Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1182
Peer-review started: December 8, 2023
First decision: December 29, 2023
Revised: January 5, 2024
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 26, 2024
Processing time: 74 Days and 2 Hours
Lung cancer (LC) is the leading cause of malignancy-related deaths worldwide. The most common sites of metastasis include the nervous system, bone, liver, respiratory system, and adrenal glands. LC metastasis in the parotid gland is very rare, and its diagnosis presents a challenge. Here, we report a case of parotid metastasis in primary LC.
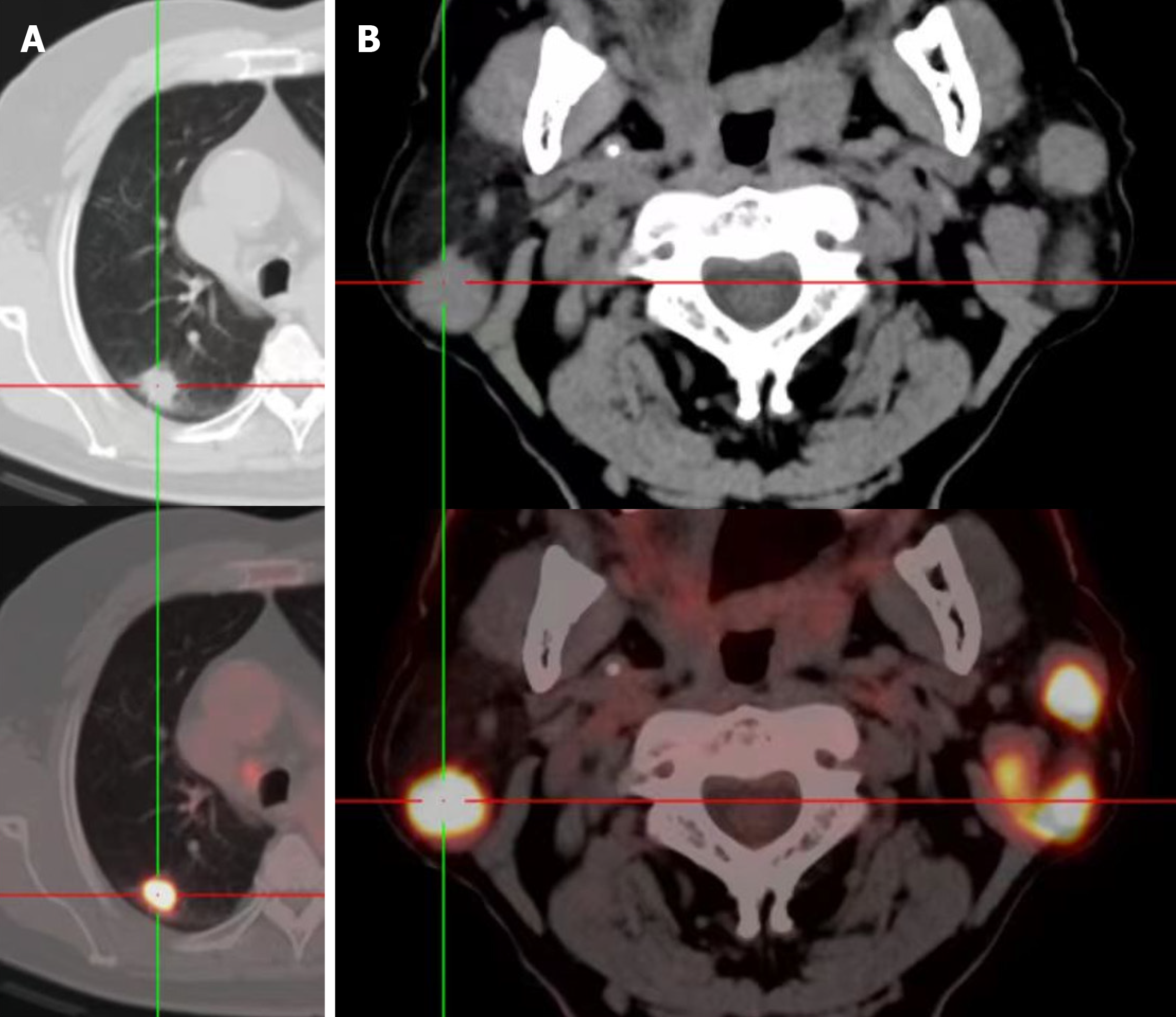
The patient was a 74-year-old male who was discovered to have bilateral facial asymmetry inadvertently two years ago. The right earlobe was slightly swollen and without pain or numbness. Computed tomography (CT) examination showed bilateral lung space-occupying lesions. Pulmonary biopsy was performed and re
This case report highlights the challenging diagnosis of parotid metastasis in LC given its rare nature. Such lesions should be differentiated from primary tumors of the parotid gland. Simple radiological imaging is unreliable, and puncture bio
Core Tip: A 74-year-old male presented with a bilateral facial asymmetry. Computed tomography examination revealed bilateral lung space-occupying lesions. Pulmonary biopsy was performed on the right-upper-lung nodule tissue and revealed the presence of adenocarcinoma. Parotid puncture biopsy was performed considering lung adenocarcinoma metastasis. This work highlights the challenging diagnosis of parotid metastasis in lung cancer and the need for biopsy in the final diagnosis.
- Citation: Yan RX, Dou LB, Wang ZJ, Qiao X, Ji HH, Zhang YC. Parotid metastasis of rare lung adenocarcinoma: A case report. World J Clin Cases 2024; 12(6): 1182-1189
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1182.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1182
Lung cancer (LC) refers to the second most common malignant tumor in the world, next to breast cancer, and is a serious threat to human life and health[1]. LC can be classified as small-cell or non-small-cell carcinoma (e.g., adenocarcinoma, squamous-cell carcinoma, and large-cell carcinoma). Non-small-cell LC (NSCLC) accounts for nearly 85% of all LCs[2]. The most common metastatic sites of LC comprise the nervous system, bone, liver, respiratory system, and adrenal glands[3]. Reports on the metastasis of LC in the parotid gland are extremely rare. In this study, we report a case of paro
A 74-year-old male presented with a bilateral facial asymmetry for 2 years.
The patient was a 74-year-old male who was discovered to have bilateral facial asymmetry inadvertently two years ago. The right earlobe was slightly swollen and without pain or numbness.
He used to be healthy, denied diabetes, heart disease, hypertension for 15 years, and denied trauma surgery.
He denied any history of exposure to special chemicals and radiation. He had a smoking history of 50 years and had quit smoking for 7 years. He denied drinking alcohol. There were no infectious diseases, metabolic diseases, diabetes, hemo
The patient’s vitals were stable upon examination. The following conditions were noted: asymmetric left and right sides of his face, swollen posterior inferior pole of the bilateral parotid glands, intact surface skin without ulceration, low local skin temperature, the left tumor measuring approximately 5.0 cm × 4.0 cm, right tumor with a size of around 3.0 cm × 3.0 cm, smooth surface, clear boundary with the surrounding tissues, good mobility, no evident spontaneous pain or tenderness, and the facial nerve showing no sign of involvement. In addition, the patient showed normal mouth opening degree, good oral hygiene condition, minimal swelling of the corresponding parotid duct opening, and normal saliva secretion.
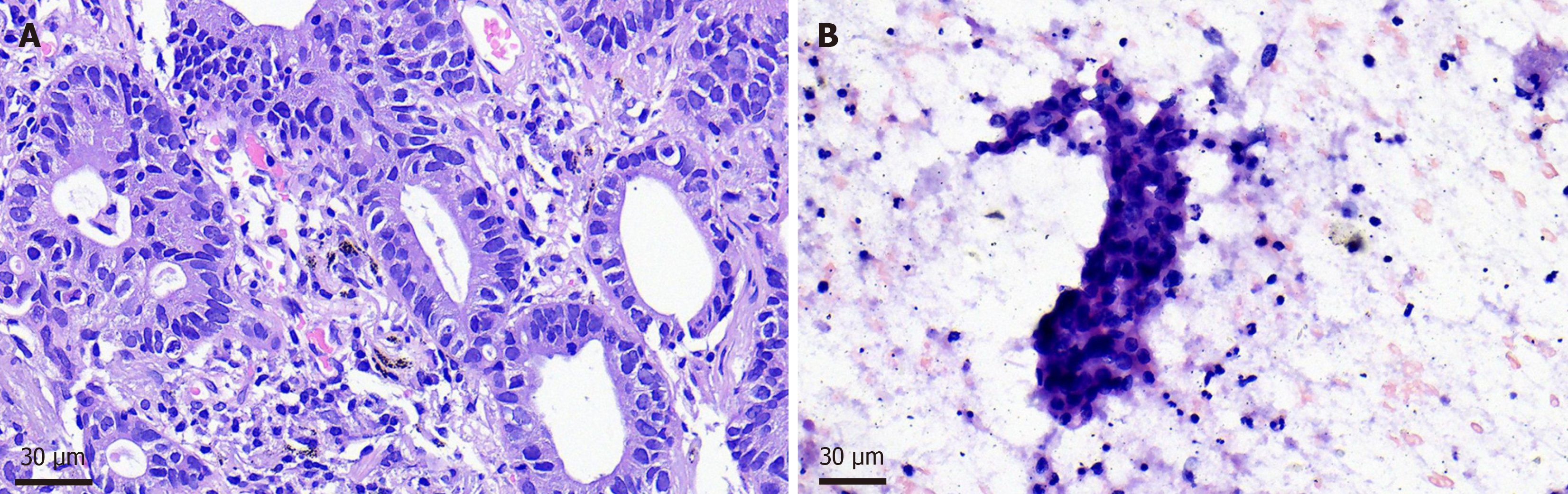
Parotid puncture biopsy: Considering lung adenocarcinoma metastasis. Pulmonary biopsy was performed: (right upper lung nodule tissue) adenocarcinoma; Gene detection of lung biopsy specimens showed EGFR gene 21 exon L858R mu
Computed tomography (CT) examination revealed bilateral lung space-occupying lesions. Pulmonary biopsy was per
The patient’s was assessed further. Color ultrasound examination indicated multiple heterogeneous hypoechoic nodules in bilateral parotid glands. The patient had smoked for 50 years and had no crucial medical history. CT of the chest re
Right lung adenocarcinoma stage 4; Bilateral parotid gland lung metastatic cancer.
The patient is currently receiving immunotherapy.
The patient’s condition was stable upon reexamination in May 2023 but showed slight progression during another assessment in August 2023.
Clinically, the metastasis of LC to the parotid gland rarely occurs. The metastatic routes of LC include direct diffusion, lymphatic metastasis, and hematogenous metastasis. In this case, metastatic signs of lymph node enlargement were observed on the neck of the patient, and parotid gland metastasis of LC was considered lymphatic. LC usually shows no distinct sequence of metastatic sites. The most common sites of metastasis comprise the nervous system, bone, liver, re
Gupta et al[4] retrospectively reviewed published literature over the past 44 years (August 1977 to December 2021) and discovered 122 documented cases of oral soft tissue metastasis (OSTM) from LC as the sole primary source (Table 1). In Sonia Gupta’s study, no difference was observed in the age of onset of OSTM, which occurred in 5-6 years, between sexes. The male majority showed a clear gender advantage. A total of 35 patients (28.7%) had a history of LC. This number is more than the average patients who present with oral soft tissue as the only metastatic site. LC is the most common pri
| Feature | |
| Total number of papers published | 120 |
| CR | 111 |
| SC | 3 |
| LTE | 3 |
| Co | 2 |
| RA | 1 |
| Total number of patients | 122 |
| World-wide distribution of cases, n (%) | |
| Japan | 31 (25.8) |
| India | 14 (11.7) |
| United States | 13 (10.8) |
| China | 10 (8.3) |
| Turkey | 9 (7.5) |
| Italy | 7 (5.8) |
| Korea = United Kingdom | 5 (4.2) |
| Brazil | 4 (3.3) |
| Morocco = Taiwan = France = Greece = Tunisia = Germany = Switzerland = Australia = Spain | 2 (1.7) |
| Israel = Beirut = Bangladesh = Victoria | 1 (0.8) |
| Gender, n (%) | |
| M | 100 (82) |
| F | 22 (18) |
| Average age of patients (range), yr | 60.8 (25-87) |
| Average age of male patients (range), yr | 61.4 (25-87) |
| Average age of female patients (range), yr | 58.3 (36-87) |
| Chief complaint, n (%) | |
| Related to oral health | 97 (79.5) |
| Not related to oral health | 22 (18) |
| Routine check-up | 3 (2.5) |
| Previous history of LC, n (%) | 35 (28.7) |
| No previous history LC, n (%) | 78 (63.9) |
| NA data on previous history of LC, n (%) | 9 (7.4) |
| Associated risk factors, n (%) | 66 (54.1) |
| S | 53 (80.3) |
| A | 9 (13.6) |
| HT | 7 (10.7) |
| As | 6 (9.1) |
| TB | 3 (4.5) |
| Others | 15 (27.2) |
| No risk factors, n (%) | 38 (31.1) |
| NA data on associated risk factors, n (%) | 18 (14.8) |
| Site of metastasis, n (%) | |
| G | 51 (41.8) |
| Max (Ant-6, Post-12, Both-1, SNA-5/R-10, L-5, Both-4, SNA-5) | 24 (47) |
| Mand (Ant-6, Post-9, Both-2, SNA-8/R-8, L-12, Both-2, SNA-3) | 25 (49) |
| SNA-2 | 2 (4) |
| T (Ant-6, Base-4, DL-6, SNA-3, Tip-2) | 21 (17.2) |
| To [Palatine-19 (R-10, L-9), Lingual-2] | 21 (17.2) |
| P (R-8, L-4, BL-2) | 14 (11.5) |
| SMG (L-3, R-1, BL-1) | 5 (4.1) |
| Pa | 3 (2.5) |
| Lip (U-1, L-1) | 2 (1.6) |
| BM | 1 (0.8) |
| RMT | 1 (0.8) |
| MS | 3 (2.5) |
| Oral soft tissues as the initial site of metastasis, n (%) | |
| Y | 74 (60.6) |
| N | 44 (36.1) |
| NA | 4 (3.3) |
| Oral soft tissues as the only site of metastasis, n (%) | |
| Y | 63 (51.6) |
| N | 54 (44.2) |
| NA | 5 (4.2) |
| Average time of detection of metastasis from diagnosis of LC | Few days to 10 yr |
| Most common clinical features, n (%) | |
| Swelling | 100 (81.9) |
| Ulceration | 13 (10.6) |
| Exophytic | 12 (9.8) |
| Pedunculated | 10 (8.2) |
| Nodules | 6 (4.9) |
| Edema | 5 (4.2) |
| Erosive | 2 (1.6) |
| BOP | 10 (8.2) |
| ST = LP = FNP | 1 (0.8) |
| Type of LC, n (%) | |
| AD | 46 (37.7) |
| SCLC | 19 (15.6) |
| MT | 17 (13.9) |
| SCC | 10 (8.2) |
| NSCLC | 8 (6.5) |
| LCC | 6 (4.9) |
| NEC | 5 (4.1) |
| Sa | 3 (2.4) |
| Ha | 2 (1.6) |
| AC | 2 (1.6) |
| AS | 2 (1.6) |
| PI | 2 (1.6) |
| LC Metastasis, n (%) | |
| IL | 55 (45.1) |
| CL | 33 (27) |
| BL | 2 (1.6) |
| NA | 32 (26.2) |
| Treatment aids, n (%) | |
| RT + CH | 31 (25.4) |
| CH | 27 (22.1) |
| RT = SU | 12 (9.8) |
| CH + RT + SU | 8 (6.5) |
| CH + SU | 2 (1.6) |
| SU + Ta = SU = Sy | 1 (0.8) |
| NG | 11 (9) |
| NA | 5 (4.1) |
| RBP | 4 (3.3) |
| STO | 2 (1.6) |
| Death, n (%) | 66 (54.1%) |
| Reasons of death, n (%) | |
| MM | 18 (27.2) |
| DC | 18 (27.2) |
| RF | 4 (6.1) |
| Others | 13 (19.7) |
| NA | 13 (19.7) |
| Average time of death from diagnosis of metastasis | 1 wk to 2.5 yr |
| Partial relief of symptoms, n (%) | 1 (0.8) |
| Favorable prognosis, n (%) | 13 (10.6) |
| TGO, n (%) | 5 (4.1) |
| LFU, n (%) | 6 (4.9) |
Of the 122 cases of LC metastasis, 14 involved the parotid gland. The submandibular and sublingual glands lack lymph nodes, and the route of metastasis is primarily blood derived. Thus, metastasis to these salivary glands is very rare. Gupta et al[4] reported five cases of LC-induced submandibular gland metastasis but found no cases involving sublingual gland metastasis.
The detection of distant metastases in the diagnosis of malignant tumors plays a crucial role in the accurate prognosis and guidance of treatment strategies[8,9]. In the case of LC, FDG-PET/CT sensitively identifies extrathoracic metastases, especially bone and adrenal lesions. However, several benign diseases (infection or inflammation) or malignant lesions that are unrelated to primary NSCLC may show strong FDG uptake, similar to distant metastases such as adenomas[10]. Studies have reported cases of misdiagnosis of Warthin tumor (WT) as a metastatic disease based solely on radiological imaging of LC patients[11]. High FDG-PET/CT uptake cannot be used to distinguish metastatic disease from WTs. WT is the second most common benign tumor in salivary glands after pleomorphic adenoma, and most of related cases occur in the parotid gland. Most of the WTs are benign, and the incidence of malignancy reaches 0.3%[11]. Differential diagnosis relies on a detailed medical history and imaging studies. Clinically, benign tumors of the parotid gland have a long cour
The diagnosis of LC parotid metastases presents a challenge because of rare nature. Lesions should be differentiated from primary parotid tumors. Radiological imaging alone is unreliable, and puncture biopsy is needed for final diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ekine-Afolabi B, United Kingdom S-Editor: Zhang H L-Editor: A P-Editor: Xu ZH
| 1. | World Health Organization; International Agency for Research on Cancer. Geneva: 2020. Lung Cancer. International Agency for Research on Cancer. [cited 2023 Nov 8]. Available from: https://www.who.int/zh/news-room/fact-sheets/detail/cancer. |
| 2. | American Cancer Society. Key statistics for lung cancer. 2018. [cited 2023 Nov 8]. Available from: https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html. |
| 3. | Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 599] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 4. | Gupta S, Jawanda MK, Kedia NB, Deb AR, Ganganna A, Saurabh K, Yadav SK, Yadav AB. Lung cancer metastasis to oral soft tissues; Systematic review of 122 cases. J Clin Exp Dent. 2022;14:e854-e874. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Irani S. Metastasis to the oral soft tissues: A review of 412 cases. J Int Soc Prev Community Dent. 2016;6:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity - pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 8. | Kwee RM, Kwee TC. Modern imaging techniques for preoperative detection of distant metastases in gastric cancer. World J Gastroenterol. 2015;21:10502-10509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (3)] |
| 9. | Lin A, Ma S, Dehdashti F, Markovina S, Schwarz J, Siegel B, Powell M, Grigsby P. Detection of distant metastatic disease by positron emission tomography with (18)F-fluorodeoxyglucose (FDG-PET) at initial staging of cervical carcinoma. Int J Gynecol Cancer. 2019;29:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Farsad M. FDG PET/CT in the Staging of Lung Cancer. Curr Radiopharm. 2020;13:195-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Yaranal PJ, T U. Squamous Cell Carcinoma Arising in Warthin's Tumour: A Case Report. J Clin Diagn Res. 2013;7:163-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Wang R, Wang T, Zhou Q. Parotid metastases from primary lung cancer: Case series and systematic review of the features. Front Oncol. 2022;12:963094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | White CK, Williams KA, Rodriguez-Figueroa J, Langer CJ. Warthin's tumors and their relationship to lung cancer. Cancer Invest. 2015;33:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |