Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1339
Peer-review started: December 2, 2023
First decision: December 28, 2023
Revised: January 9, 2024
Accepted: February 7, 2024
Article in press: February 7, 2024
Published online: March 6, 2024
Processing time: 89 Days and 23.6 Hours
Bradycardia-induced cardiomyopathy (BIC), which is a disease resulting from bradycardia, is characterized by cardiac chamber enlargement and diminished cardiac function. The correction of bradycardia can allow for significant improvements in both cardiac function and structure; however, this disease has been infrequently documented. In this case, we conducted a longitudinal follow-up of a patient who had been enduring BIC for more than 40 years to heighten awareness and prompt timely diagnosis and rational intervention.
A woman who presented with postactivity fatigue and dyspnea was diagnosed with bradycardia at the age of 7. Since she had no obvious symptoms, she did not receive any treatment to improve her bradycardia during the 42-year follow-up, except for the implantation of a temporary pacemaker during labor induction surgery. As time progressed, the patient's heart gradually expanded due to her low ventricular rate, and she was diagnosed with BIC. In 2014, the patient developed atrial fibrillation, her ventricular rate gradually increased, and her heart shape gradually returned to normal. This report describes the cardiac morphological changes caused by the heart rate changes in BIC patients older than 40 years, introduces another possible outcome of BIC, and emphasizes the importance of early intervention in treating BIC.
BIC can induce atrial fibrillation, causing an increased ventricular rate and leading to positive cardiac remodeling.
Core Tip: Bradycardia-induced cardiomyopathy (BIC) is a disease caused by bradycardia and characterized by enlargement of the heart chambers and decreased cardiac function. Increasing the ventricular rate significantly improved the associated symptoms and cardiac remodeling; however, this disease has rarely been reported. This case report describes a woman with BIC who, with little therapeutic intervention to improve bradycardia, had an increase in her ventricular rate due to the onset of atrial fibrillation, after which her cardiac enlargement due to a low ventricular rate was reversed. This case also emphasized the importance of early intervention for BIC.
- Citation: Gao DK, Ye XL, Duan Z, Zhang HY, Xiong T, Li ZH, Pei HF. Cardiac remodeling in patients with atrial fibrillation reversing bradycardia-induced cardiomyopathy: A case report. World J Clin Cases 2024; 12(7): 1339-1345
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1339.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1339
Prolonged tachyarrhythmia and bradyarrhythmia can significantly impact cardiac function. While tachyarrhythmia-induced cardiomyopathy (TIC) has garnered considerable attention in recent studies, evidence suggests that bradycardia also plays a role in cardiomyopathy progression. Fabritz et al[1] were the pioneers in introducing the concept of "Bradycardiomyopathy". The pathogenesis of bradycardia-induced cardiomyopathy (BIC) may be linked to a prolonged ventricular diastolic phase, volume overload, abnormal atrial and ventricular excitation and contraction sequences, adverse myocardial perfusion, neuroendocrine function alterations, among other factors. Despite these insights, reports on bradycardia cardiomyopathy remain scarce. In our longitudinal follow-up spanning over 40 years, we tracked a female patient with BIC, documenting the transformation of her cardiac chambers and morphology from abnormal to normal as heart rate increased, predominantly without pacing therapy. This endeavor aims to heighten awareness and prompt timely diagnosis and rational intervention for BIC.
A 7-year-old female presented with postactivity cardiac fatigue and dyspnea and was admitted to the general medical unit of our hospital in December 1980. After being discharged from the hospital, she was followed up at our hospital for a long time because of her arrhythmia.
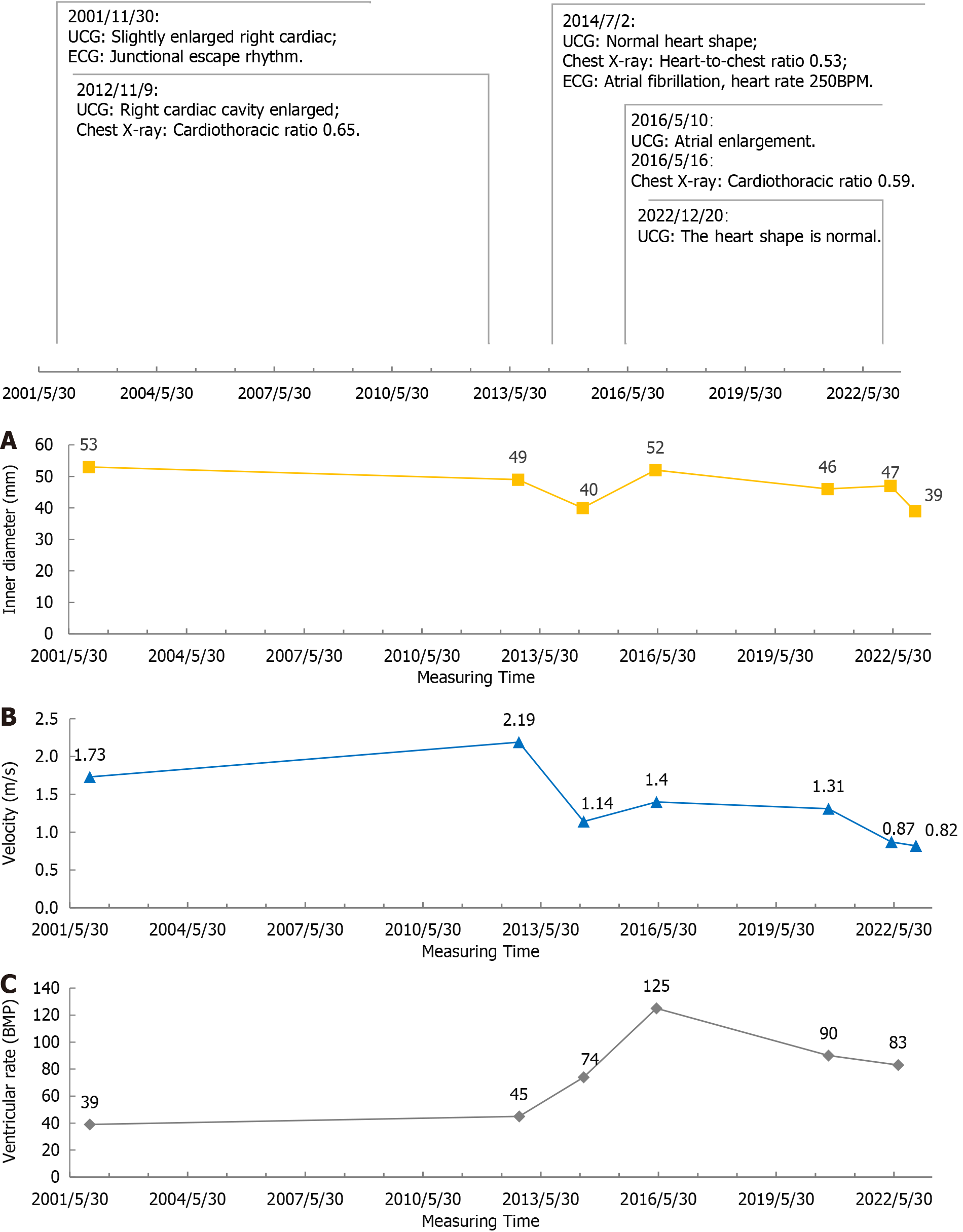
The female presented with postactivity fatigue and dyspnea at the age of 7, and electrocardiographic (ECG) assessment revealed arrhythmia and bradycardia, culminating in a diagnosis of "myocarditis and sick sinus syndrome". Her symptoms of fatigue and dyspnea improved after treatment with atropine and prednisone (details unknown), but her ventricular rate remained low. Throughout the course of treatment, there were no episodes of syncope or amaurosis. After discharge, she was followed up in our hospital for a long time because of her low heart rate. Follow-up ECG, echocardiography (UCG) and chest X-ray showed that she had bradycardia and cardiac enlargement. Nine years earlier (2014), the patient developed atrial fibrillation, and the ventricular rate gradually increased while the dilated heart gradually returned to normal, during which the patient did not experience any cardiac symptoms.
The patient had no history of congenital heart disease and had never developed hypertension, diabetes, valvular heart disease, hyperthyroidism, hypothyroidism, hyperlipidemia or other diseases related to cardiac remodeling. In 2001, the patient could not bear a pregnancy because of her bradycardia, so she chose induced abortion, and a temporary pacemaker was installed before the operation. In November 2012, the patient was admitted to the hospital for uterine fibroid therapy. Due to her bradycardia, the patient had to have another temporary pacemaker placed before surgery. She did not use specific drugs, had no history of smoking or drinking, and had no history of radiation or toxin exposure.
The female’s menstruation was reported as normal (the patient had menarche at the age of 12, with a menstrual cycle of 28-30 d, and each menstrual period lasted for 3-5 d). She became menopausal in February 2020. The periods were regular, and the menstrual flow was moderate, without dysmenorrhea. No familial occurrences of a similar disease were identified.
The female laboratory examinations did not reveal any abnormalities.
On June 2, 1981 after prednisone and atropine treatment, UCG showed enlargement of left atrium and left ventricle (details unknown).
On November 30, 2001, UCG suggested that the ventricular rate was 39 beats per minute (BPM), right atrium and right ventricle were slightly enlarged, right atrial (RA) transverse diameter: 53 mm; aortic valve (AV) peak systolic flow velocity: 1.73 m/s.
On November 9, 2012, UCG hint: Right heart enlargement, RA: 49 mm, AV: 2.19 m/s; chest X-ray hint: Cardiothoracic ratio 0.65.
On July 02, 2014, UCG suggested that all chambers of the heart were normal, and RA: 40 mm, AV: 1.14 m/s; chest X-ray showed that the cardiothoracic ratio was 0.53.
On May 10, 2016, UCG hint: Atrial enlargement, RA: 52 mm, AV: 1.40 m/s; on May 16, 2016, chest X-ray indicated that the heart was enlarged, the cardiothoracic ratio was 0.59.
On September 27, 2020, UCG indicated that the atrium was slightly enlarged, RA: 46 mm, AV: 1.31 m/s.
On May 6, 2022, UCG suggested: Atrial enlargement, RA: 47 mm, AV: 0.87 m/s.
UCG on December 20, 2022 showed the following: the patient’s heart morphology was normal, RA: 39 mm, and AV: 0.82 m/s, with minimal trace regurgitation in the bicuspid, tricuspid, and pulmonary valves.
The patient's early ECG manifestations when she was 8 years old included sinus arrest, a junctional escape rhythm with a rate of approximately 40 BPM, sporadic frequent ventricular premature beats, brief bursts of ventricular tachycardia, and infrequent sinus arrest with first-degree atrioventricular block. Notably, two prolonged gaps were observed, with the lengthiest RR interval reaching 4680 ms. The suggestion that she undergo artificial pacemaker implantation was made due to these findings, but the recommendation was declined by the patient.
In 2001, the patient was unable to endure a pregnancy due to her bradycardia and opted for induced abortion with the preoperative installation of a temporary pacemaker. A unipolar electrocardiogram derived from the temporary pacemaker electrodes situated in the elevated right atrium revealed sinus arrest with intermittent atrial electrical activity (various A-waves), near-complete atrioventricular dissociation, and a junctional escape rhythm. Stimulation of the enlarged right atrium with S1S1 (940 ms) induced atrial agitation, with 1:1 conduction to the ventricle, while S1R was prolonged to 310 ms.
Upon admission for uterine fibroids on November 9, 2012, the patient exhibited an ECG depicting junctional escape rhythm at 45 BPM.
ECG assessments on July 2, 2014 showed atrial fibrillation with atrial rates of 250 BPM and a mean ventricular rate of 74 BPM.
The ECG measured on May 10, 2016 showed: Atrial fibrillation, with a mean ventricular rate 125 BPM.
On September 27, 2020, the ECG indicated atrial fibrillation with a mean heart rate of 90 BPM.
On July 8, 2022, the ECG revealed atrial flutter with a ventricular rate of 83 BPM.
The patient was diagnosed with a BIC.
The patient was treated with prednisone and atropine for a short period when symptoms became apparent at the age of 7 years, and subsequently received a temporary pacemaker implant during induction of labor and uterine fibroid surgery. She did not undergo any treatment to increase the ventricular rate, including pacemaker implantation, thereafter. Even after atrial fibrillation occurred, not only did radiofrequency ablation combined with pacemaker implantation not be performed, but anticoagulation treatment was even refused.
We chronologically organized the ECG and UCG data collected over 20 years (Figure 1). Our analysis revealed that the patient exhibited bradycardia until the age of 40 (before 2014), which was marked by cardiomegaly and dilated chambers, as indicated by UCG and chest radiographs. Subsequently, the patient experienced a transition to atrial flutter/atrial fibrillation, leading to a gradual increase in the ventricular rate. Remarkably, without any specific treatment, the cardiac morphology gradually normalized over this period. Notably, significant alterations in the RA transverse diameter and peak systolic flow velocity of the AV were observed, both of which normalized with an increasing ventricular rate[2]. This observation suggested that an elevated ventricular rate can effectively reverse a dilated heart in patients with BIC, leading to improvements in the patient’s hemodynamics and cardiac function. Considering the patient's age-related high risk of developing tachyarrhythmia-induced cardiomyopathy, we strongly advocated for cardiac radiofrequency ablation in this patient.
In recent years, multiple studies have underscored a significant correlation between cardiomyopathies and arrhythmias, with the well-established concept of TIC. Radiofrequency ablation has emerged as an efficacious intervention for a growing number of patients experiencing tachyarrhythmias, fostering improvements in heartbeat and the restoration of cardiac morphology and function. Conversely, investigations into BIC, a disease resulting from abnormal heart rates characterized by cardiac chamber enlargement and diminished cardiac function, are limited. Historical research in animals revealed that chronic complete high-grade atrioventricular block led to ventricular cardiomyocyte hypertrophy[3]. Sasaki et al[4] documented a case of restrictive cardiomyopathy and paroxysmal atrial fibrillation, wherein the transition to sinus rhythm induced right heart failure, later alleviated by pacemaker implantation and pharmacological maintenance of sinus rhythm. Kováts et al[5] reported sinus arrest with escape rhythm (40 BPM) in a hypertensive patient on β-blockers, highlighting elevated N-terminal B-type brain natriuretic peptide precursor, which normalized upon discontinuation of β-blockers. Fabritz et al[1] proposed "bradycardiomyopathy" in an animal study, linking severe bradycardia to dilatation, fibrosis, and atrioventricular dysfunction. Caliskan et al[6] reported significant improvement in a male patient with sinus bradycardia and non-dense cardiomyopathy after pacemaker implantation, establishing a causal link. Our documented case of BIC, spanning over 40 years with a gradual shift in cardiac morphology from abnormal to normal, represents unique empirical data, offering the first extended observational insight into the natural evolution of BIC.
Similar to the diagnosis of TIC, the retrospective diagnosis of BIC hinges on specific criteria. Diagnostic parameters for BIC include: (1) A history of prolonged bradycardia; (2) manifestation of pertinent symptoms and signs, encompassing weakness, dizziness, amaurosis, and, in severe cases, fainting, along with signs and symptoms of heart failure; (3) ultrasound confirmation of enlarged cardiac chambers in the context of prolonged bradycardia, while maintaining a generally normal left ventricular ejection fraction; and (4) normalization of cardiac chambers and function following pacing treatment. The augmentation of ventricular rate through pacemaker implantation or alternative methods has been demonstrated to yield substantial improvements in cardiac function and structure in such patients[7]. Notably, the patient in question initially exhibited symptoms of cardiac dysfunction during the early stages of bradycardia diagnosis. The disease's evolution paralleled that of other arrhythmia-related cardiomyopathies, with the increasing ventricular rate facilitating a partial recovery of heart morphology and function. This correlation underscores the close association between cardiomyopathy-like changes and bradycardia, substantiating the diagnosis of BIC.
In this case, beginning with the childhood diagnosis of sick sinus node syndrome, the patient progressively exhibited manifestations of bradycardia, sinus arrest, junctional escape beats, and first-degree atrioventricular block. Despite meeting criteria for pacemaker implantation, as evidenced by the need for implantation after atrial fibrillation radiofrequency ablation[8], the patient's refusal of radiofrequency ablation and subsequent prolonged period without cardiac-related symptoms allowed for the observation of cardiac morphologic changes associated with increased heart rate. Chronic bradycardia in this patient resulted in a relative increase in ventricular filling time, causing elevated ventricular wall tension, cardiac off-center hypertrophy, and an increase in the transverse diameter of the right atrium. Additionally, prolonged bradycardia activated the sympathetic nervous system, inducing vasoconstriction, increased myocardial contractility, elevated resistance to cardiac efflux, raised peak flow velocity of the AV, and promoted myocardial fibrosis, potentially triggering atrial fibrillation[9]. Following the onset of AF, the patient's ventricular rate gradually increased, a strategy shown to mitigate deleterious cardiac remodeling by maintaining a heart rate below 110 BPM in patients with early atrial fibrillation[10,11]. Remarkably, without intervention, cardiac remodeling reversed progressively with the rising ventricular rate, culminating in the restoration of normal cardiac morphology and function. The RA transverse diameter and peak aortic systolic flow velocity gradually normalized, suggesting that pacing therapy or an increased ventricular rate due to atrial fibrillation/atrial flutter can ameliorate abnormal cardiac morphology and function in patients with BIC. As the patient ages, the potential for the recurrence of atrial enlargement and ventricular modifications in response to heart rate changes, possibly progressing towards TIC, poses a notable risk of AF-induced stroke. Consequently, we strongly advocate for cardiac radiofrequency ablation and the routine use of non-vitamin K antagonist oral anticoagulants before and after resuscitation[10]. Increased awareness of BIC and early intervention holds the potential to positively impact the prognosis of this disease.
The landscape of bradycardia treatment underwent a paradigm shift in 1958 when the Swedish cardiac surgeon Ake Senning pioneered the first pacemaker implantation in human history[12]. This landmark development has significantly limited the opportunities for observing the progression of bradycardia to BIC. While the evolution from arrhythmia to arrhythmogenic cardiomyopathy typically spans months to years, recurrent arrhythmias can hasten the onset of heart failure, accompanied by a rapid decline in ventricular function. Early diagnosis and effective arrhythmia management have shown considerable potential in improving ventricular function and alleviating clinical symptoms. Prompt identification of electromechanical dysfunction in individuals with a normal left ventricular ejection fraction is now achievable through the assessment of cardiac electrical and mechanical function using acoustic cardiography (ACG)[13,14]. The ACG serves as a valuable early screening tool for heart failure.
The presented case is subject to certain limitations. The first limitation is the lack of ECGs prior to the patient reaching 8 years of age, precluding a definitive determination of congenital sinus node dysfunction and subtle cardiac insufficiency during that period. The second limitation is the absence of empirical evidence from the initial hospitalization supporting the diagnosis of myocarditis. This report describes the cardiac morphological changes caused by the heart rate changes in BIC patients older than 40 years and introduces another possible outcome of BIC. However, we still need to remain vigilant about BIC.
The documented cardiac structural changes in this case indicate that BIC can induce atrial fibrillation, causing an increased ventricular rate and leading to positive cardiac remodeling; these changes are believed to be inherent because no therapeutic interventions aimed at cardiac remodeling are implemented in response to the detected heart rate abnormality, with few similar instances reported in the literature. The etiology of BIC remains uncertain and is further complicated by the scarcity of prospective studies. The gradual, often unnoticed development of this condition may lead to a potential underestimation of its incidence. Therefore, maintaining an enhanced level of vigilance for individuals with a BIC is of paramount importance in clinical practice.
We thank all the authors who participated in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G, Albania S-Editor: Liu H L-Editor: A P-Editor: Zhao S
| 1. | Fabritz L, Kirchhof P, Fortmüller L, Auchampach JA, Baba HA, Breithardt G, Neumann J, Boknik P, Schmitz W. Gene dose-dependent atrial arrhythmias, heart block, and brady-cardiomyopathy in mice overexpressing A(3) adenosine receptors. Cardiovasc Res. 2004;62:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Fang LG, Li ZA, Yang Y, Wang H, Wang JH, Shu XH, Sun K, Chen M, Yao MQ, Yin HN, Han RL, Liu HL, Hong H, Shi BF, Tian JW, Zhang CM, Ren WD, Yang J, Zhou Y, Gao DH, Zhang Y, Zhang M, Yao GH, Kang WQ, Wang J, kang CS, Duan YY, Zhang J, Zhou Q, Xu D, Zhao BW, Liu XM, Zhen ZL, Ran HT, Li R, Liu YN, Zhang XS, Li JG, Yuan JJ, Qin SC, Deng YB, Xie MX, Fei HW, Wei YL, Wu Y, Wu J, Ba T, Mu YM, Guo KX, Tong MH, Yan RL, Na LS, Zhang SD, Zhou QC, Zhu CY, Yin LX, Deng Y, Li S, Tang H, Che ZL, Ding YC, Zhen YH, Xu SZ, Ma XJ, Zhang QF, Gao DM. [Guidelines for Echocardiography and Measurement in Chinese Adults]. Zhonghua Chaosheng Yingxiangxue Zazhi. 2016;25:645-666. [DOI] [Full Text] |
| 3. | Volders PG, Sipido KR, Vos MA, Kulcsár A, Verduyn SC, Wellens HJ. Cellular basis of biventricular hypertrophy and arrhythmogenesis in dogs with chronic complete atrioventricular block and acquired torsade de pointes. Circulation. 1998;98:1136-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Sasaki N, Yasumura Y, Uemura N, Hanatani A, Nakatani S, Yamagishi M, Miyatake K. Restrictive cardiomyopathy with right-sided dominant heart failure after sinus conversion from atrial fibrillation: case report. Circ J. 2003;67:969-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kováts T, Wettstein A, Nagy E, Tomcsányi J. Bradycardia can induce increased serum natriuretic peptide-level. Int J Cardiol. 2008;123:e43-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Caliskan K, Balk AH, Jordaens L, Szili-Torok T. Bradycardiomyopathy: the case for a causative relationship between severe sinus bradycardia and heart failure. J Cardiovasc Electrophysiol. 2010;21:822-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Xiang HJ, Zhan HJ, Wang FJ. Bradycardia-induced cardiomyopathy. Shiyong Xindianxue Zazhi. 2015;24:16-17+21. [DOI] [Full Text] |
| 8. | Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM; ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 1145] [Article Influence: 286.3] [Reference Citation Analysis (1)] |
| 9. | Li X, Garcia-Elias A, Benito B, Nattel S. The effects of cardiac stretch on atrial fibroblasts: analysis of the evidence and potential role in atrial fibrillation. Cardiovasc Res. 2022;118:440-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6452] [Article Influence: 1613.0] [Reference Citation Analysis (0)] |
| 11. | Smit MD, Crijns HJ, Tijssen JG, Hillege HL, Alings M, Tuininga YS, Groenveld HF, Van den Berg MP, Van Veldhuisen DJ, Van Gelder IC; RACE II Investigators. Effect of lenient vs strict rate control on cardiac remodeling in patients with atrial fibrillation data of the RACE II (RAte Control Efficacy in permanent atrial fibrillation II) study. J Am Coll Cardiol. 2011;58:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Cooley DA. In memoriam. Tribute to Ake Senning, pioneering cardiovascular surgeon. Tex Heart Inst J. 2000;27:234-235. [PubMed] |
| 13. | Chung TL, Liu YH, Huang JC, Wu PY, Chen SC, Chang JM. Changes in acoustic cardiographic parameters before and after hemodialysis are associated with overall and cardiovascular mortality in hemodialysis patients. Sci Rep. 2021;11:1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Walia R, Chang SL, Lin YJ, Lo LW, Hu YF, Chao TF, Chung FP, Liao JN, Lin CY, Chang YT, Lin CH, Te ALD, Yamada S, Chen SA, Tsao HM. Early detection of electromechanical dysfunction in patients with idiopathic premature ventricular contractions. Pacing Clin Electrophysiol. 2019;42:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |