Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1111
Peer-review started: November 27, 2023
First decision: December 19, 2023
Revised: December 28, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: February 26, 2024
Processing time: 85 Days and 0.3 Hours
Neuroendocrine neoplasms of the female genital tract are rare.
To enhance our clinical understanding of neuroendocrine carcinoma (NEC) of the ovary.
A retrospective review was conducted on 12 patients diagnosed with NEC of the ovary, analyzing clinicopathological characteristics, treatment modalities, and survival status.
The median age at diagnosis was 34.5 years (range: 20 to 62 years). Among the 12 cases, 9 were small cell carcinoma of the ovary and 3 were large cell NEC. Five cases were stage I tumors, one case was stage IV, and six cases were stage III. Eleven patients underwent surgery as part of their treatment. All patients received adjuvant chemotherapy. Among the 12 patients, one patient received radiotherapy, and one patient with a BRCA2 mutation was administered PARP inhibitor maintenance after chemotherapy. The median progression-free survival was 13 months, and the median overall survival was 19.5 months. Four cases remained disease-free, while eight cases experienced tumor recurrence, including three cases that resulted in death due to disease recurrence.
NEC of the ovary is a rare condition that is more common in women of child
Core Tip: Through the analysis and summary of 12 cases of ovarian neuroendocrine carcinoma, we found that the incidence of ovarian neuroendocrine carcinoma is low and the prognosis is poor. Surgery is the cornerstone of treatment, and some patients may benefit from comprehensive treatment.
- Citation: Xing XY, Zhang W, Liu LY, Han LP. Clinical analysis of 12 cases of ovarian neuroendocrine carcinoma. World J Clin Cases 2024; 12(6): 1111-1119
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1111.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1111
Neuroendocrine neoplasms (NENs) of the female genital tract are rare, accounting for only 1%-2% of gynecological malignancies[1]. Among these, primary ovarian NENs are even rarer, as most gynecological NENs are located in the uterine cervix. NENs are a heterogeneous group of diseases. Neuroendocrine carcinoma (NEC) of the ovary represents the highly malignant end of this spectrum, characterized by high-grade tumors that often exhibit aggressive clinical behavior and unfavorable outcomes[2]. NEC of the ovary can be further classified into two subtypes: Large cell neuroendocrine carcinoma (LCNEC) and small cell carcinoma of the ovary (SCCO). Diagnosis of NEC primarily relies on histology, which includes immunohistochemistry. Common markers used for diagnosis include chromogranin A (Cg-A), synaptophysin (Syn), neuron- specific enolase, and CD56. The histologic origin of NEC is believed to be either from the ovarian surface epithelium or associated with the dedifferentiation of a de novo carcinoma, as they have often been associated with epithelial neoplasms[3,4]. Currently, there are no established treatment guidelines for NEC of the ovaries due to limited knowledge about this rare entity. Most patients are treated based on guidelines for ovarian epithelial malignancies, which typically involve debulking surgery followed by adjuvant chemotherapy (carboplatin and paclitaxel). The lack of clinical data and evidence-based protocols may contribute to unsatisfactory outcomes in relation to this condition.
In this study, we presented a series of 12 cases of NEC of the ovary, which is one of the largest series reported from a single institution. We analyzed the clinical, pathological, radiological, and survival data of these cases to provide a reference for future therapeutic approaches.
A database search was conducted to identify patients with primary ovarian NEC who were treated in the Department of Gynecology at the First Affiliated Hospital of Zhengzhou University from August 2015 to May 2020. A total of 12 cases were identified with a confirmed diagnosis of NEC through postoperative pathology and the pathological interpretation was conducted by a pathologist specialized in gynecologic malignancies and verified by a second gynecological pathologist. Patients with incomplete clinical data and/or who were lost to follow-up were excluded from the study.
Clinical data were obtained through a retrospective chart review, which included information such as age, chief symptoms, auxiliary examination results, FIGO stage, pathology, and treatment. The follow-up period was defined as the time between the initial diagnosis of NEC of the ovary and the last date of contact or death, and the follow-up included routine gynecological examination, computed tomography (CT)/ positron emission tomography contrast-enhanced and blood tumor marker detection [antigen (CA)-125, HE4 and CA-199]. The performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) score standard. Tumors were staged according to the 2014 FIGO staging classification for ovarian carcinomas. The follow-up period concluded on May 31, 2023. The collection and analysis of data were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2023-KY-0892-002).
From August 2015 to May 2020, we searched 2048 records of patients with ovarian cancer, and the incidence of NEC of the ovary among all ovarian malignancies in this series was 0.59% (12/2048). The median age at diagnosis was 34.5 years (ranging from 20 to 62 years). None of the patients had a family history of ovarian cancers. The initial presentations included abdominal pain (5/12), pelvic mass (4/12), vaginal bleeding (2/12), and abdominal distention (1/12). None of the patients in the study group presented with paraneoplastic syndrome. Eleven out of twelve cases showed an increase in preoperative cancer CA-125 levels, with a median level of 146.6 U/mL (range: 38.63 to 401.2 U/mL; normal range: 35 U/mL). However, after treatment, all elevated tumor markers returned to normal levels. None of the patients showed an increase in CA-199 or HE4 levels. Based on the FIGO 2014 staging standard, 5 cases were classified as stage IA, 1 case as stage IVB, and 6 cases as stage IIIC disease. The clinical features can be found in Table 1.
| Case | Age (yr) | Symptom | CA-125 (U/mL) | Size (cm) | Location | Diagnosis | Stage |
| 1 | 28 | Mass | 91.4 | 12 | Right | SCCO | IA |
| 2 | 62 | Pain | 38.63 | 14 | Left | LCNEC | IA |
| 3 | 29 | Pain | 46.4 | 8 | Right | SCCO | IA |
| 4 | 37 | Mass | 42.13 | 8 | Right | SCCO | IA |
| 5 | 20 | Pain | 401.2 | 20 | Bilateral | SCCO | IC |
| 6 | 54 | Bloating | 170.6 | 5 | Bilateral | LCNEC | IIIB |
| 7 | 26 | Pain | 150.7 | 5 | Bilateral | SCCO | IIIC |
| 8 | 31 | Mass | 61.25 | 10 | Left | SCCO | IIIC |
| 9 | 32 | Pain | 146.6 | 10 | Bilateral | SCCO | IIIC |
| 10 | 46 | Mass | 191 | 13 | Bilateral | LCNEC | IIIC |
| 11 | 58 | Bleeding | 14.5 | 4 | Bilateral | SCCO | IIIC |
| 12 | 62 | Bleeding | 166.5 | 15 | Bilateral | SCCO | IVB |
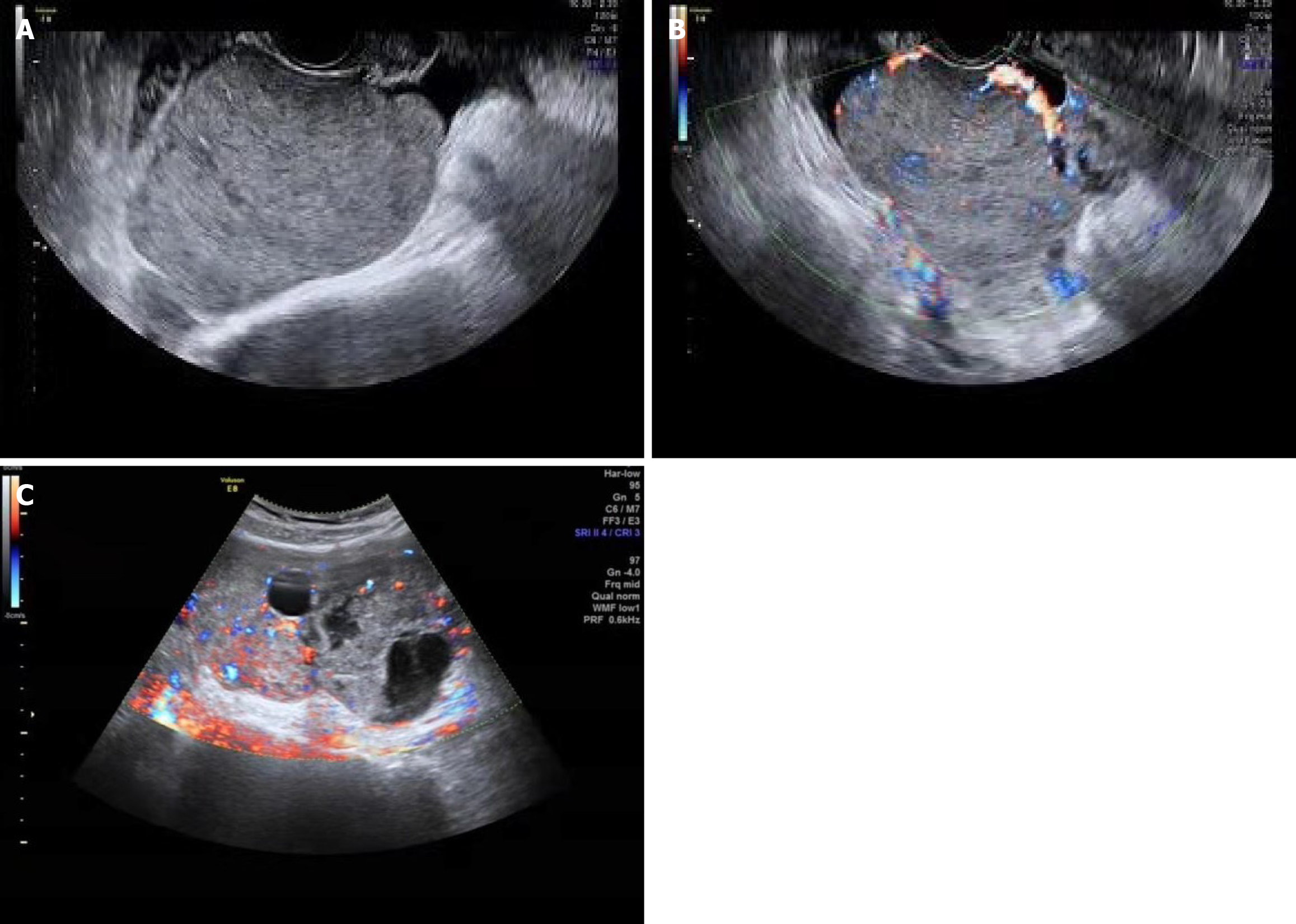
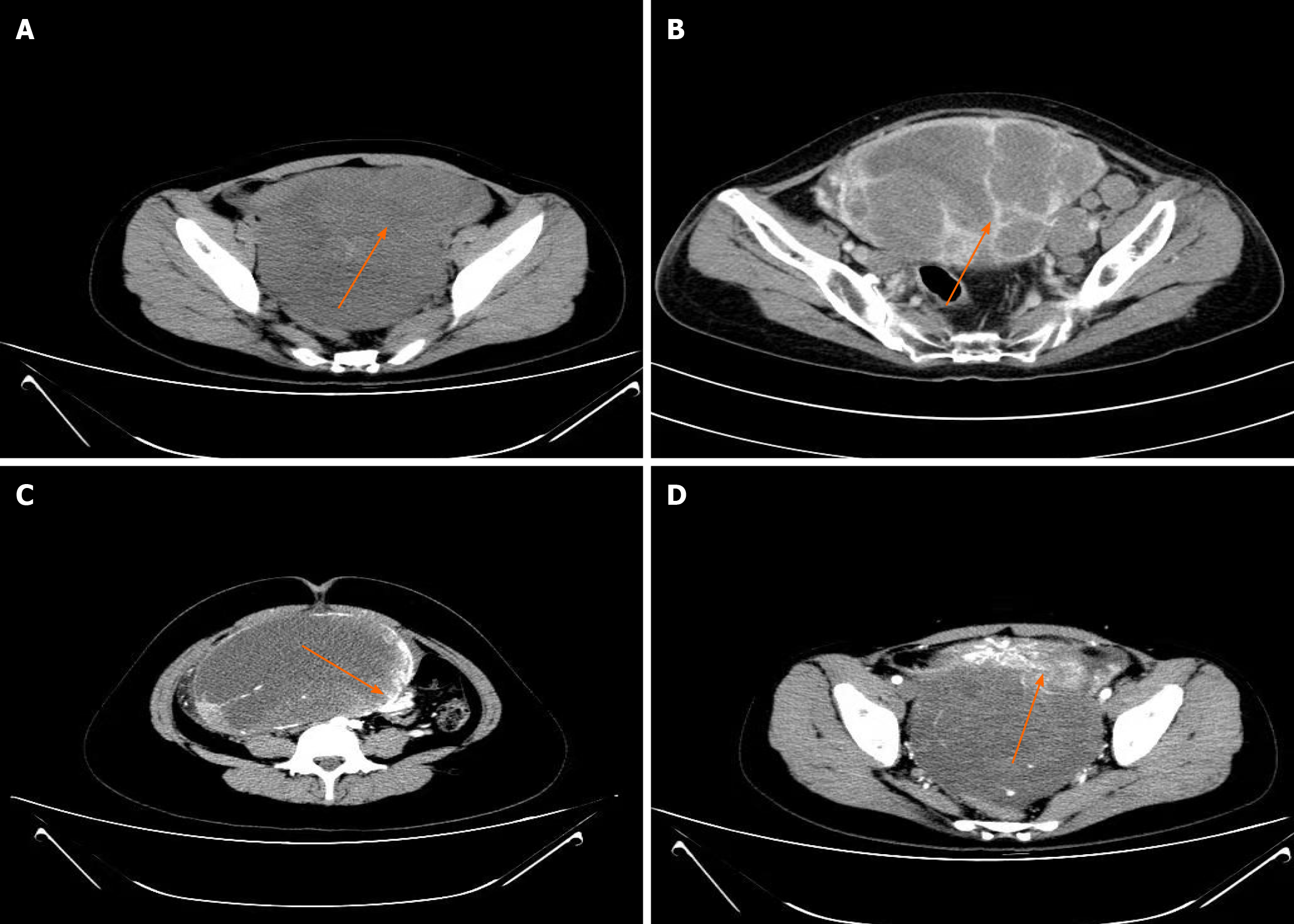
Abdominal ultrasound (US) was performed on all 12 patients, and CT was performed on 10 patients. Abdominal ultrasound showed that the masses were typically solid or cystic-solid with abundant blood supply within the lesion, occupying the pelvic cavity. The borders of the masses were clearly defined with irregular contours (Figure 1). The CT scan typically revealed a soft tissue mass with varying density in the pelvic cavity. Contrast-enhanced CT demonstrated slight enhancement or uneven enhancement of the solid components (Figure 2).
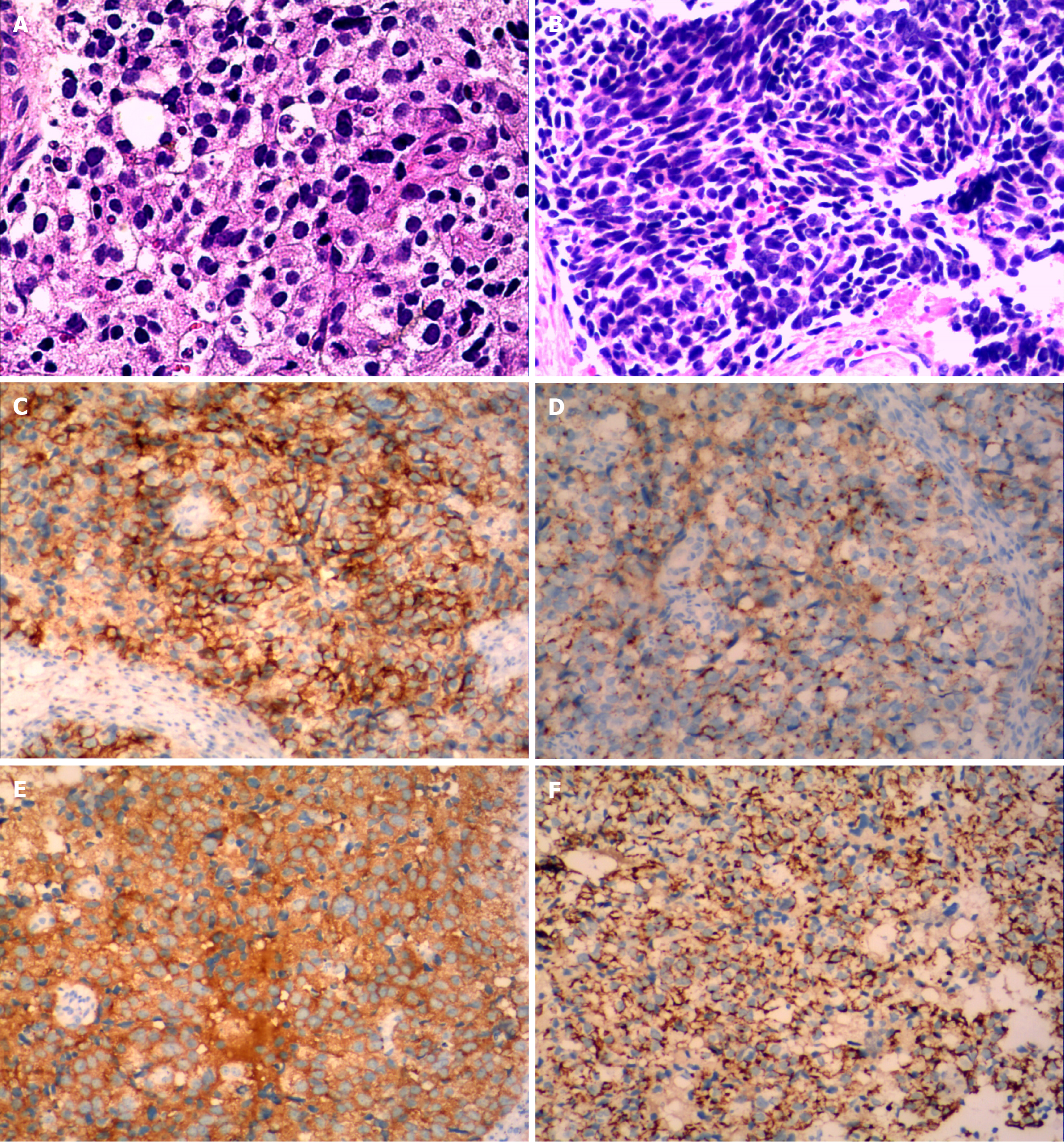
Excluding metastatic ovarian cancer, a total of 12 patients with ovarian NEC were identified. Out of the 12 primary ovarian NEC patients, 9 had SCCO and 3 had LCNEC. Three cases of LCNEC were pure large cell carcinoma. The median greatest dimension of the ovarian tumor was 10.4 cm, ranging from 4 to 20 cm. The tumor was unilateral in 5 cases and bilateral in 7 cases (Figure 3). The neoplastic cells were positive for CD56 in all cases (Figure 3C), Cg-A in 5 out of 10 cases (Figure 3D), and Syn in 9 out of 11 cases (Figure 3E). Additionally, cytokeratin (CK) was positive in 5 out of 6 cases (Figure 3F), with CK7 being positive in 4 out of 6 cases, epithelial memberane antigen being positive in 6 out of 7 cases, and PAX-8 being positive in 4 out of 8 cases.
Surgery was performed by gynecological oncologists with the primary goals of achieving a complete tumor resection and adequate staging, following the FIGO guidelines. Primary surgery was conducted in 10 patients, with one patient having received neoadjuvant chemotherapy (TCx3), and one patient (case 12) declining surgery due to extensive metastasis. Out of the 11 patients, 8 underwent bilateral salpingo-oophorectomy (BSO) and hysterectomy. Fertility was preserved in two patients (case 3 and 5), while one patient (case 7) only underwent omentectomy and peritoneal biopsy as their family refused debulking surgery. Systemic pelvic and paraaortic lymphadenectomy was performed in all 9 patients. To achieve optimal tumor debulking, omentectomy was performed in all nine patients, with additional extra-ovarian debulking in five patients. After surgery, 10 out of 11 patients showed no visible tumors. Prior to chemotherapy, all patients were evaluated for their performance status using the ECOG 0-1 scale. The chemotherapy regimens used are listed in Table 2. The time interval between surgery and the start of chemotherapy ranged from 2 to 4 wk. Six patients received etoposide/cisplatin (EP), while the remaining cases were treated with paclitaxel/cisplatin (TP) or carboplatin (TC), as indicated. Among the 6 patients treated with TP or TC, patient 10, who requested genetic testing, was found to have a BRCA2 mutation. After chemotherapy, this patient received PARP inhibitor maintenance. However, the tumor recurred 2 months post-chemotherapy. On the other hand, patient 11 received radiotherapy after chemotherapy and remained disease-free during the 49-month follow-up period.
| Case | Operation | Chemotherapy | Recurrence | PFS/month | Survival state | Follow-up/month |
| 1 | BSO/TAH/OM/LN | EP6 | No | 34 | NED | 34 |
| 2 | BSO/TAH/OM/LN | TC6 | No | 37 | NED | 37 |
| 3 | USO | EP7 | Yes, brain | 9 | DOD | 10 |
| 4 | BSO/TAH/OM/LN | EP6 | Yes, liver | 13 | AWD | 13 |
| 5 | BSO/OM /LN | EP6 | Yes, bones | 17 | AWD | 25 |
| 6 | NACT+BSO/TAH/OM/LN | TC8 | Yes, pelvic | 13 | AWD | 15 |
| 7 | OM/PB | TC3 | Yes, pelvic | 3 | DOD | 10 |
| 8 | BSO/TAH/OM/LN | EP6 | No | 15 | NED | 26 |
| 9 | BSO/TAH/OM/LN | TP6 | Yes, lymph | 6 | AWD | 17 |
| 10 | BSO/TAH/OM/LN | TP6 +PARPi | Yes, liver | 9 | AWD | 22 |
| 11 | BSO/TAH/OM/LN | TC6+ Radiotherapy | No | 49 | NED | 49 |
| 12 | - | EP6 | Yes, pelvic | 6 | DOD | 9 |
The follow-up time for the 12 cases ranged from 9 to 49 months, with a median of 19.5 months. Out of the patients, 3 expired due to tumor recurrence, while 9 patients were still alive during the follow-up period. Among the patients, 8 experienced recurrence and/or disease progression, with recurrences observed in the liver, bones, pelvis, and inguinal lymph nodes. Due to the limited number of cases, we were unable to determine the impact of FIGO stage, tumor pathological type, and different chemotherapies on prognosis (Table 2).
Patients with NEC of the ovary tend to be younger than those with other histologic subtypes of ovarian cancer. Some studies have also suggested that NEC of the ovary can occur after menopause[5]. In our study, the main symptoms observed were abdominal pain and pelvic mass. Additionally, a majority of the cases (7 out of 12) were diagnosed at an advanced stage, which aligns with findings in the existing literatures[5]. The rate of lymph node metastasis in NEC of the ovary has rarely been reported in previous studies. In our study, only one case showed lymph node metastasis, indicating a potentially low rate of lymph node metastasis for this type of tumor. This suggests that lymphatic metastasis may not be the primary route of metastasis for NEC of the ovary.
The diagnosis of NEC of the ovary often lacks specific findings on US and CT scans alone[6]. However, CT scans can provide valuable information about the location, size, and presence of distant metastasis of the lesion. Therefore, CT scans are of great significance for preoperative staging and determining surgical methods. In this study, it was observed that serum CA-125 levels increased in most cases (11 out of 12), suggesting a potential link between CA-125 and NEC of the ovary. Previous studies have confirmed that CA-125 levels are closely associated with epithelial ovarian cancers and can be utilized for monitoring and diagnosing such cancers[7]. The elevation of serum CA-125 in ovarian NEC has been reported in the literature[8]. In our study, CA-125 levels increased in 11 out of 12 cases, with an average of 126.7 U/mL. However, the CA-125 level in ovarian NEC was found to be lower than that in ovarian epithelial carcinoma[9]. Therefore, further studies are needed to analyze the relationship between CA-125 and NEC of the ovary. Ascitic fluid cytology was also performed, and only two cases showed the presence of malignant cells in ascites or peritoneal lavage fluid. This demonstrates that identifying malignant cells derived from NEC of the ovary in ascitic fluid may be challenging.
Histopathological analysis of tissue specimens is crucial for diagnosing NEC of the ovary. Immunohistochemistry is necessary to confirm the diagnosis[4]. The results of immunohistochemistry revealed that tumors expressed at least one neuroendocrine marker (Cg-A, CD56, or Syn). In this study, CD56 was expressed in all cases, suggesting that it was the most sensitive marker for demonstrating the neuroendocrine nature of these tumors. However, the lack of specificity of CD56 was observed as it was also expressed in nonendocrine tissues such as renal tubules, ovarian sex cord-stromal, and thyroid follicular cells. For this reason, some authors did not recommend relying solely on CD56 to demonstrate neuroendocrine components[10-12]. Therefore, immunohistochemistry plays a crucial role in the diagnosis.
Small cell carcinoma of the ovary is further classified as small cell carcinoma of the ovary-hypercalcemic type (SCCOHT) and small cell ovarian carcinoma of the pulmonary type (SCCOPT)[13]. Due to the differences in age of onset, clinical manifestations, and molecular mechanisms between SCCOPT and SCCOHT, SCCOPT is included in the chapter of neuroendocrine Neoplasms of the Female Reproductive System for the first time in the 2020 edition of World Health Organization[5]. SCCOPT should be differentiated from ovarian metastatic small cell lung cancer by combining medical history and imaging data. When small cell carcinoma is found in the lung and ovary at the same time, the lung tumor is considered to be the primary lesion. When lung small cell carcinoma metastasizes to the ovary, it mostly does not involve the surface of the ovary. The presence or absence of other ovarian epithelial tumors can also be used as a differential direction. In addition, SCCOPT should be distinguished from SCCOHT. The mean age of onset of SCCOHT is 24 years, bilateral ovaries are rarely involved, hypercalcemia occurs in two-thirds of patients, and loss of SMARCA4 protein expression is specific for the diagnosis of SCCOHT[14]. However, only one of the nine patients in our paper with SCCO underwent SMARCA4 detection and was diagnosed as SCCOHT. In the future, we will pay more attention to the detection of SMARCA4 to guide the clinicopathological classification.
The current main treatment for NEC of the ovary is typically surgical resection followed by adjuvant chemotherapy. However, there is currently no standard guideline in place[15]. The goal of surgical resection is to remove all visible lesions. A recent study has shown that surgeries can significantly improve survival rates, therefore complete surgical resection should be recommended as the primary treatment option. Some young patients may wish to preserve their fertility. However, the use of fertility-sparing surgery is a topic of debate. There have been a few small case series reporting successful pregnancies in patients who underwent unilateral salpingo-oophorectomy (USO) and received adjuvant chemotherapy[16,17]. In another study, 26 patients underwent USO, but none of them resulted in a successful pregnancy[18]. Of these cases, two opted for fertility-sparing surgery. Unfortunately, one patient who had a BSO passed away after 10 months of treatment, and the other patient who had a USO experienced recurrence after 12 months. It is worth noting that postoperative chemotherapy can potentially impact ovarian function[19]. To safeguard the ovary from the harmful effects of chemotherapy, clinical practice has incorporated techniques such as oocyte or embryo cryopreservation, as well as cryopreservation of the ovarian cortex. However, performing these methods may be challenging and the future use of cryopreserved germ cells is still uncertain[20]. Therefore, additional studies should be conducted to evaluate the feasibility of fertility-sparing surgery for patients with ovarian NEC.
The present study refers to adjuvant therapies for lung small cell carcinoma, specifically chemotherapy and radiation[2]. The literature suggests that EP and TP are the main chemotherapy regimens[16,21]. Although small sample studies and case reports indicate potential benefits of chemotherapy, the lack of prospective studies hinders the availability of convincing evidence regarding its effect on the prognosis of patients with ovarian NEC. In this study, 6 patients were treated with EP, and 6 patients received PT. Out of the total 8 patients, progression or recurrence was observed. Among these, 3 patients relapsed due to platinum resistance, while 2 patients showed progression during chemotherapy. These findings indicate a poor response of these tumors to the chemotherapy. Hence, further investigation is required to determine the sensitivity of patients to platinum drugs and identify the optimal chemotherapy regimen.
Radiotherapy has been infrequently utilized in the treatment of ovarian cancers[2]. In our study, only one patient received radiation therapy and was followed up for 49 months without any recurrence of the disease. The role of radiotherapy in the management of NEC of the ovary is still not well-established, although some reports have suggested potential benefits[22,23]. Therefore, further investigation is warranted to explore the potential of radiotherapy in NEC of the ovary. Currently, there is limited research on targeted therapy in NEC of the ovary. In this study, a single case with a BRCA2 germline mutation was treated with a PARP inhibitor. However, the patient experienced recurrence after 2 months, suggesting that PARP inhibitors may not be beneficial for patients with NEC of the ovary. Further recruitment of additional cases is necessary to investigate the role of PARP inhibitors in treating NEC of the ovary.
The 1-, 3- and 5- year survival rates of ovarian NEC were 58.3%, 33.3% and 27.6%, respectively[5]. Limited studies have been conducted on the prognosis of ovarian neuroendocrine cancer, with tumor staging being considered an important prognostic factor. The median follow-up time of this study was 19.5 months. Three patients died due to disease recurrence, four patients survived without evidence of recurrent disease, and five patients survived with tumor. Among patients with relapse or progression, four patients showed an increase in CA-125 levels. Further data are needed to confirm whether serum CA-125 levels can be used as an indicator for disease monitoring.
NEC of the ovary is a rare and aggressive malignant disease with a poor prognosis. The diagnosis primarily relies on histopathological analysis of tissue specimens, and immunohistochemistry plays a crucial role in both diagnosis and differential diagnosis. Surgical resection is the preferred treatment option, and adjuvant chemotherapy along with potential radiotherapy can potentially extend the survival of certain cases. Considering the rarity of primary ovarian NEC, a future multicenter study is necessary to gain further understanding of this uncommon disease.
Primary ovarian neuroendocrine carcinoma (NEC) are rare and there are no established treatment guidelines for NEC of the ovaries due to limited knowledge about this rare entity.
Primary ovarian NEC are rare, and we want to know more about it.
We retrospectively analyzed the clinicopathological features, treatment and survival of 12 patients with ovarian NEC, and hope to enhance our clinical understanding of NEC of the ovary.
The clinical data of 12 patients with ovarian NEC in our hospital were retrospectively analyzed.
Among more than 2000 patients with ovarian cancer during the same period, we identified 9 cases of small cell ovarian cancer and 3 cases of large cell NEC. Eleven patients underwent surgery, all of whom received adjuvant chemotherapy, and 1 patient with a BRCA2 mutation received PARP inhibitor maintenance therapy after chemotherapy. The median progression-free survival was 13 months, and the median overall survival was 19.5 months. Four patients were alive without tumor recurrence, 8 patients had tumor recurrence, and 3 of them died of tumor recurrence.Abdominal ultrasound showed that the masses were typically solid or cystic-solid with abundant blood supply within the lesion, occupying the pelvic cavity. The borders of the masses were clearly defined with irregular contours. The computed tomography (CT) scan typically revealed a soft tissue mass with varying density in the pelvic cavity. Contrast-enhanced CT demonstrated slight enhancement or uneven enhancement of the solid components. The neoplastic cells were positive for CD56 in all cases, chromogranin A in 5 out of 10 cases, and synaptophysin in 9 out of 11 cases. Additionally, cytokeratin (CK) was positive in 5 out of 6 cases, with CK7 being positive in 4 out of 6 cases, epithelial memberane antigen being positive in 6 out of 7 cases, and PAX-8 being positive in 4 out of 8 cases.
NEC of the ovary is a rare condition that is more common in women of childbearing age and is associated with aggressive behavior and poor clinical outcomes. Surgical resection remains the mainstay of treatment, with some patients benefiting from adjuvant chemoradiation therapy.
In my opinion, the future research direction may be pathogenesis and immunotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moyana T, Canada S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Rouzbahman M, Clarke B. Neuroendocrine tumors of the gynecologic tract: select topics. Semin Diagn Pathol. 2013;30:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Huang W, Bao Y, Luo X, Yao L, Yuan L. Neuroendocrine neoplasms of the ovary: an analysis of clinicopathological characteristics and prognosis with a focus on histological grading. Endocrine. 2022;77:188-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Virarkar M, Vulasala SS, Morani AC, Waters R, Gopireddy DR, Kumar S, Bhosale P, Lall C. Neuroendocrine Neoplasms of the Gynecologic Tract. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Howitt BE, Kelly P, McCluggage WG. Pathology of Neuroendocrine Tumours of the Female Genital Tract. Curr Oncol Rep. 2017;19:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Zhu Y, Meng F, Fang H, Zhang Z, Wang L, Zheng W. Clinicopathologic characteristics and survival outcomes in neuroendocrine carcinoma of the ovary. Int J Gynecol Cancer. 2020;30:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Choi YD, Lee JS, Choi C, Park CS, Nam JH. Ovarian neuroendocrine carcinoma, non-small cell type, associated with serous carcinoma. Gynecol Oncol. 2007;104:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Piatek S, Panek G, Lewandowski Z, Bidzinski M, Piatek D, Kosinski P, Wielgos M. Rising serum CA-125 levels within the normal range is strongly associated recurrence risk and survival of ovarian cancer. J Ovarian Res. 2020;13:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Hu D, Ma D, Zhang ZJ, Zhang Y, Huang K, Li X. Prognosis comparison between small cell carcinoma of ovary and high-grade serous ovarian cancer: A retrospective observational cohort study. Front Endocrinol (Lausanne). 2023;14:1103429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Zhang R, Siu MKY, Ngan HYS, Chan KKL. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 117] [Reference Citation Analysis (0)] |
| 10. | Gupta P, Bagga R, Rai B, Srinivasan R. Primary pure large cell neuroendocrine carcinoma of the ovary: histopathologic and immunohistochemical analysis with review of the literature. Int J Clin Exp Pathol. 2021;14:1000-1009. [PubMed] |
| 11. | Gupta P, Kapatia G, Gupta N, Dey P, Rohilla M, Gupta A, Rai B, Suri V, Rajwanshi A, Srinivasan R. Small Cell Carcinoma of the Ovary: Clinicopathologic and Immunohistochemical Analysis of 7 New Cases of a Rare Malignancy. Int J Surg Pathol. 2021;29:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | McCluggage WG, McKenna M, McBride HA. CD56 is a sensitive and diagnostically useful immunohistochemical marker of ovarian sex cord-stromal tumors. Int J Gynecol Pathol. 2007;26:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Li Y, Wu Y, Zhang Y, Li X. Case report: Strategies for improving outcomes in patients with primary ovarian small-cell neuroendocrine carcinoma. Front Oncol. 2022;12:954289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Tischkowitz M, Huang S, Banerjee S, Hague J, Hendricks WPD, Huntsman DG, Lang JD, Orlando KA, Oza AM, Pautier P, Ray-Coquard I, Trent JM, Witcher M, Witkowski L, McCluggage WG, Levine DA, Foulkes WD, Weissman BE. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type-Genetics, New Treatment Targets, and Current Management Guidelines. Clin Cancer Res. 2020;26:3908-3917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Pang L, Guo Z. Primary neuroendocrine tumors of the ovary: Management and outcomes. Cancer Med. 2021;10:8558-8569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Pang L, Chen J, Chang X. Large-cell neuroendocrine carcinoma of the gynecologic tract: Prevalence, survival outcomes, and associated factors. Front Oncol. 2022;12:970985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Dykgraaf RH, de Jong D, van Veen M, Ewing-Graham PC, Helmerhorst TJ, van der Burg ME. Clinical management of ovarian small-cell carcinoma of the hypercalcemic type: a proposal for conservative surgery in an advanced stage of disease. Int J Gynecol Cancer. 2009;19:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Callegaro-Filho D, Gershenson DM, Nick AM, Munsell MF, Ramirez PT, Eifel PJ, Euscher ED, Marques RM, Nicolau SM, Schmeler KM. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): A review of 47 cases. Gynecol Oncol. 2016;140:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Cosgrove CM, Salani R. Ovarian effects of radiation and cytotoxic chemotherapy damage. Best Pract Res Clin Obstet Gynaecol. 2019;55:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Sonigo C, Beau I, Binart N, Grynberg M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | He Y, Zhao H, Li XM, Yin CH, Wu YM. A clinical analysis of small-cell neuroendocrine carcinoma of the gynecologic tract: report of 20 cases. Arch Gynecol Obstet. 2019;299:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Callegaro-Filho D, Burke TW, Eifel PJ, Ramirez PT, Euscher EE, Schmeler KM. Radiotherapy for recurrent small cell carcinoma of the ovary: A case report and review of the literature. Gynecol Oncol Rep. 2015;11:23-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Georgescu TA, Bohiltea RE, Munteanu O, Furtunescu F, Lisievici AC, Grigoriu C, Gherghiceanu F, Vlădăreanu EM, Berceanu C, Ducu I, Iordache AM. Emerging Therapeutic Concepts and Latest Diagnostic Advancements Regarding Neuroendocrine Tumors of the Gynecologic Tract. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |