Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1296
Peer-review started: October 8, 2023
First decision: December 1, 2023
Revised: January 2, 2024
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 6, 2024
Processing time: 145 Days and 0.8 Hours
Pancreatic cancer is a highly malignant disease. After decades of treatment progress, the current five-year survival rate for patients is still less than 10%. For later-line treatment, the treatment options are even more limited. Anti-angiogenic drugs can improve progression-free survival in patients with advanced pancreatic cancer. Preclinical data show that fruquintinib might improve the prognosis of advanced pancreatic cancer by targeting angiogenesis and lymphopoiesis, improving the abnormal vascular structure, and modulating the tumour immune microenvironment.
We present two cases of third-line fruquintinib monotherapy that brought an extraprolonged progress-free survival (PFS) of 10 months. Patient 1 took adjuvant gemcitabine-based and first-line nab-paclitaxel-based chemotherapy and then used local radiotherapy combined with programmed cell death 1 receptor (PD-1). Each line lasted approximately 7 months. Moreover, the patient took third-line fruquintinib, which was followed by stable disease for 10 months, during which no additional adverse effect was observed. The patient later refused to take fruquintinib due to difficulty urinating and lower abdominal pain after the coronavirus disease 2019 (COVID-19) infection. The patient died in February 2023. Patient 2 also took two prior lines of chemotherapy and then local radiotherapy combined with S-1. After confirmed disease progression, the patient experienced a continuous partial response after using fruquintinib monotherapy in the third line. After the patient had COVID-19 in December 2022, fruquintinib was discontinued. The patient died in January 2023 due to disease progression.
Both cases achieved a PFS benefit from later-line single-agent fruquintinib therapy. With its better safety profile, fruquintinib may be worth exploring and studying in more depth as a later-line treatment for pancreatic cancer patients.
Core Tip: These two cases achieved a progress-free survival benefit from later-line single-agent fruquintinib therapy. With its better safety profile, fruquintinib may be worth exploring and studying in more depth as a later-line treatment for pancreatic cancer patients. This is the first literary report on Fruquintinib used in metastasis pancreatic cancers.
- Citation: Wu D, Wang Q, Yan S, Sun X, Qin Y, Yuan M, Wang NY, Huang XT. Extended survival with metastatic pancreatic cancer under fruquintinib treatment after failed chemotherapy: Two case reports. World J Clin Cases 2024; 12(7): 1296-1304
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1296.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1296
Pancreatic cancer is a major factor in cancer-related deaths worldwide Despite the endeavors of researchers and clinicians, the outlook for pancreatic cancer remains grim, with an overall 5-year survival rate of less than 10%[1]. Among all the treatments, surgical resection is the only curative treatment for pancreatic cancer patients, but recurrence after resection of pancreatic cancer remains a major concern.
Most recurrent pancreatic cancer patients receive systemic chemotherapy as a standard treatment. According to the current guidelines, patients with pancreatic cancer and distant metastases, along with a good performance status, are recommended to undergo FOLFIRINOX therapy or a combination of gemcitabine plus nab-paclitaxel as their first-line treatment[2,3]. However, no global consensus exists regarding the most suitable regimen for postoperative disease recurrence in patients who have already received postoperative adjuvant chemotherapy, nor for the third-line treatment of metastatic pancreatic cancer patients.
Chemotherapy is most often used for metastatic pancreatic cancers, while other treatments, including immunotherapy, targeted therapy, and radiotherapy, have only made modest progress over the past decade. Anti-angiogenic therapy for pancreatic cancer is a controversial topic. As neovascularization is associated with the aggressive nature of malignancies, anti-angiogenic therapy has proven successful in multiple cancers, such as colorectal cancer, non-small lung cancer, renal cell carcinoma, and others. Pancreatic ductal adenocarcinoma is a hypovascular tumour in a hypoxic microenvironment, making pancreatic ductal adenocarcinoma a potential target for anti-angiogenic therapy. Multiple clinical trials of anti-angiogenic agents have been carried out for pancreatic cancer treatments, but the results have been disappointing[4]. With a prolonged progress-free survival but no improvement in overall survival (OS), further studies about anti-angiogenic treatment in pancreatic cancers remained.
Here we present two cases of pancreatic ductal carcinoma with over 10 months of PFS after third-line anti-angiogenic treatment. These two cases were both from an investigator-initiated trial, NCT05257122.
Case 1: A 67-year-old man presented with persistent pain in the left upper abdomen, with carbohydrate antigen 19-9 (CA19-9) > 1000 U/mL in November 2019.
Case 2: A 70-year-old man presented with a pancreatic mass by computer tomography in April 2019.
Case 1: In 2019, the patient was diagnosed with pancreatic cancer and underwent the standard pancreaticoduodenectomy procedure. The post-operation immunohistochemistry tests showed carcinoembryonic antigen (+), CA19-9 (+), CK20 (small amount +), p53 (30% +), Ki67 (30%), C-erbB-2 (-), and S100 (-). The patient received four cycles of adjuvant chemotherapy with gemcitabine and S-1 (tegafur, gimeracil and oteracil potassium capsules). During the regular follow-up, recurrences were found in pelvic lymph nodes. Starting October 17, 2020, the patient received first-line treatment with 4 cycles of paclitaxel-albumin with irinotecan. The patient was assessed as having a partial response (PR) after four cycles of treatment. Then, he received seven subsequent cycles of raltitrexed (brand name Tomudex, 5 mg d1 q3w) as main
The patient returned to our hospital on August 13, 2021 with recurrent lower abdominal pain. Imaging revealed disease progression of the metastatic lesions. Given the patient's good performance status (PS 1), we initiated concurrent radiotherapy (30 fractions administered from August 17 to December 5, 2021) with a programmed cell death 1 receptor (PD-1) inhibitor, administered every 3 weeks. Repeat computed tomography (CT) after completing therapy demonstrated a partial response by RECIST v1.1 criteria. The CA19-9 level had gradually fallen to 19.1 U/mL. PD-1 inhibitor maintenance therapy was continued for 7 cycles from August 23, 2021 to January 4, 2022. However, on February 21, 2022, an upper abdominal contrast-enhanced CT scan revealed a 3.1 cm × 1.7 cm mass in front of the anterior descending segment of the duodenum, with an enlarged lymph node detected behind the aorta abdominalis. The response was classified as progressive disease (PD).
Case 2: The patient underwent the standard pancreaticoduodenectomy procedure on May 10, 2019. Postoperative pathology indicated grade II ductal adenocarcinoma with nerve invasion. No cancer involvement was seen in the pancreatic cut margin, and no metastasis of peripancreatic lymph nodes was observed (0/4). The patient received six cycles of adjuvant chemotherapy with gemcitabine and S-1.
During regular follow-up, including CT scanning, recurrences were found in celiac lymph nodes in February 2020.
Therefore, he received second-line treatment with 6 cycles of paclitaxel-albumin with gemcitabine and 30 subsequent radiotherapy sessions.
While initially showing disease stabilization after second-line treatment, the patient developed PD on February 23, 2022. CT scanning showed multiple nodules in the abdomen and pelvis, and his abdominal lymph nodes were enlarged. Metastasis was considered. We saw abdominal and pelvic effusion and bilateral pleural effusion.
Case 1: History of hypertension for 8 years, history of peptic ulcer and multiple polyps in the colon, operated for foot trauma 2 years ago.
Case 2: History of hypertension for 20 years, self-claimed of manageable blood pressure. History of diabetes for more than 2 years, self-claimed of manageable blood glucose control.
Cases 1 and 2: No personal or familial history of any specific disease.
Cases 1 and 2: The patient was responsive, aware, and had clear cognition with vital signs that were steady. Examination of the lungs showed normal vesicular sounds on both sides. The heart’s auscultation revealed no irregularities, murmurs, or supplementary sounds. Pulses on all four limbs could be felt and were of normal strength.
Case 1: Laboratory results indicated a CA19-9 value of 125.3 U/mL. All other parameters, including complete blood count, aspartate aminotransferase, alkaline phosphatase, bilirubin, serum electrolytes, creatinine, and urea, were within normal ranges.
Case 2: Laboratory examination revealed CA19-9 193.6 U /mL. All other parameters, including complete blood count, aspartate aminotransferase, alkaline phosphatase, bilirubin, serum electrolytes, creatinine, and urea, were within normal ranges.
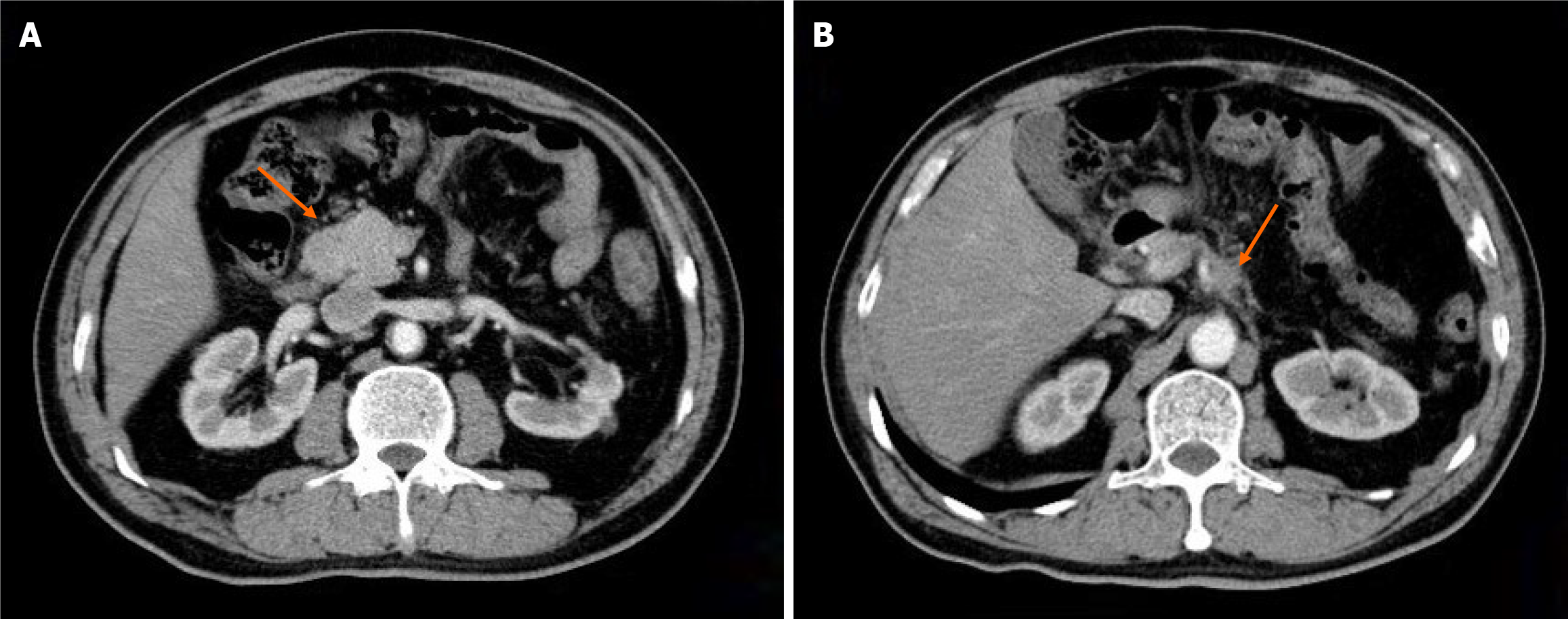
Case 1: CT revealed one reinforcing nodule anterior to the descending portion of the duodenum, considered metastatic; soft tissue shadows adjacent to the celiac trunk (Figure 1).
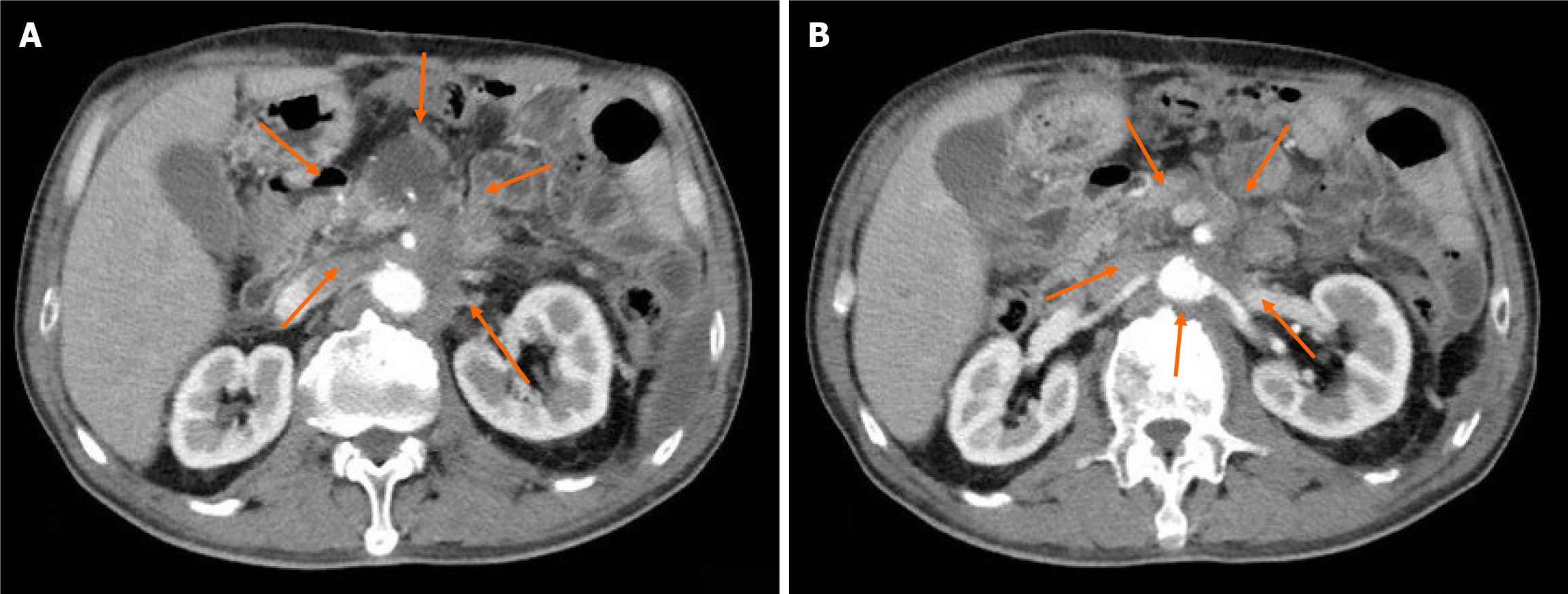
Case 2: CT revealed postoperative changes in pancreatic cancer, revealing cystic foci near the portal vein, multiple abdominopelvic nodules, potential metastasis, and enlarged abdominal lymph nodes.
Both cases were recurrent pancreatic cancer.
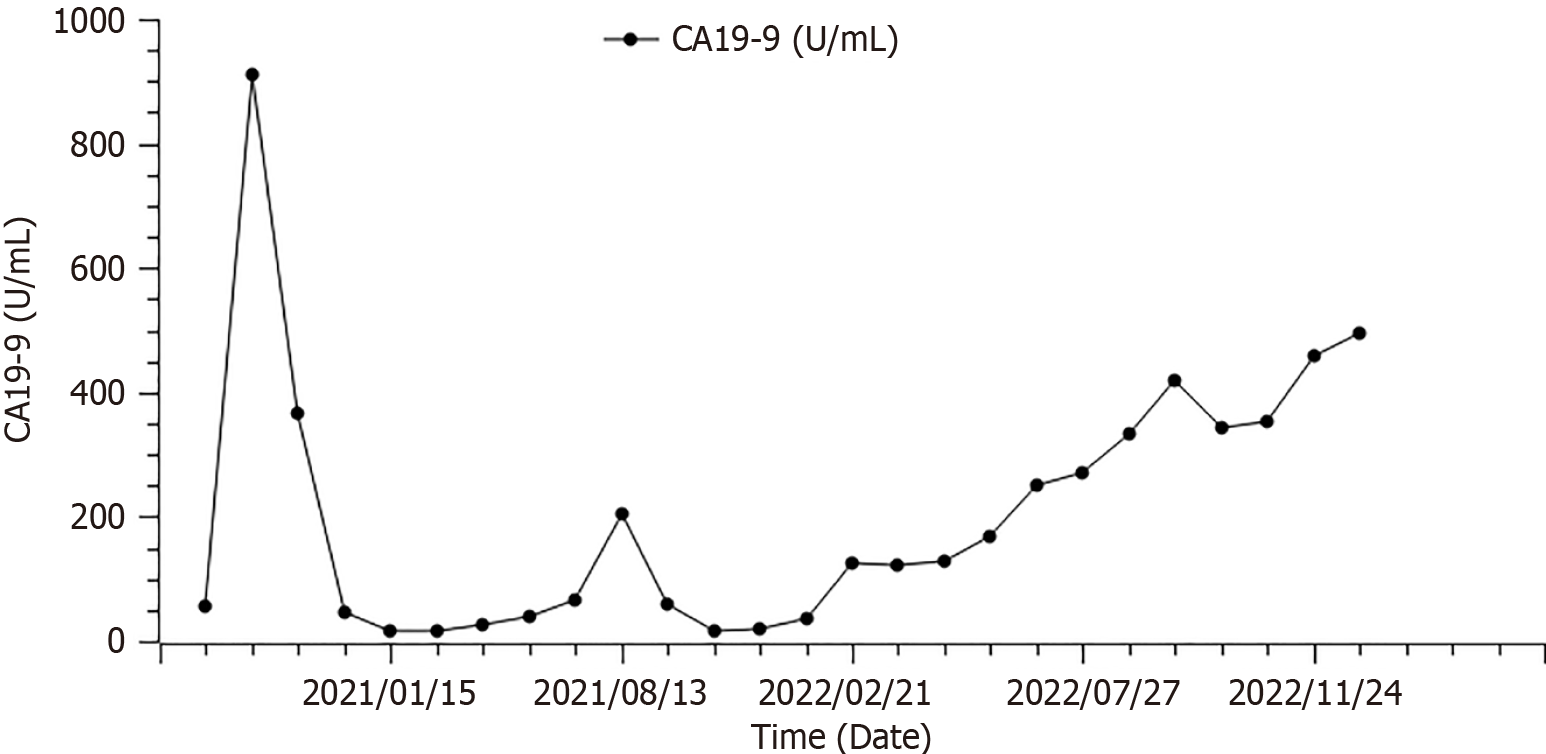
From February 26, 2022, the patient received fruquintinib (5 mg p.o. qd d1-21 q4w) as the third-line treatment. Throughout the follow-up period, his CA19-9 levels were checked at four-week intervals, and CT scans were scheduled every eight weeks. The CA19-9 levels consistently stayed between 123 U/mL and 344 U/mL (Figure 2), with the imaging revealing no notable alterations in the lesions. The response after 2 cycles of treatment was stable disease (Figure 3).
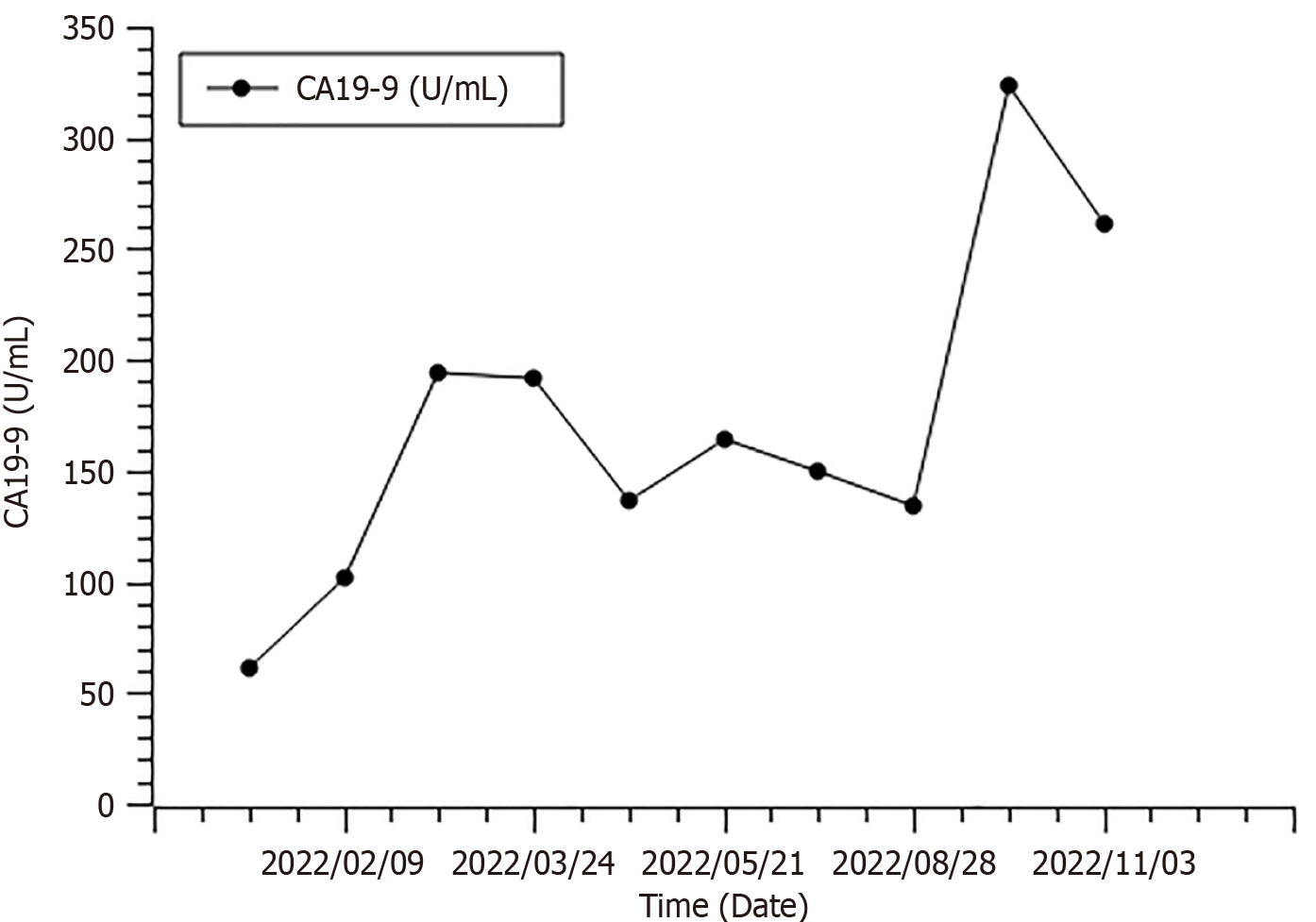
The patient refused further chemotherapy and was enrolled in an exploratory clinical trial with fruquintinib (Figure 4). Starting February 24, 2022, the patient received fruquintinib as the third-line treatment. The therapeutic schedule was 5 mg of fruquintinib orally once daily for 21 d in a 4-wk cycle. His CA19-9 level was tested every 4 wk (Figure 5), and a CT scan was performed every 8 wk during the follow-up. A PR according to RECIST 1.1 criteria was achieved at the assessment in June 2022 (targeted lesions: 4.7 to 2.9 cm). The response continues to the end of treatment (Figure 6). During this process, the main adverse event was occasional diarrhoea without treatment interruption. The patient showed a durable and safe response for nearly 1 year.
On December 13, 2022, the patient came to our hospital complaining of dysuria and recurrence of lower abdominal pain; the CT scan showed that the response to treatment was PD. CA19-9 had markedly increased to 494.6 U/mL. His PS status had diminished rapidly to 3. The patient finally died of multiple-organ dysfunction resulting from pulmonary infection.
The patient was infected with coronavirus disease 2019 (COVID-19) in mid-December 2022. He stopped taking fruquintinib and was given symptomatic supportive treatment. The patient died on January 1, 2023.
Pancreatic cancer has an extremely poor prognosis, with a median survival of only about 10 months with treatment and 5 to 6 months without. This bleak outlook stems largely from late-stage diagnosis in many patients and a low proportion of candidates eligible for tumor resection surgery[1]. According to current guideline recommendations, the first-line regimen of chemotherapy, gemcitabine plus nab-paclitaxel and FOLFILINOX [the combination of folinic acid, also called leucovorin (LV), with 5-fluorouracil (5-FU), irinotecan and oxaliplatin] is the highest recommendation[3]. However, for patients with metastatic pancreatic cancer (mPC), median OS with 1L therapy increased by only 3 months between 1986 and 2016[5].
Treatment options for advanced stages of pancreatic cancer are notably scarce. The sole approved regimen for mPC in a second-line (2L) context is a combination therapy consisting of liposomal irinotecan with 5-fluorouracil and leucovorin (5-FU/LV). This approval followed the progression of the disease after initial treatment with gemcitabine-based therapies. The endorsement stems from the outcomes of the phase III NAPOLI-1 trial, which compared the efficacy of liposomal irinotecan both as a monotherapy (n = 151) and in combination with 5-FU/LV (n = 117), against the administration of 5-FU/LV alone (n = 149) in patients with mPC who had previously undergone first-line (1L) treatment with gemcitabine-based chemotherapy. The combination achieved the primary endpoint, showing a median overall survival of 6.1 months, surpassing the 4.2 months observed with 5-FU/LV alone [unstratified hazard ratio (HR) of 0.67 (95%CI): 0.49–0.92, P = 0.012]. Additionally, a significant enhancement in median progression-free survival (PFS) was observed, with the combination therapy yielding 3.1 months compared to 1.5 months for the 5-FU/LV alone group [unstratified HR of 0.56 (95%CI: 0.41–0.75); P = 0.0001][6].
To date, no phase III study has improved the third-line treatment for mPC. Real-world data demonstrate that only a small proportion of patients (13%) reach a third-line treatment[7]. The current choice of third-line treatment for advanced pancreatic cancer is mainly differentiated from the front-line chemotherapy regimens: Treatment regimens not used in the front line are applied in the third line. In mPC, later-line treatment options for different mechanisms are still being tried. With the rapid introduction of many novel anti-angiogenic drugs in the past decade, only the combination of gemcitabine and erlotinib have improved the overall survival by two weeks for pancreatic cancer patients[8]. Recently, the Japanese investigator conducted a retrospective study with later-line Erlotinib plus gemcitabine, with the PFS ranging from 1.4 months to 2.1 months. The OS ranged from 3.5 months to 5.4 months[9]. Another retrospective study showed an mPFS of 4.4 months (range 0.6-15.4 months) for mPC patients using nano-liposomal irinotecan (nal-IRI) plus 5-fluorouracil/L-leucovorin (5-FU/L-LV) (only 19 out of 68 patients received third-line treatment)[10].
As for the anti-vascular endothelial growth factor receptor (Anti-VEGFR) pathway, some preclinical studies indicated that anti-VEGF inhibitors could significantly reduce pancreatic cancer cell growth and metastasis. Bevacizumab, a monoclonal antibody that targets VEGF-A, failed to improve PFS over gemcitabine monotherapy. Tyrosine kinase inhibitors (TKIs), including sorafenib, sunitinib, axitinib and vandetanib, all failed to demonstrate overall survival benefits compared to standard chemotherapy with mPFS ranging from 43 d to 3.2 months. Anti-VEGF TKIs may benefit some patients with advanced pancreatic cancer but hardly translate into an OS benefit in phase III studies. The failure of anti-VEGF therapy in pancreatic cancers seems to demonstrate that pancreatic cancer patients will not benefit from this kind of treatment. The hypothesis for this clinical phenomenon is that anti-angiogenic therapy renders tumors with a more invasive phenotype and easily triggers metastasis to distant organs[4]. There also were discussions focused on the relationship between vascularity and metastatic colonization. The analysis of genes and their expression in primary tumours and metastasis samples revealed that there are complex patterns of the expression of angiogenic and antiangiogenic factors, which differ between tumour sites and patients[11]. In pancreatic cancers, also reported the metastasis sites reacted to treatment differ from primary sites[12]. So investigators are also trying to find more appropriate applications for anti-angiogenic pathway drugs in pancreatic cancer treatment.
Here we present two cases of third-line fruquintinib monotherapy that brought a long PFS (more than 10 months). In both cases, the patients were treated surgically and later underwent two lines of systemic therapy. Patient 1 took adjuvant gemcitabine-based chemotherapy, and after 7 months, the CT scan indicated disease progression. Considering the former chemotherapy regimen, we shifted to nab-paclitaxel plus irinotecan. After four cycles, tumour assessment showed a partial response. To maintain the treatment benefit, we used raltitrexed as maintenance treatment for 7 months, but the patient experienced disease progression again. Therefore, at that time, the available chemotherapy agent was limited, and we considered using a PD-1 antibody combined with local radiotherapy. The disease remained stable for 6 months. For third-line therapy, we chose fruquintinib monotherapy. The patient started fruquintinib (5 mg, qd, d1-21, q4w) in February 2022. During the course of treatment, the patient's tumour index was slowly elevated, but the disease was assessed as stable disease. Due to the patient's difficulty urinating and lower abdominal pain after infection with the new coronavirus, the patient refused to take fruquintinib and died in February 2023. Patient 2, after starting adjuvant gemcitabine combined with S-1 in May 2019 and taking it for 6 cycles, review in April 2021 suggested new lymph node metastases. He was switched to nab-paclitaxel combined with gemcitabine regimen, which was discontinued due to intolerance to the regimen. He started local radiation therapy combined with S-1 treatment in August 2021. Disease progression was suggested in March 2022, so he started on monotherapy with fruquintinib. CT scan suggested patient sustained disease remission after treatment. He discontinued fruquintinib in December 2022 after contracting COVID-19. The patient died in January 2023 due to disease progression.
Fruquintinib uniquely inhibits the VEGFR kinase family, including VEGFR1, VEGFR2, and VEGFR3, with remarkable potency and selectivity. It powerfully suppresses VEGFR-1, VEGFR-2, and VEGFR-3 with IC50 values of 33, 35, and 0.5 nmol/L, respectively. Though displaying slight activity versus other kinases such as RET, FGFR-1, and c-kit (IC50 of 128-458 nmol/L), fruquintinib leaves untargeted all other tested kinases, with associated IC50s topping 1000 nmol/L. Thereby, fruquintinib achieves exquisitely specific blockade of VEGFR signaling, without meaningful off-target interactions. Preclinical data showed that fruquintinib might improve the prognosis of advanced pancreatic cancer by targeting angiogenesis and lymphopoiesis, improving the abnormal vascular structure, and modulating the tumour immune microenvironment. The anti-tumour activity of fruquintinib has been demonstrated in clinical trials involving a range of solid tumour types[13]. Two phase III studies (FRESCO study, FRESCO 2 study) demonstrated that fruquintinib improved both PFS and OS in mCRC patients who had undergone at least two lines of standard treatment[14,15]. A phase I/II trial of fruquintinib in combination with paclitaxel [fruquintinib (4 mg qd po), paclitaxel 80 mg/m2 weekly therapy for a total of 3 wk on/1 wk off for a 4-wk cycle] evaluated 28 patients with advanced gastric cancer. The results showed that fruquintinib combined with paclitaxel had an objective response rate of 35.7% and an 8-wk disease control rate of 67.9%; on RP2D, more than 50% of patients had PFS exceeding 16 wk and OS exceeding 7 months[16].
We present these two metastatic pancreatic cancer cases to demonstrate that the use of fruquintinib in later lines of treatment for advanced pancreatic cancer resulted in exciting long-lasting tumour control with a manageable safety profile. We like to present these two cases as a reflection of the complex reality heterogeneity of therapeutic responses. Due to the lack of biomarker testing data, we still need to investigate the population that would benefit the most from fruquintinib.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Tumor Support and Rehabilitation Therapy of the Chinese Society of Clinical Oncology.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Lithuania; Kitamura K, Japan; Vyshka G, Albania S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1518] [Article Influence: 253.0] [Reference Citation Analysis (1)] |
| 2. | Yang YM, Bai XL, Bian DP, Cai SW, Chen RF, Cao F, Dai MH, Fang CH, Fu DL, Ge CL, Guo XC, Hao CY, Hao JH, Huang HG, Jian ZX, Jin G, Li F, Li HM, Li SP, Li WQ, Li YX, Li HZ, Liang TB, Liu XB, Lou WH, Miao Y, Mou YP, Peng CH, Qin RY, Shao CH, Sun B, Tan G, Tian XD, Wang HZ, Wang L, Wang W, Wang WL, Wei JM, Wu HH, Wu WM, Wu Z, Xu JY, Yan CQ, Yin XY, Yu XJ, Yuan CH, Zhang TP, Zhang JX, Zhou J, Zhao YP. Guidelines for the diagnosis and treatment of pancreatic cancer in China (2021). Yixian Bingxue Zazhi. 2021;4:49-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 692] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 4. | Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, Li H, Zhang SR, Xu JZ, Qi ZH, Ni QX, Yu XJ, Liu L. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22:15-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 5. | Zhao Z, Liu W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol Cancer Res Treat. 2020;19:1533033820962117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 6. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 829] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 7. | Hegewisch-Becker S, Aldaoud A, Wolf T, Krammer-Steiner B, Linde H, Scheiner-Sparna R, Hamm D, Jänicke M, Marschner N; TPK-Group (Tumour Registry Pancreatic Cancer). Results from the prospective German TPK clinical cohort study: Treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int J Cancer. 2019;144:981-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2775] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 9. | Mie T, Sasaki T, Takeda T, Okamoto T, Mori C, Furukawa T, Yamada Y, Kasuga A, Matsuyama M, Ozaka M, Sasahira N. Treatment outcomes of erlotinib plus gemcitabine as late-line chemotherapy in unresectable pancreatic cancer. Jpn J Clin Oncol. 2021;51:1416-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yasuoka H, Naganuma A, Kurihara E, Kobatake T, Ijima M, Tamura Y, Suzuki Y, Hoshino T, Ishida F, Hosaka H, Hatanaka T, Yoshida S, Aihara R, Hosouchi Y, Ishii N, Araki K, Shirabe K, Uraoka T, Kakizaki S. Efficacy and Safety of the Combination of Nano-Liposomal Irinotecan and 5-Fluorouracil/L-Leucovorin in Unresectable Advanced Pancreatic Cancer: A Real-World Study. Oncology. 2022;100:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 11. | Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187-2196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Zou JY, Wang Z, Wang Y. Fruquintinib: a novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag Res. 2019;11:7787-7803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Dasari A, Sobrero A, Yao J, Yoshino T, Schelman W, Yang Z, Chien C, Kania M, Tabernero J, Eng C. FRESCO-2: a global Phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Future Oncol. 2021;17:3151-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 16. | Xu RH, Zhang DS, Shen L, Li J, Huang J, Gong JF, Guo WJ, Zhang Y, Fan SH, Li K, Hua Y, Su WG. A Phase I/II trial of fruquintinib in combination with paclitaxel for second-line treatment in patients with advanced gastric cancer. J Clin Oncol. 2017;35:128-128. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |