Wang D, Zhou X. Pulmonary mucoepidermoid carcinoma: A clinicopathological study of 45 patients. Thorac Cancer. 2022;13:2385-2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Huo Z, Wu H, Li J, Li S, Wu S, Liu Y, Luo Y, Cao J, Zeng X, Liang Z. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One. 2015;10:e0143169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Fink DD, Lomas AM, Roden AC, Shah PC, Zreik RT. Primary mucoepidermoid carcinoma of the lung with prominent clear cells. Proc (Bayl Univ Med Cent). 2017;30:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Okumura Y, Nakano S, Murase T, Ueda K, Kawakita D, Nagao T, Kusafuka K, Urano M, Yamamoto H, Kano S, Tsukahara K, Okami K, Hanai N, Iwai H, Kawata R, Tada Y, Nibu KI, Inagaki H. Prognostic impact of CRTC1/3-MAML2 fusions in salivary gland mucoepidermoid carcinoma: A multiinstitutional retrospective study. Cancer Sci. 2020;111:4195-4204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Li X, Guo Z, Liu J, Wei S, Ren D, Chen G, Xu S, Chen J. Clinicopathological characteristics and molecular analysis of primary pulmonary mucoepidermoid carcinoma: Case report and literature review. Thorac Cancer. 2018;9:316-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Falk N, Weissferdt A, Kalhor N, Moran CA. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol. 2016;23:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Hsieh CC, Sun YH, Lin SW, Yeh YC, Chan ML. Surgical outcomes of pulmonary mucoepidermoid carcinoma: A review of 41 cases. PLoS One. 2017;12:e0176918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Cheng DL, Hu YX, Hu PQ, Wen G, Liu K. Clinicopathological and multisection CT features of primary pulmonary mucoepidermoid carcinoma. Clin Radiol. 2017;72:610.e1-610.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Zhu F, Wang W, Hou Y, Shi J, Liu Z, He D, Bai C, Li S, Jiang L. MAML2 rearrangement in primary pulmonary mucoepidermoid carcinoma and the correlation with FLT1 expression. PLoS One. 2014;9:e94399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Yamamoto T, Nakajima T, Suzuki H, Tagawa T, Iwata T, Mizobuchi T, Yoshida S, Nakatani Y, Yoshino I. Surgical treatment of mucoepidermoid carcinoma of the lung: 20 years' experience. Asian Cardiovasc Thorac Ann. 2016;24:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Li Q, Wei X, Wang Y, Liu C, Fan B, Lv C, Si W, Li M. Pulmonary mucoepidermoid carcinoma in the Chinese population: A clinical characteristic and prognostic analysis. Front Oncol. 2022;12:916906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 12. | Tamura K, Uchimura K, Furuse H, Imabayashi T, Matsumoto Y, Tsuchida T. Mucoepidermoid carcinoma cured by a combination of high-frequency snare and photodynamic therapy: A case report. Thorac Cancer. 2023;14:1306-1310. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |