Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1290
Peer-review started: September 7, 2023
First decision: December 7, 2023
Revised: December 18, 2023
Accepted: February 7, 2024
Article in press: February 7, 2024
Published online: March 6, 2024
Processing time: 175 Days and 13.7 Hours
Toxic epidermal necrolysis (TEN) is a life-threatening dermatological emergency mainly induced by drug hypersensitivity reactions. Standard management includes discontinuation of culprit drug and application of immunomodulatory therapy. However, mortality remains high due to complications like septic shock and multiorgan failures. Innovative approaches for skin care are crucial. This report introduces borneol-gypsum, a traditional Chinese drug but a novel dre
A 38-year-old woman diagnosed with eosinophilic granulomatosis with polyangiitis experienced gangrenous complications and motor nerve invol
Borneol-gypsum dressing is a promising adjuvant that could significantly improve TEN management, skin regeneration, and patient comfort.
Core Tip: This case report highlights the innovative use of Borneol-Gypsum dressing in the skin management of toxic epidermal necrolysis (TEN). Despite the standard treatments, the patient's condition improved remarkably after supplementary application of this dressing, showcasing its potential in halting disease progression and fostering skin regeneration. The borneol-gypsum dressing not only relieved pain of exfoliated skin but enhanced patient cooperation during dress changing, suggesting it could be a promising dermatological supplement for further care in TEN.
- Citation: Yang LW, Zhang LJ, Zhou BB, Lin XY, Chen YT, Qin XY, Tian HY, Ma LL, Sun Y, Jiang LD. Efficacy of borneol-gypsum in skin regeneration and pain control in toxic epidermal necrolysis: A case report. World J Clin Cases 2024; 12(7): 1290-1295
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1290.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1290
Toxic epidermal necrolysis (TEN) is a severe skin injury most frequently caused by drugs and characterized by widespread necrosis and shedding of the skin. Secondary infections are a leading cause of TEN’s high mortality rate of up to 30%[1]. Traditional treatment for TEN involves discontinuing all suspected drugs and administering high doses of glucocorticoids and human immunoglobulin. Recent studies have demonstrated that the use of tumour necrosis factor (TNF) antagonists can improve the prognosis of TEN patients. Proper skin care and skin barrier reconstruction also play crucial roles in reducing the risk of infections and further lowering the mortality rate[2-4]. Borneol, a component of traditional Chinese medicine, has been shown to have protective effects on the skin and to facilitate wound healing. Borneol targets the TRPM8 pathway to provide pain relief and promotes skin healing by reducing inflammation and activating the p38-COX-2-PGE2 signalling pathway[5,6]. A recent case study reported successful treatment of drug-induced TEN through the use of traditional treatments, casting, and borneol application, resulting in improved survival rates and reduced infection risk.
Rash for one year, one-month foot drop, erythema and blisters for one week.
A 38-year-old female patient visited our Rheumatology Clinic complaining of recurrent itchy erythema on her lower limbs that had persisted for a year. A month before her admission, she experienced discolouration and severe pain in her right ring and little fingers, scoring 9 on the visual analogue scale, accompanied by limping and right foot drop. The patient’s complete blood count indicated a significant increase in eosinophils, and ultrasonography of her limbs revealed multiple embolisms in both radial arteries, anterior tibial arteries, and dorsal foot arteries. Electromyography indicated peripheral nerve damage in multiple areas of both lower limbs, suggesting axonal degeneration. After excluding clonal haematological malignancies through bone marrow aspiration and biopsy, she was diagnosed with eosinophilic granulomatosis with polyangiitis (EGPA) based on the American College of Rheumatology/European League Against Rheumatism 2021 criteria. The patient began a comprehensive treatment regimen, which included intravenous methylprednisolone (1 g/d for 3 d, followed by 80 mg/d for another 3 d, which was subsequently orally tapered starting from 1 mg/kg/d), intravenous cyclophosphamide (0.8 g/month), oral warfarin, beraprost sodium, and vitamin B12. Two weeks later, her foot drop improved, and there was no further progression of necrosis in her fingers, although her pain persisted. The patient was then discharged with a prescription of duloxetine for pain management. Three days postdischarge, she developed progressive red rashes on her limbs, chest, and abdomen, which evolved into large, ruptured, exudative blisters. Additionally, she experienced erosion and congestion in the mucous membranes of her lips and eyes, along with intensified pain, necessitating a return visit to the clinic.
The patient’s medical history included bronchial asthma and allergic rhinitis.
The patient had no significant personal or family history.
Upon admission, the patient had a temperature of 37.8 °C, a blood pressure of 99/53 mmHg, and a heart rate of 129 beats per minute. Oral examination revealed mucosal erosion and crusting with darkening of the lips. She exhibited diffuse, dark erythema on her face, along with ulcerative lesions on her eyelids. The conjunctiva was congested and exudative, while the cornea was still clear. Her trunk showed widespread erythema with lax blisters and bullae, some of which were ulcerated and eroded, and a positive Nikolsky sign was observed. The upper limbs were marked with multiple dark erythematous patches and blisters. On the patient’s lower limbs, there were widespread erythematous patches and blisters and some lesions resembling soybean-sized, dark red blisters or targetoid lesions. Diffuse erythema and blisters were present on the soles of her feet. Notably, there was evident necrosis on her right ring and little fingers.
Routine blood tests revealed a white blood cell count of 13.81 × 109/L (90.0% neutrophils, 0.04 × 109/L eosinophils), a haemoglobin level of 110 g/L, and a platelet count of 451 × 109/L. The faecal occult blood test was positive/negative. Liver function tests revealed alanine aminotransferase levels of 25 U/L, aspartate aminotransferase levels of 31 U/L, total bilirubin of 4.0 μmol/L, albumin of 29 g/L, and a blood glucose level of 11.2 mmol/L. The renal function parameters included a serum urea nitrogen concentration of 12.6 mmol/L and a creatinine concentration of 82 μmol/L. Her electrolyte levels were potassium 3.1 mmol/L, sodium 135 mmol/L, chloride 100 mmol/L, and serum bicarbonate 19 mmol/L. The erythrocyte sedimentation rate was 88 mm/h, and the hypersensitive C-reactive protein concentration was 30.6 mg/L. The serum amyloid A concentration was 525.0 mg/L. The cytokine levels were as follows: TNF, 55.80 pg/mL; interleukin (IL)-1β, 14.0 pg/mL; IL-2 receptor, 664.0 U/mL; and IL-6, 28.40 pg/mL. The procalcitonin concentration was 0.05 ng/mL. Blood cultures for aerobic and anaerobic bacteria were negative. The Score of TEN was calculated as 4.
The patient did not undergo any imaging studies as part of the diagnostic evaluation.
TEN and EGPA.
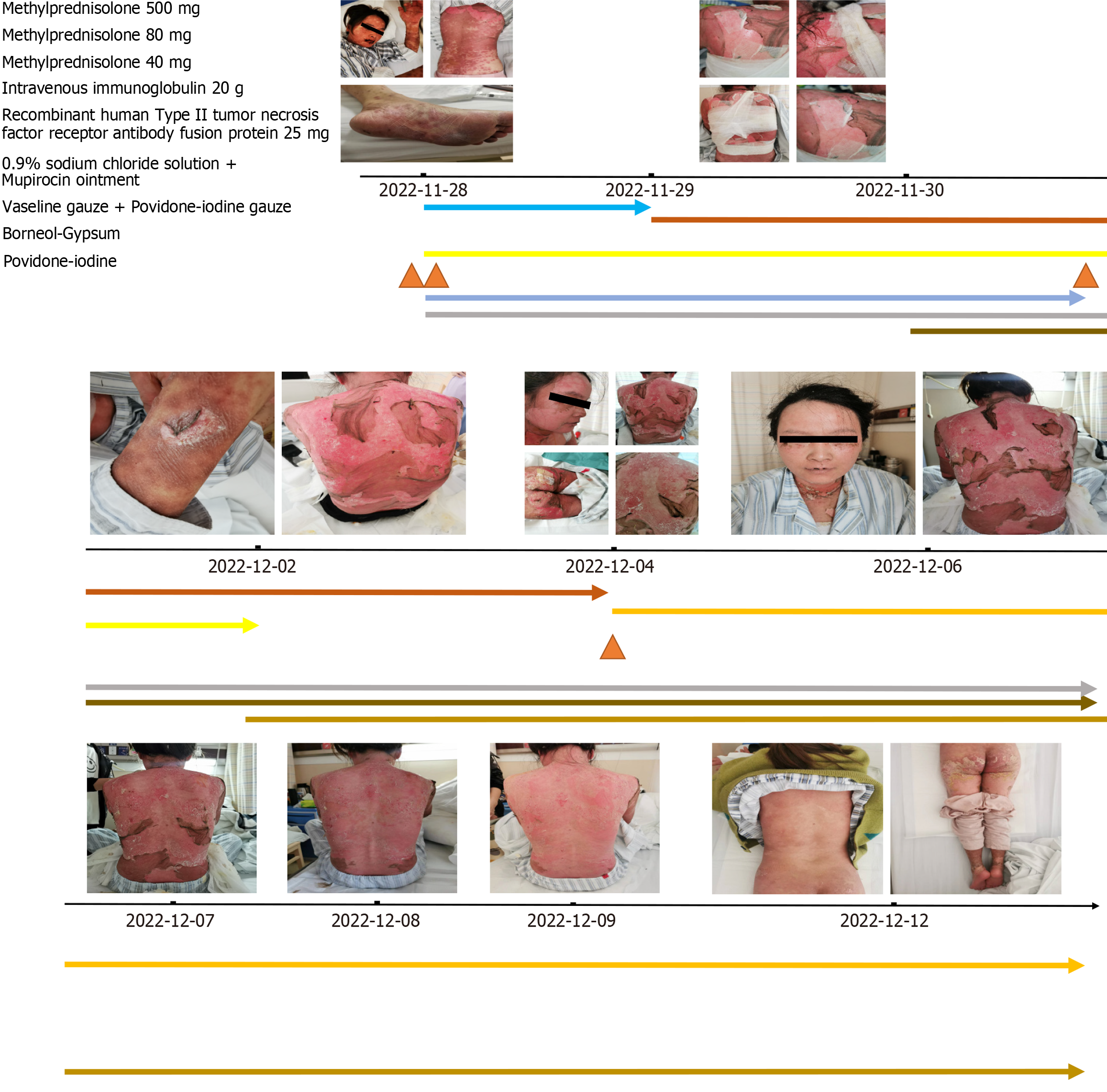
The suspected allergenic drug duloxetine was discontinued, and the room temperature was maintained between 28 and 30 °C. Following dermatological and ophthalmological consultations, the patient received a single dose of 500 mg methylprednisolone, which was tapered to 80 mg/d and adjusted to 40 mg/d after 6 d. This treatment was accompanied by 20 g/d of intravenous immunoglobulin for 5 d and an initial dose of 50 mg of recombinant human type II TNF receptor antibody fusion protein, followed by 25 mg every 3 d. Meropenem (1.5 g daily) was used for anti-infective therapy, and omeprazole (80 mg/d) was prescribed for stress ulcer prevention. The patient's hydration and nutritional needs were addressed with 3500-4000 mL of 0.9% saline solution daily, along with a high-calorie, protein-rich liquid diet. Rigorous monitoring of blood glucose levels ensured that the levels remained within acceptable ranges. Ophthalmological care included bidaily administration of tobramycin and dexamethasone eye drops supplemented with twice-daily carbomer eye gel and four daily polyvinyl alcohol applications to both eyes. Daily saline washes were conducted for eye hygiene, and mupirocin ointment was applied to the lips thrice daily. Extensive blisters on the limbs and trunk were managed with fluid drainage, and regular saline washes were used for cleansing the skin lesions. The affected regions were treated with a combination of mupirocin, petrolatum gauze, and povidone iodine gauze. Additionally, diluted potassium permanganate (1 g/8000 mL) was used for sitz baths. On days two and three, the patient experienced persistent serous discharge from the skin on the back, accompanied by considerable pain. The skin care regimen was adjusted to include mupirocin, petrolatum gauze, povidone iodine gauze, gypsum, and borneol on the ruptured areas. This adjustment led to a marked decrease in discharge and alleviation of pain. The treatment approach consisted of gypsum, borneol, mupirocin, petrolatum gauze, and povidone iodine gauze (Figure 1).
By the fifth day, new skin had formed in the affected areas, prompting continued treatment with regular changes in the wound dressings. Significant improvement in the skin lesions was evident by the eighth day, at which point new skin growth occurred. By Days 12 to 14, the skin lesions had completely resolved. The patient has been under regular follow-up for a year, with no recurrence of skin lesions.
TEN is a severe and potentially fatal disease caused by widespread shedding of the skin, severe systemic infection, toxic symptoms, and multiple-organ failure, leading to a high mortality rate[7]. Despite a lack of standard treatment, strong evidence supporting the use of glucocorticoid and human gamma globulin approaches is lacking. In recent years, TNF inhibitors (TNFis) have been tested for use in treating TEN, but large-scale data are still lacking[8]. Hence, proper supportive care and attentive nursing are crucial in treating TEN with the aim of reducing its mortality.
In this case, we treated the patient with TEN by an intensive immunosuppression regimen that included high-dose glucocorticoids and standard-dose TNFis. To improve the effectiveness of skincare, a combination of gypsum and borneol was used in combination for basic management. Borneol, a natural substance commonly used in food and traditional Chinese medicine for pain management and anaesthesia, has been shown to have anti-inflammatory effects, as well as the ability to reduce monocyte infiltration and the number of Th1 and Th17 cells[5,9,10]. Borneol also has antiadhesive properties, reducing bacterial attachment and biofilm formation, and can enhance the efficacy of antibiotics[11,12]. Moreover, borneol has been reported to inhibit oedema and severe skin erythema[6,13,14]. The use of gypsum, commonly used to treat scalds, has been shown to improve exudate absorption, wound healing, and muscle regeneration, and calcium ions play crucial roles in burn healing and anti-inflammatory effects[15-17]. The combination of gypsum and borneol effectively reduced local pain, facilitated skin lesion repair, reduced patient discomfort, improved patient compliance with dressing changes, and reduced the risk of infection. This approach may be an important treatment strategy for patients with TENs.
Overall, more attention should be given to the prevention of TENs in the management of patients with EGPA. A history of allergy and high levels of eosinophilic granulocytes are the prominent features of EGPA, leading to high frequencies of allergic reactions in patients with EGPA. Regular visits, health education, careful physical examination and drug monitoring may help to reduce the incidence of TEN.
TEN, a serious skin injury, can be managed more effectively by adding recombinant human TNF receptor II antibody fusion protein and borneol-gypsum dressings to conventional therapy. This approach not only controls disease progression and fosters skin regeneration, but it also minimizes local pain and enhances patient compliance, which potentially reduces infection risks.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Betlloch-Mas I, Spain; Oley MH, Indonesia S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J Invest Dermatol. 2016;136:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, Serrano-Reyes C. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63. [PubMed] |
| 5. | Wang S, Zhang D, Hu J, Jia Q, Xu W, Su D, Song H, Xu Z, Cui J, Zhou M, Yang J, Xiao J. A clinical and mechanistic study of topical borneol-induced analgesia. EMBO Mol Med. 2017;9:802-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Ji J, Zhang R, Li H, Zhu J, Pan Y, Guo Q. Analgesic and anti-inflammatory effects and mechanism of action of borneol on photodynamic therapy of acne. Environ Toxicol Pharmacol. 2020;75:103329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin Rev Allergy Immunol. 2018;54:147-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 8. | Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, Chang CJ, Su SC, Hui RC, Chin SW, Huang LF, Lin YY, Chang WY, Fan WL, Yang CY, Ho JC, Chang YC, Lu CW, Chung WH; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 9. | Wang YY, Ryu AR, Jin S, Jeon YM, Lee MY. Chlorin e6-Mediated Photodynamic Therapy Suppresses P. acnes-Induced Inflammatory Response via NFκB and MAPKs Signaling Pathway. PLoS One. 2017;12:e0170599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ge YB, Wang ZG, Xiong Y, Huang XJ, Mei ZN, Hong ZG. Anti-inflammatory and blood stasis activities of essential oil extracted from Artemisia argyi leaf in animals. J Nat Med. 2016;70:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Luo L, Li G, Luan D, Yuan Q, Wei Y, Wang X. Antibacterial adhesion of borneol-based polymer via surface chiral stereochemistry. ACS Appl Mater Interfaces. 2014;6:19371-19377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Sun X, Qian Z, Luo L, Yuan Q, Guo X, Tao L, Wei Y, Wang X. Antibacterial Adhesion of Poly (methyl methacrylate) Modified by Borneol Acrylate. ACS Appl Mater Interfaces. 2016;8:28522-28528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Zhang XG, Shan C, Zhu JZ, Bao XY, Tong Q, Wu XF, Tang XC, Xue T, Liu J, Zheng GQ, Wang Y. Additive Neuroprotective Effect of Borneol with Mesenchymal Stem Cells on Ischemic Stroke in Mice. Front Physiol. 2017;8:1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Zhang Z, Luo Z, Bi A, Yang W, An W, Dong X, Chen R, Yang S, Tang H, Han X, Luo L. Compound edaravone alleviates lipopolysaccharide (LPS)-induced acute lung injury in mice. Eur J Pharmacol. 2017;811:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Xu T, Xu YJ, Xu XX, Wu YQ, Xu RA. [Effects of gypsum and saimei'an powder on wound healing]. Zhongguo Yiyao Zhinan. 2011;9:251-252. [DOI] [Full Text] |
| 16. | Fan H, Song XF, Xu YX, Yu JG. [Effects of Calcination on the Stabilization of Calcium Sulfate Whisker]. Huadong Ligong Daxue Xuebao, Ziran Kexueban. 2019;45:388-395. [DOI] [Full Text] |
| 17. | Shan Y, Li C, Wu Y, Li Q, Liao J. Hybrid cellulose nanocrystal/alginate/gelatin scaffold with improved mechanical properties and guided wound healing. RSC Adv. 2019;9:22966-22979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |