Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1190
Peer-review started: December 18, 2023
First decision: January 10, 2024
Revised: January 15, 2024
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: February 26, 2024
Processing time: 63 Days and 23.8 Hours
Retroperitoneal high-grade serous carcinoma (HGSC) of unknown origin is a sporadic tumor that can originate from ovarian cancer. Herein, we report the case of a woman with retroperitoneal HGSC of unknown origin and describe how she was diagnosed and treated.
A 71-year-old female presented with the tumor marker CA125 elevated to 1041.9 U/mL upon a regular health examination. Computed tomography revealed retroperitoneal lymph node enlargement. Subsequently, positron emission tomography scanning revealed lesions with increased F-18 fluorodeoxyglucose uptake at the nodes. As a result, she underwent laparoscopic lymph node resection, and pathology revealed metastatic adenocarcinoma with CK7(+), PAX8(+), WT1(+), PR(-), and p53 mutational loss of expression, indicating that the origin may be from the adnexa. The patient was admitted to our ward and underwent laparoscopic staging; however, the pathological results were negative. Under the suspicion of retroperitoneal HGSC of unknown origin, chemotherapy and targeted therapy were initiated. Tumor marker levels decreased after treatment.
We present a case of HGSC of unknown origin managed using retroperitoneal lymphadenectomy, staging surgery, chemotherapy, and targeted therapy.
Core Tip: We report a case of high-grade serous carcinoma (HGSC) of unknown origin in a postmenopausal woman treated with lymphadenectomy and chemotherapy. We provide updated information regarding the symptoms, signs, diagnosis, treatment, and prognosis of HGSCs of unknown origin. Based on our experience, we report our strategy to diagnose and treat this condition.
- Citation: Hsieh WL, Ding DC. Management of retroperitoneal high-grade serous carcinoma of unknown origin: A case report. World J Clin Cases 2024; 12(6): 1190-1195
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1190.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1190
Primary retroperitoneal carcinoma is sporadic. To date, only 16 cases of retroperitoneal serous carcinoma and 10 cases of high-grade serous carcinoma (HGSC) have been reported[1]. Retroperitoneal carcinoma shows a similar histological subtype and sex preference to ovarian carcinoma and, therefore, may share a similar pathogenesis[2]. Serous carcinomas are classified as HGSC or low-grade serous carcinoma[2]. A notable percentage of HGSCs develop within the secretory epithelial cells of the tubal fimbria[3].
The pathogenesis of retroperitoneal serous carcinoma appears to be associated with endosalpingiosis and a remnant Müllerian tract[4,5]. Several gene mutations have been associated with retroperitoneal serous carcinoma; KRAS, NRAS, and TP53 play a crucial role in high-grade transformation, and BRCA gene mutations have been reported to be relevant to endosalpingiosis[6]. Retroperitoneal carcinoma presents with nonspecific signs and symptoms, making its diagnosis difficult[7]. Evaluating tumor markers, such as CA125, which has high sensitivity in retroperitoneal serous carcinoma, can reveal the disease[7]. Imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), can be used for diagnosis[8].
Complete tumor resection in patients without ruptured capsules improves outcomes[9]. Furthermore, surgery may be done for better results, including exploration of the abdominal cavity and bilateral salpingo-oophorectomy with hysterectomy[1]. HGSC is sensitive to chemotherapy consisting of paclitaxel and carboplatin[10,11]. The role of targeted therapy for retroperitoneal HGSC of unknown origin has not yet been revealed; however, it is worth investigating further. Targeted therapies, including vascular endothelial growth factor (VEGF) and poly ADP-ribose polymerase (PARP) inhibitors, have been proven to be efficient with carboplatin-placitaxel treatment in ovarian HGSC[12].
Herein, we report the case of a woman with retroperitoneal HGSC of unknown origin and describe how she was diagnosed and treated.
Increased tumor marker CA125 level.
A 71-year-old female underwent a regular health examination at Da-Lin Tzu Chi Hospital, which revealed an elevation in the tumor marker CA125 (1041.9 U/mL) at 4 months before admission. No other discomfort was reported. Pelvic ultrasound did not reveal any abnormalities, and a subsequent CT scan (2 months before admission) revealed two enlarged retroperitoneal lymph nodes. A PET scan was performed and reported increased F-18 fluorodeoxyglucose (FDG) uptake in lesions located in the bilateral abdominal para-aortic region, which was probably the primary malignant or metastatic nodule.
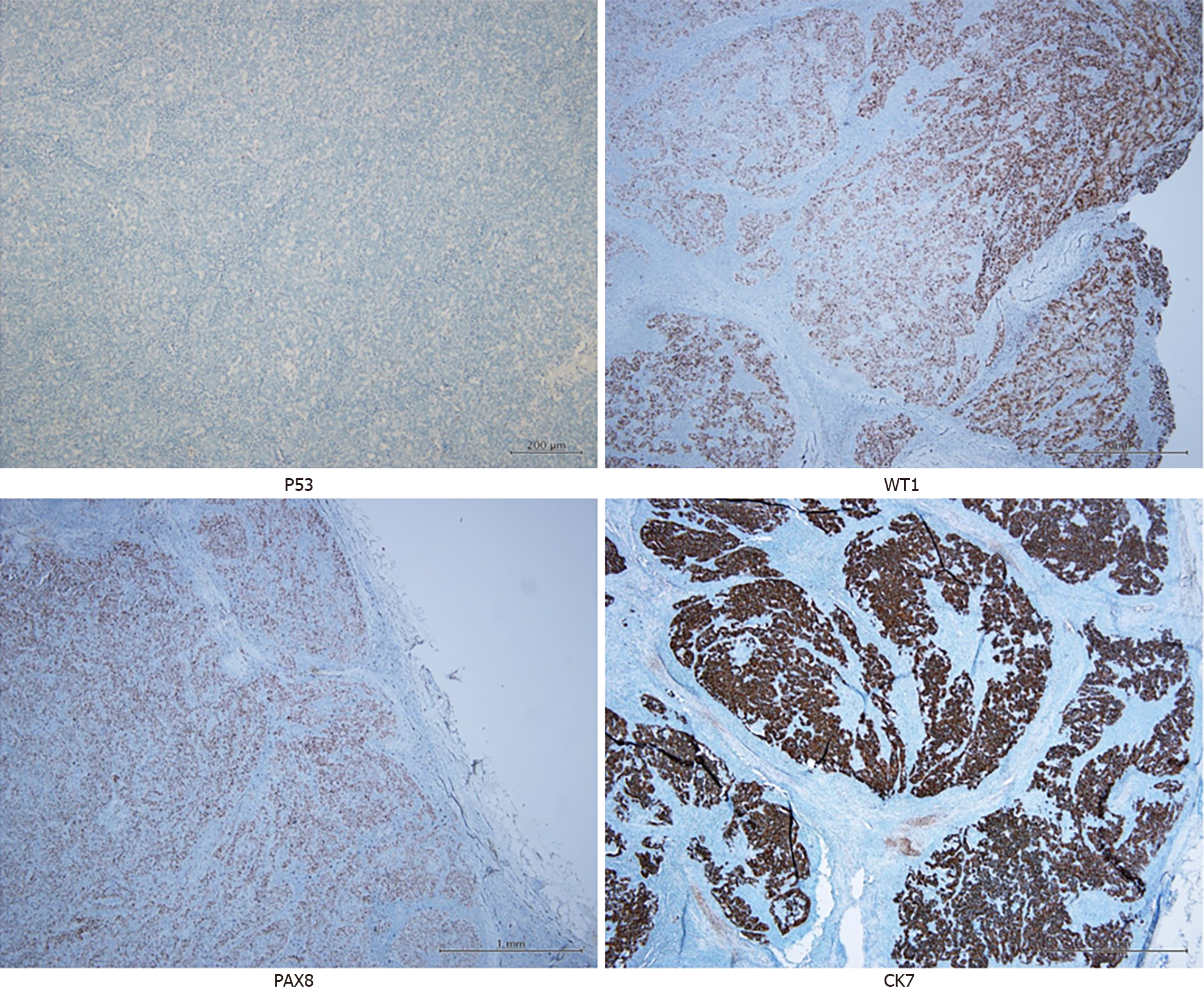
Consequently, the patient visited our general surgery department for further evaluation. Laparoscopic lymph node biopsy (two lymph nodes in the suprapancreatic region and one lymph node in the right pleural area) was performed 1 month before admission, which revealed metastatic adenocarcinoma, with CK7(+), CDX2(-), GATA(-), TTF-1(-), PAX8(+), WT1(+), PR(-), and p53 mutational loss of expression (Figure 1). Next-generation sequencing did not detect any clinically significant variants or biomarkers. The metastatic lesions were suspected to have originated from a gynecological organ, and the patient was referred to our outpatient department for further evaluation.
Gastric ulcers were diagnosed using panendoscopy performed 4 months before admission. The patient was under medical treatment and was regularly followed up. Sicca syndrome and interstitial cystitis were regularly followed up at the Da-Lin Tzu Chi Hospital. She also underwent tympanic membrane perforation/tympanoplasty 10 years previously, and had undergone cholecystectomy 3 years prior to presentation for cholelithiasis.
The patient experienced menarche at the age of 13 years and menopause at the age of 54 years, without receiving menopausal hormonal therapy. She delivered one child and had a spontaneous abortion. The patient had no history of oral contraceptive use. No previous hysterectomy, salpingectomy, or tubectomy was performed. Her mother had diabetes mellitus and there was no gynecological family history.
Pelvic examination revealed no abnormalities. Physical examinations of the abdomen, chest, heart, and musculoskeletal system did not reveal any significant findings. The neurological examination results were normal. Pelvic examination revealed atrophy of the vagina and cervix. No palpable masses are observed.
Four and one month before admission, her CA125 levels were 1041.9 and 1742.0 U/mL, respectively, showing a remarkable elevation of the tumor marker. After retroperitoneal lymphadenectomy, the value decreased to 17.8 U/mL. On the other hand, her creatinine ranged from 0.53 to 0.6 mg/dL, and her estimated glomerular filtration rate was 111.13–120.86 mL/min.
Two months prior to admission, pelvic ultrasonography did not reveal any abnormalities. CT performed on the same day revealed two lymph nodes of up to 26 mm in the para-aortic retroperitoneum, some lung nodules, a liver mass, and a calcified uterine myoma. Subsequently, a PET scan was arranged, and two enlarged lymph nodes in the bilateral abdominal para-aortic region were reported with intense uptake (maximum standardized uptake value: 9.3 and 10.0, right and left, respectively). One month before admission, the ultrasound showed an anteverted uterus sized 3.7 cm × 2.1 cm, with calcified myoma sited at the right lateral wall; the bilateral adnexa was invisible without discovering ascites.
HGSC of unknown origin with suprapancreatic and right pleural region lymph node metastasis was the final diagnosis.
After discussion with the patient, the possible origin of HGSC was suggested to have been the adnexa. Therefore, the patient was admitted to our ward and laparoscopic staging surgery was performed.
Laparoscopic staging surgery including total hysterectomy, bilateral salpingo-oophorectomy, and bilateral pelvic lymph node dissection. We did not find any abnormalities in the pelvic or retroperitoneal cavity. Histopathological examination did not reveal carcinoma in the specimen.
Chemotherapy with paclitaxel and carboplatin has been suggested for HGSCs of unknown origin. Three weeks after the surgery, paclitaxel was administered for the first time; however, chemotherapy was discontinued immediately after the development of tachycardia, dyspnea, and desaturation. As no chemotherapy was administered, a PARP inhibitor (Lynparza) was prescribed. After 3 wk, she was admitted and received the first course of Lipodox and Avastin; no side effects were noted.
The value of CA125 after this admission and the first course of failed chemotherapy was 69.7 and 58.7 U/mL, respectively. CA125 levels increased slightly after staging surgery and in the first month after lymphadenopathy. The patient is undergoing follow-up to determine the outcomes of subsequent chemotherapy and targeted therapy.
Primary retroperitoneal carcinoma, a rare tumor, represents 0.1%-0.2% of all malignancies, which is in contrast to the more prevalent ovarian cancer in females[1]. While its pathogenesis remains unknown, there is a potential association with ovarian carcinoma, evidenced by similarities in histological subtype and sex preference, although differences in histological subtypes may exist[13]. Carcinomas with an unidentified primary source, particularly when confined to the retroperitoneal space, may be classified as primary retroperitoneal carcinomas, as illustrated in situations where the carcinoma is exclusively found in the lymph nodes, resembling a carcinoma of unknown origin[1]. To date, only 16 cases of retroperitoneal serous carcinoma and 10 cases of HGSC have been reported[1].
The development of retroperitoneal serous carcinoma is believed to be linked to endosalpingiosis and a remnant Müllerian tract, with two main theories explaining the mechanism of endosalpingiosis: The tubal escape theory and Müllerian metaplasia theory[4,5]. According to the tubal escape theory, shed tubal epithelium may either be implanted on the peritoneal surface or spread through the lymphatic system to reach a lymph node[5]. According to the Müllerian metaplasia theory, dormant cells located outside the Müllerian tract, which includes the fallopian tube, endometrium, and endocervix, retain the ability to develop benign glands resembling those of the fallopian tube[4]. Additionally, endosalpingiosis can result from displacement of the primitive tubal tissue outside the fallopian tube[14].
Various gene mutations, including KRAS, NRAS, TP53, and BRCA, are associated with retroperitoneal serous carcinoma; TP53 mutation (loss of expression) was identified in our case to be relevant to the disease[15]. Among 10 patients with retroperitoneal HGSC, TP53 mutations have been noted in seven patients[1].
The diagnosis of retroperitoneal carcinoma is difficult because of its nonspecific symptoms and signs. Common symptoms of retroperitoneal carcinoma include abdominal discomfort and a palpable mass[7]. Of those patients with asymptomatic retroperitoneal carcinoma, imaging studies and evaluation of tumor markers may incidentally detect the disease. CA125 has a high sensitivity in retroperitoneal serous carcinoma, with 90% of cases showing an elevation[7]. CT and MRI revealed the location, size, shape, and thickness of the wall rather than the histological subtype of the tumor. Furthermore, PET scans can be helpful in discriminating between benign and malignant masses[16]. Therefore, surgery is the only way to provide a definitive diagnosis[17]. Our case is the first to report tumor marker elevation during a regular health examination without other noted symptoms. Subsequently, a CT scan revealed two enlarged lymph nodes and PET revealed that the lymph nodes had increased FDG uptake.
Surgery is necessary to diagnose retroperitoneal carcinoma. Furthermore, complete tumor resection in patients without ruptured capsules improves outcomes[9]. In patients with retroperitoneal serous carcinoma, it is crucial to explore the abdominal cavity using diaphragmatic implants and detect peritoneal recurrence after tumor resection[1]. Patients with retroperitoneal serous carcinoma may undergo bilateral salpingo-oophorectomy with hysterectomy because of the potential presence of concurrent adnexal serous carcinomas, including intraepithelial carcinoma[1]. Previous studies have revealed that 9 of 10 patients with retroperitoneal HGSC underwent surgical treatment[1]. Our patient was also treated with surgical resection of the lymph nodes followed by staging surgery.
The drug selection for chemotherapy after surgery depends on the histological subtype. HGSC is sensitive to chemotherapy consisting of paclitaxel and carboplatin[10,11]. The role of targeted therapy for retroperitoneal HGSC of unknown origin remains unknown; however, considering its histological type and pathogenic similarity to high-grade ovarian serous carcinoma, it is worth investigating further. Targeted therapies include VEGF and PARP inhibitors, which have been proven to be efficient in combination with carboplatin-paclitaxel treatment[12]. Ten retroperitoneal HGSC patients were treated with chemotherapy (eight with carboplatin and paclitaxel, one with docetaxel and carboplatin) and one with combined chemotherapy with nivolumab or avastin[1]. Our patient was also treated with Lynparza (a PARP inhibitor), Lipodox, and Avastin (a VEGF inhibitor).
Patients diagnosed with retroperitoneal serous carcinoma exhibit low survival rates, with 53% and 18% disease-free survival at 2 and 5 years, respectively[1]. Notably, patients with nodal-type retroperitoneal serous carcinoma may experience more favorable survival outcomes[11], similar to patients with serous carcinoma of the ovary, fallopian tube, or peritoneum who present with lymph node metastasis and minimal peritoneal disease[13]. The patient’s prognosis is to follow.
Our case report involved only a single case of retroperitoneal HGSC, limiting the generalizability of the clinical manifestations, diagnosis, and treatment of our findings. We anticipate that future research with a larger sample size or more extensive trials will provide a better understanding of the disease.
In conclusion, we present a case of HGSC of unknown origin that was managed using retroperitoneal lymphadenectomy, staging surgery, chemotherapy, and targeted therapy. Ongoing monitoring is essential to evaluate patient prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Zhang G, China S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Otsuka I. Primary Retroperitoneal Carcinomas: New Insights into Pathogenesis and Clinical Management in Comparison with Ovarian Carcinomas and Carcinoma of Unknown Primary. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 2. | Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 825] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 3. | Otsuka I. Mechanisms of High-Grade Serous Carcinogenesis in the Fallopian Tube and Ovary: Current Hypotheses, Etiologic Factors, and Molecular Alterations. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Sessine MS, Zhai Y, Tipton C, McCool K, Kuick R, Connolly DC, Fearon ER, Cho KR. Lineage tracing suggests that ovarian endosalpingiosis does not result from escape of oviductal epithelium. J Pathol. 2019;249:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Gallan AJ, Antic T. Benign müllerian glandular inclusions in men undergoing pelvic lymph node dissection. Hum Pathol. 2016;57:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Cao L, Nguyen D, Lu H. TP53 mutations in epithelial ovarian cancer. Transl Cancer Res. 2016;5:650-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Myriokefalitaki E, Luqman I, Potdar N, Brown L, Steward W, Moss EL. Primary retroperitoneal mucinous cystadenocarcinoma (PRMCa): a systematic review of the literature and meta-analysis. Arch Gynecol Obstet. 2016;293:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Engbersen MP, Van Driel W, Lambregts D, Lahaye M. The role of CT, PET-CT, and MRI in ovarian cancer. Br J Radiol. 2021;94:20210117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Tokai H, Nagata Y, Taniguchi K, Matsumura N, Kitasato A, Tokunaga T, Takeshita H, Kuroki T, Maeda S, Ito M, Fujioka H. The long-term survival in primary retroperitoneal mucinous cystadenocarcinoma: a case report. Surg Case Rep. 2017;3:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Chae YK, Saleem N, Roh Y, Bilal H, Viveiros P, Sukhadia B, Lin X, Sheikh MM, Park LC. Exceptional response to chemotherapy followed by concurrent radiotherapy and immunotherapy in a male with primary retroperitoneal serous Adenocarcinoma: a case report and literature review. BMC Cancer. 2019;19:748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Otsuka I, Honma K. FDG PET/CT in Primary Retroperitoneal Serous Carcinoma. Clin Nucl Med. 2023;48:625-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Gadducci A, Guarneri V, Peccatori FA, Ronzino G, Scandurra G, Zamagni C, Zola P, Salutari V. Current strategies for the targeted treatment of high-grade serous epithelial ovarian cancer and relevance of BRCA mutational status. J Ovarian Res. 2019;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1:61-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 14. | Homsi MJ, Dadlani A, Khazai B, Anendaga CM, Bakhru S, Flaherty F. Diffuse abdominal and pelvic endosalpingiosis: A case report. Radiol Case Rep. 2022;17:3515-3518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Boyarskikh UA, Gulyaeva LF, Avdalyan AM, Kechin AA, Khrapov EA, Lazareva DG, Kushlinskii NE, Melkonyan A, Arakelyan A, Filipenko ML. Spectrum of TP53 Mutations in BRCA1/2 Associated High-Grade Serous Ovarian Cancer. Front Oncol. 2020;10:1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Narayanan P, Sahdev A. The role of (18)F-FDG PET CT in common gynaecological malignancies. Br J Radiol. 2017;90:20170283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Kohada Y, Teishima J, Hattori Y, Kurimura Y, Fujii S, Sadahide K, Fukuoka K, Ueno T, Kitano H, Goto K, Hieda K, Shinmei S, Sentani K, Inoue S, Hayashi T, Yasui W, Matsubara A. Serous adenocarcinoma of retroperitoneum: a case report. Int Cancer Conf J. 2017;6:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |